Novel Amphiphilic Polyfluorene-Graft-(Polymethacrylic Acid) Brushes: Synthesis, Conformation, and Self-Assembly
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of 2.7-Dibromo-9,9-Bis(3-Hydroxylpropyl)-Fluorene (1)
2.2. Synthesis of Poly[(9,9-Bis(3-Hydroxylpropyl)-Fluorene)-Alt-(9,9-Dioctylfluorene)] (P1)
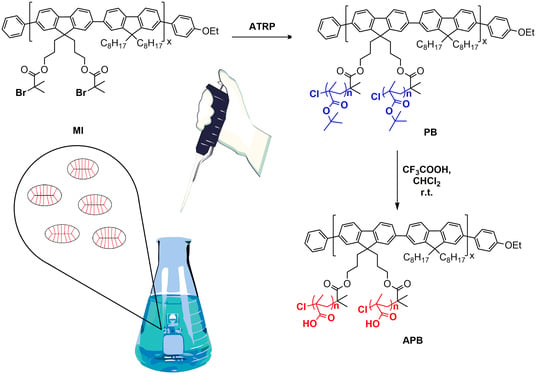
2.3. Synthesis of Macroinitiator MI
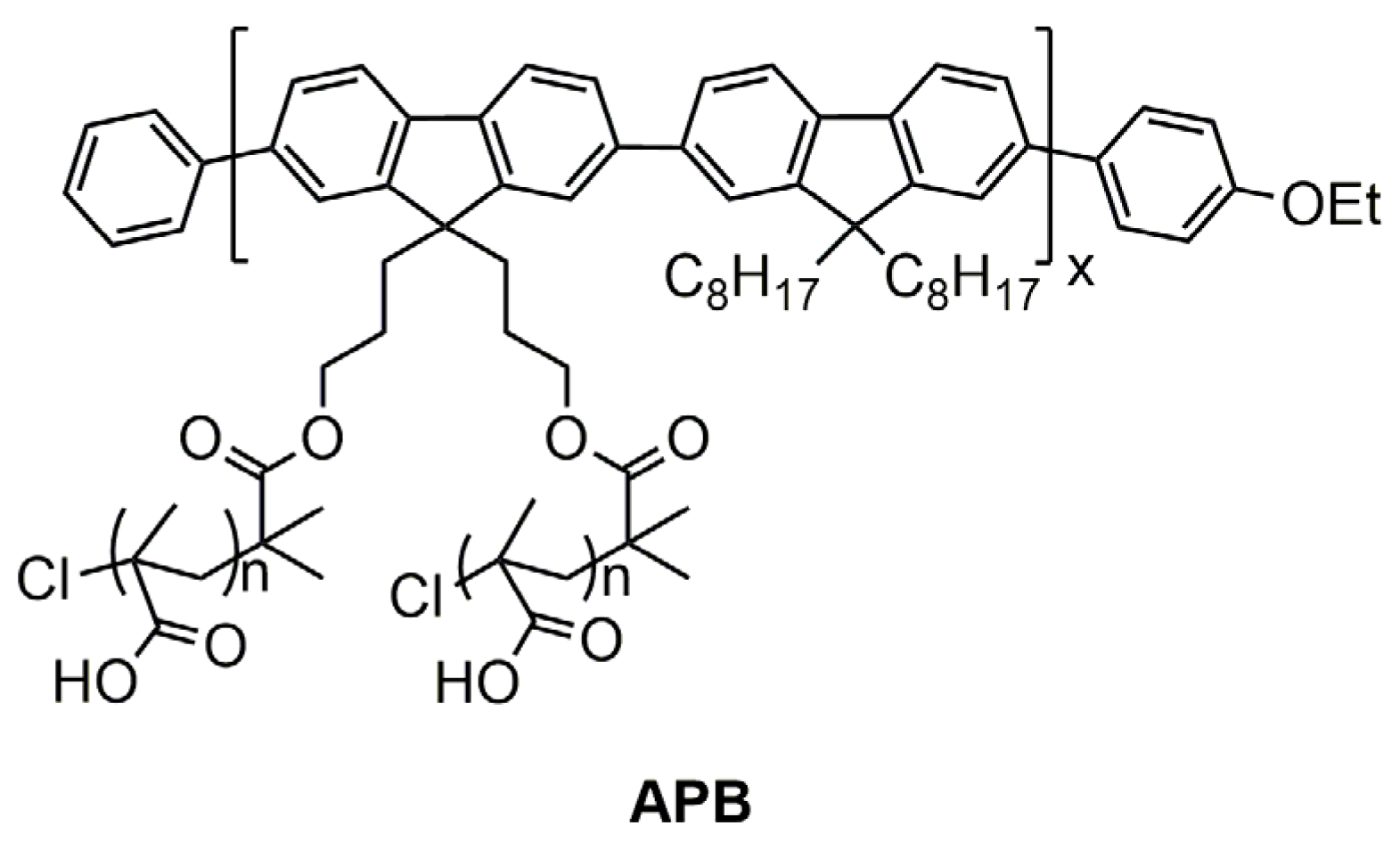
2.4. Synthesis of Polymer Brush with Polyfluorene Backbone and Poly-Tert-Butyl Methacrylate Said Chains (PB)
2.5. Synthesis of Amphiphilic Polymer Brush with Polyfluorene Backbone and Poly Methacrylic Acid Said Chains (APB) and Preparation of APB Nanoparticles
2.6. Determination of Molar Masses, Hydrodynamic Characteristics and Investigation of Self-Assembly of Molecular Brushes in Dilute Solutions
3. Results and Discussion
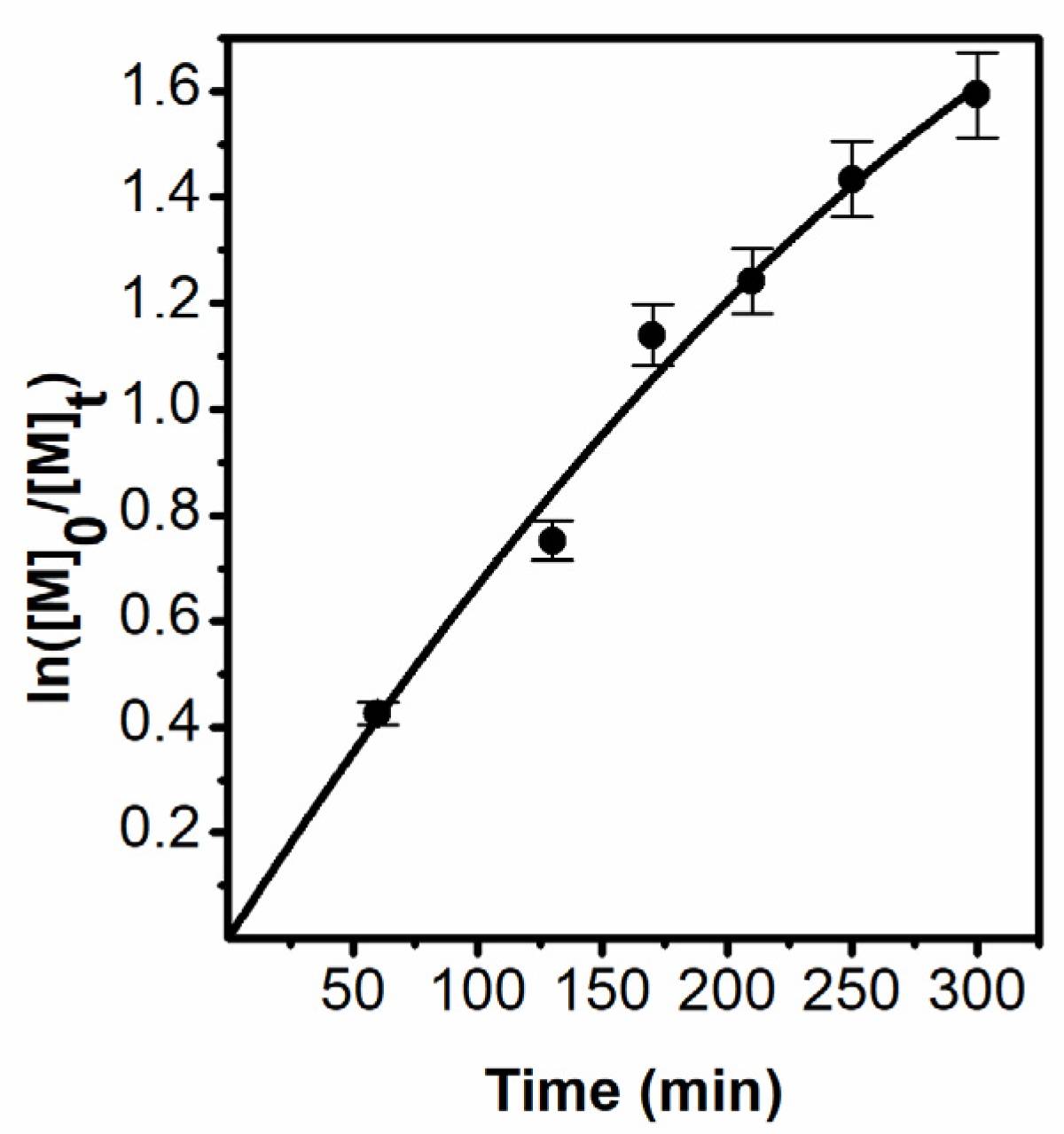
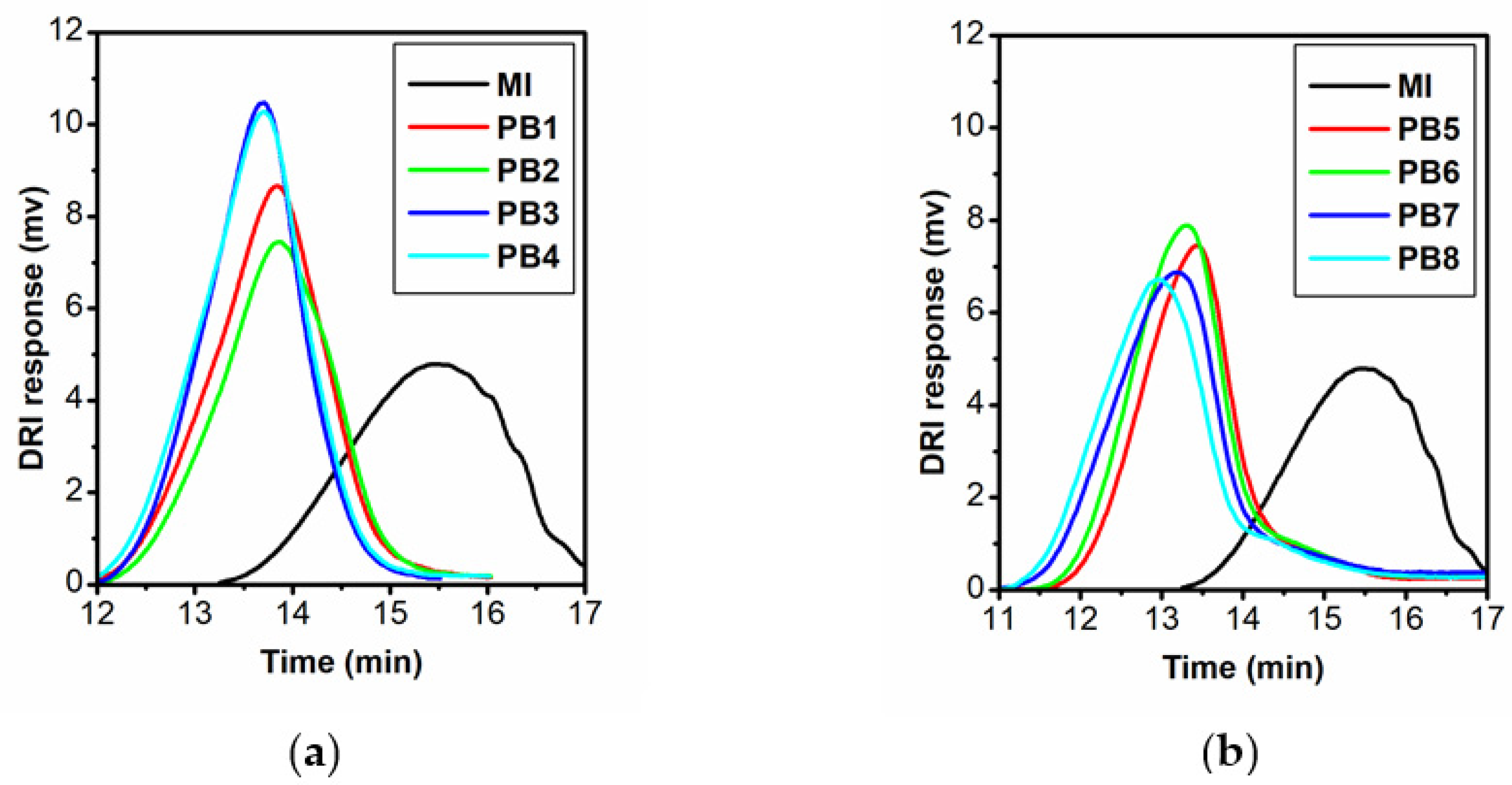
3.1. Synthes of Polymer Brushes
3.2. Molecular Hydrodynamic Characteristics of MI, PB, and APB
3.3. Study of the Thermal Characteristics of Polymer Brushes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheng, G.; Böker, A.; Zhang, M.; Krausch, G.; Müller, A.H.E. Amphiphilic cylindrical core-shell brushes via a ‘grafting from’ process using ATRP. Macromolecules 2001, 34, 6883–6888. [Google Scholar] [CrossRef]
- Lu, X.; Wei, A.; Fan, Q.; Wang, L.; Chen, P.; Dong, X.; Huang, W. Macroporous foam of reduced graphene oxides prepared by lyophilization. Mater. Res. Bull. 2012, 47, 4335–4339. [Google Scholar] [CrossRef]
- Zhou, L.; Geng, J.; Wang, G.; Liu, J.; Liu, B. A water-soluble conjugated polymer brush with multihydroxy dendritic side chains. Polym. Chem. 2013, 4, 5243–5251. [Google Scholar] [CrossRef]
- Meleshko, T.K.; Ivanov, I.V.; Kashina, A.V.; Bogorad, N.N.; Simonova, M.A.; Zakharova, N.V.; Filippov, A.P.; Yakimansky, A.V. Diphilic Macromolecular Brushes with a Polyimide Backbone and Poly(methacrylic acid) Blocks in Side Chains. Polym. Sci.-Ser. B. 2018, 60, 35–50. [Google Scholar] [CrossRef]
- Borodinov, N.; Gil, D.; Savchak, M.; Gross, C.E.; Yadavalli, N.S.; Ma, R.; Tsukruk, V.V.; Minko, S.; Vertegel, A.; Luzinov, I. En Route to Practicality of the Polymer Grafting Technology: One-Step Interfacial Modification with Amphiphilic Molecular Brushes. ACS Appl. Mater. Interfaces. 2018, 10, 13941–13952. [Google Scholar] [CrossRef]
- Ivanov, I.V.; Meleshko, T.K.; Kashina, A.V.; Yakimansky, A.V. Amphiphilic multicomponent molecular brushes. Russ. Chem. Rev. 2019, 88, 1248–1290. [Google Scholar] [CrossRef]
- Simonova, M.; Filippov, A.; Nosova, G.; Zhukova, E.; Litvinova, L.; Berezin, I.; Yakimansky, A. Carbazole-functionalized polyfluorenes: Synthesis and conformational properties in chloroform solution and β-phase formation in copolyfluorene films. Mater. Today Chem. 2021, 22, 100553. [Google Scholar] [CrossRef]
- Lian, X.; Wu, D.; Song, X.; Zhao, H. Synthesis and self-assembly of amphiphilic asymmetric macromolecular brushes. Macromolecules 2010, 43, 7434–7445. [Google Scholar] [CrossRef]
- Teulère, C.; Ben-Osman, C.; Barry, C.; Nicolaÿ, R. Synthesis and self-assembly of amphiphilic heterografted molecular brushes prepared by telomerization. Eur. Polym. J. 2020, 141, 110080. [Google Scholar] [CrossRef]
- Filippov, A.P.; Belyaeva, E.V.; Krasova, A.S.; Simonova, M.A.; Meleshko, T.K.; Ilgach, D.M.; Bogorad, N.N.; Yakimansky, A.V.; Larin, S.V.; Darinskii, A.A. Conformations of molecular brushes based on polyimide and poly(methyl methacrylate) in selective solvents: Experiment and computer simulation. Polym. Sci.-Ser. A. 2014, 56, 393–404. [Google Scholar] [CrossRef]
- Filippov, A.P.; Belyaeva, E.V.; Krasova, A.S.; Simonova, M.A.; Tarabukina, E.B.; Meleshko, T.K.; Ilgach, D.M.; Bogorad, N.N.; Yakimansky, A.V. Synthesis and investigation of the solution behavior of graft block copolymers of polyimide and poly(methyl methacrylate). Polym. Sci.-Ser. A 2014, 56, 1–9. [Google Scholar] [CrossRef]
- Tarabukina, E.; Amirova, A.; Belyaeva, E.; Krasova, A.; Simonova, M.; Filippov, A.; Meleshko, T.; Ilgach, D.; Bogorad, N.; Yakimansky, A. Conformational Characteristics of Polyimide Initiator for the Synthesis of Poly(Methylmethacrylate) Grafted Block-Copolymers. J. Macromol. Sci. Part B 2013, 52, 1545–1557. [Google Scholar] [CrossRef]
- Yakimansky, A.V.; Meleshko, T.K.; Ilgach, D.M.; Bauman, M.A.; Anan’Eva, T.D.; Klapshina, L.G.; Lermontova, S.A.; Balalaeva, I.V.; Douglas, W.E. Novel regular polyimide-graft-(polymethacrylic acid) brushes: Synthesis and possible applications as nanocontainers of cyanoporphyrazine agents for photodynamic therapy. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 4267–4281. [Google Scholar] [CrossRef]
- Grimsdale, A.C.; Chan, K.L.; Martin, R.E.; Jokisz, P.G.; Holmes, A.B. Synthesis of light-emitting conjugated polymers for applications in electroluminescent devices. Chem. Rev. 2009, 109, 897–1091. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Liu, L.; Yang, Q.; Lv, F.; Wang, S. Water-soluble conjugated polymers for imaging, diagnosis, and therapy. Chem. Rev. 2012, 112, 4687–4735. [Google Scholar] [CrossRef]
- Nosova, G.I.; Ilgach, D.M.; Berezin, I.A.; Zhukova, E.V.; Kopylova, T.N.; Nikonova, E.N.; Gadirov, R.M.; Smyslov, R.Y.; Yakimansky, A.V. White electroluminescence from polyfluorenes copolymerized with carbazole derivatives of Nile Red and 1,8-naphthalimide. Mendeleev Commun. 2017, 27, 265–267. [Google Scholar] [CrossRef]
- Ilgach, D.M.; Nosova, G.I.; Kopylova, T.N.; Nikonova, E.N.; Gadirov, R.M.; Smyslov, R.Y.; Litvinova, L.S.; Yakimansky, A.V. Polyfluorene copolymers containing 2,5-difluoro-1,4-phenylene chains and carbazole conjugates with 1,8-naphthalimides for stable blue OLEDs. Mendeleev Commun. 2017, 27, 357–359. [Google Scholar] [CrossRef]
- Zhang, Z.; Fan, Q.; Sun, P.; Liu, L.; Lu, X.; Li, B.; Quan, Y.; Huang, W. Highly selective anionic counterion-based fluorescent sensor for Hg 2+ by grafted conjugated polyelectrolytes. Macromol. Rapid Commun. 2010, 31, 2160–2165. [Google Scholar] [CrossRef]
- Zhang, Z.; Lu, X.; Fan, Q.; Hu, W.; Huang, W. Conjugated polyelectrolyte brushes with extremely high charge density for improved energy transfer and fluorescence quenching applications. Polym. Chem. 2011, 2, 2369–2377. [Google Scholar] [CrossRef]
- Liu, X.; Shi, L.; Zhang, Z.; Fan, Q.; Huang, Y.; Su, S.; Fan, C.; Wanga, L.; Huang, W. Monodispersed nanoparticles of conjugated polyelectrolyte brush with high charge density for rapid, specific and label-free detection of tumor marker. Analyst 2015. [Google Scholar] [CrossRef] [PubMed]
- Gu, P.; Liu, X.; Tian, Y.; Zhang, L.; Huang, Y.; Su, S.; Feng, X.; Fan, Q.; Huang, W. A novel visible detection strategy for lysozyme based on gold nanoparticles and conjugated polymer brush. Sens. Actuators B Chem. 2017, 246, 78–84. [Google Scholar] [CrossRef]
- Balcı Leinen, M.; Klein, P.; Sebastian, F.L.; Zorn, N.F.; Adamczyk, S.; Allard, S.; Scherf, U.; Zaumseil, J. Spiropyran-Functionalized Polymer–Carbon Nanotube Hybrids for Dynamic Optical Memory Devices and UV Sensors. Adv. Electron. Mater. 2020, 6, 1–10. [Google Scholar] [CrossRef]
- Zhou, M.; He, Z.; Chen, Y.; Zhu, L.; Li, L.; Li, J. Synthesis, Self-assembly, and Fluorescence Application of Bottlebrush Polyfluorene-g-Polycaprolactone with Conjugated Backbone and Crystalline Brushes. Macromol. Rapid Commun. 2021, 42, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Müllner, M. Molecular Polymer Brushes in Nanomedicine. Macromol. Chem. Phys. 2016, 217, 2209–2222. [Google Scholar] [CrossRef]
- Yang, C.; Huang, S.; Wang, X.; Wang, M. Theranostic unimolecular micelles of highly fluorescent conjugated polymer bottlebrushes for far red/near infrared bioimaging and efficient anticancer drug delivery. Polym. Chem. 2016, 7, 7455–7468. [Google Scholar] [CrossRef]
- Liu, F.; Zhao, X.; Zhang, X.; Zhang, X.; Peng, J.; Yang, H.; Deng, K.; Ma, L.; Chang, C.; Wei, H. Fabrication of theranostic amphiphilic conjugated bottlebrush copolymers with alternating heterografts for cell imaging and anticancer drug delivery. Polym. Chem. 2018, 9, 4866–4874. [Google Scholar] [CrossRef]
- Ji, Y.; Lu, F.; Hu, W.; Zhao, H.; Tang, Y.; Li, B.; Hu, X.; Li, X.; Lu, X.; Fan, Q.; et al. Tandem activated photodynamic and chemotherapy: Using pH-Sensitive nanosystems to realize different tumour distributions of photosensitizer/prodrug for amplified combination therapy. Biomaterials 2019, 219, 1193933. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Li, L.; Jin, A.J.; Huang, W.; Chen, X. Rational design of semiconducting polymer brushes as cancer theranostics. Mater. Horizons. 2020, 7, 1474–1494. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Lv, F.; Liu, L.; Wang, S. Development of A Thermo-Responsive Conjugated Polymer with Photobleaching-Resistance Property and Tunable Photosensitizing Performance. Macromol. Rapid Commun. 2020, 41, 1–5. [Google Scholar] [CrossRef]
- Yang, C.; Liu, H.; Zhang, Y.; Xu, Z.; Wang, X.; Cao, B.; Wang, M. Hydrophobic-Sheath Segregated Macromolecular Fluorophores: Colloidal Nanoparticles of Polycaprolactone-Grafted Conjugated Polymers with Bright Far-Red/Near-Infrared Emission for Biological Imaging. Biomacromolecules 2016, 17, 1673–1683. [Google Scholar] [CrossRef]
- Zhang, Z.; Tan, M.; Kong, L.; Lu, X.; Sun, P.; Mo, H.; Fan, Q.; Huang, W. Application of Nanoscale Zwitterionic Polyelectrolytes Brush with High Stability and Quantum Yield in Aqueous Solution for Cell Imaging. J. Chem. 2020, 2020, 1942791. [Google Scholar] [CrossRef]
- Müllner, M.; Müller, A.H.E. Cylindrical polymer brushes—Anisotropic building blocks, unimolecular templates and particulate nanocarriers. Polymer (Guildf) 2016, 98, 389–401. [Google Scholar] [CrossRef]
- Simonova, M.; Ivanov, I.; Meleshko, T.; Kopyshev, A.; Santer, S.; Yakimansky, A.; Filippov, A. Self-assembly of molecular brushes with polyimide backbone and amphiphilic block copolymer side chains in selective solvents. Polymers 2020, 12, 2922. [Google Scholar] [CrossRef] [PubMed]
- Krasova, A.; Belyaeva, E.; Tarabukina, E.; Filippov, A.; Meleshko, T.; Ilgach, D.; Bogorad, N.; Yakimansky, A. Synthesis and solution properties of loose polymer brushes having polyimide backbone and methylmethacrylate side chains. Macromol. Symp. 2012, 316, 32–42. [Google Scholar] [CrossRef]
- Simonova, M.; Kamorin, D.; Kazantsev, O.; Nepomnyashaya, M.; Filippov, A. Conformation, self-organization and thermoresponsibility of polymethacrylate molecular brushes with oligo(ethylene glycol)-block-oligo(propylene glycol) side chains. Polymers 2021, 13, 2715. [Google Scholar] [CrossRef]
- Siddique, S.; Chow, J.C.L. Application of nanomaterials in biomedical imaging and cancer therapy. Nanomaterials 2020, 10, 1700. [Google Scholar] [CrossRef] [PubMed]
- Fery-Forgues, S.; Lavabre, D. Are fluorescence quantum yields so tricky to measure? A demonstration using familiar stationery products. J. Chem. Educ. 1999, 76, 1260–1264. [Google Scholar] [CrossRef]
- Demas, J.N.; Crosby, G.A. The measurement of photolumineseence quantum yields. A Rev. J. Phys. Chem. 1971, 75, 991–1024. [Google Scholar] [CrossRef]
- Yamakawa, H.; Fujii, M. Translational friction coefficient of wormlike chains. Macromolecules 1973, 6, 407–415. [Google Scholar] [CrossRef]
- Yamakawa, H.; Fujii, M. Intrinsic viscosity of wormlike chains. Determination of the shift factor. Macromolecules 1974, 7, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa, H.; Yoshizaki, T. Transport coefficients of helical wormlike chains. 3. Intrinsic viscosity. Macromolecules 1980, 13, 633–643. [Google Scholar] [CrossRef]
- Terao, K.; Hayashi, S.; Nakamura, Y.; Norisuye, T. Solution properties of polymacromonomers consisting of polystyrene. Polym. Bull. 2000, 44, 309–316. [Google Scholar] [CrossRef]
- Nemoto, N.; Nagai, M.; Koike, A.; Okada, S. Diffusion and sedimentation studies on poly (macromonomer) in dilute solution. Macromolecules 1995, 28, 3854–3859. [Google Scholar] [CrossRef]
- Lanson, D.; Schappacher, M.; Deffieux, A.; Borsali, R. Synthesis of Comblike Poly(styrene-b-isoprene) Block Copolymers and Their Properties in Good and Selective Solvents. Macromolecules 2006, 39, 7107–7114. [Google Scholar] [CrossRef]
- Kanemaru, E.; Terao, K.; Nakamura, Y.; Norisuye, T. Dimensions and viscosity behavior of polyelectrolyte brushes in aqueous sodium chloride. A polymacromonomer consisting of sodium poly (styrene sulfonate). Polymer 2008, 49, 4174–4179. [Google Scholar] [CrossRef][Green Version]
- Ronova, I.A.; Bruma, M. Influence of chemical structure on glass transition temperature of polyimides. Struct. Chem. 2010, 21, 1013–1020. [Google Scholar] [CrossRef]
- Terao, K.; Takeo, Y.; Tazaki, M.; Nakamura, Y.; Norisuye, T. Polymacromonomer consisting of polystyrene. Light scattering characterization in cyclohexane. Polym. J. 1999, 31, 193–198. [Google Scholar] [CrossRef]
- Terao, K.; Nakamura, Y.; Norisuye, T. Solution properties of polymacromonomers consisting of polystyrene. 2. Chain dimensions and stiffness in cyclohexane and toluene. Macromolecules 1999, 32, 711–716. [Google Scholar] [CrossRef]
- Tsukahara, Y.; Kohjiya, S.; Tsutsumi, K.; Okamoto, Y. On the intrinsic viscosity of poly(macromonomer)s. Macromolecules 1994, 27, 1662–1664. [Google Scholar] [CrossRef]
- Wintermantel, M.; Gerle, M.; Fischer, K.; Schmidt, M.; Wataoka, I.; Urakawa, H.; Kajiwara, K.; Tsukahara, Y. Molecular bottlebrushes. Macromolecules 1996, 29, 978–983. [Google Scholar] [CrossRef]
- Tsvetkov, V.N. Rigid-Chain Polymers; Plenum: New York, NY, USA, 1989. [Google Scholar]
- Tsvetkov, V.N.; Lavrenko, P.N.; Bushin, S.V. A hydrodynamic invariant of polymeric molecules. Russ. Chem. Rev. 1982, 51, 975–993. [Google Scholar] [CrossRef]
- Tsvetkov, V.N.; Lavrenko, P.N.; Bushin, S.V. Hydrodynamic invariant of polymer-molecules. J. Polym. Sci. Polym. Chem. Ed. 1984, 22, 3447–3486. [Google Scholar] [CrossRef]
- Shpyrkov, A.A.; Tarasenko, I.I.; Pankova, G.A.; Il’ina, I.E.; Tarasova, E.V.; Tarabukina, E.B.; Vlasov, G.P.; Filippov, A.P. Molecular mass characteristics and hydrodynamic and conformational properties of hyperbranched poly-L-lysines. Polymer Sci. Ser. A 2009, 51, 250–258. [Google Scholar] [CrossRef]
- Filippov, A.P.; Zamyshlyayeva, O.G.; Tarabukina, E.B.; Simonova, M.A.; Kozlov, A.V.; Semchikov, Y.u.D. Structural and conformational properties of hyperbranched copolymers based on perfluorinated germanium hydrides. Polymer Sci. Ser. A 2012, 54, 319–329. [Google Scholar] [CrossRef]
- Filippov, A.P.; Belyaeva, E.V.; Tarabukina, E.B.; Amirova, A.I. Behavior of hyperbranched polymers in solutions. Polymer Sci. Ser. C 2011, 53, 107–117. [Google Scholar] [CrossRef]
- Filippov, A.P.; Krasova, A.S.; Tarabukina, E.B.; Kashina, A.V.; Meleshko, T.K.; Yakimansky, A.V. The effect of side chain length on hydrodynamic and conformational characteristics of polyimide-graft-polymethylmethacrylate copolymers in thermodynamically good solutions. J. Polym. Res. 2016, 23, 1–9. [Google Scholar] [CrossRef]

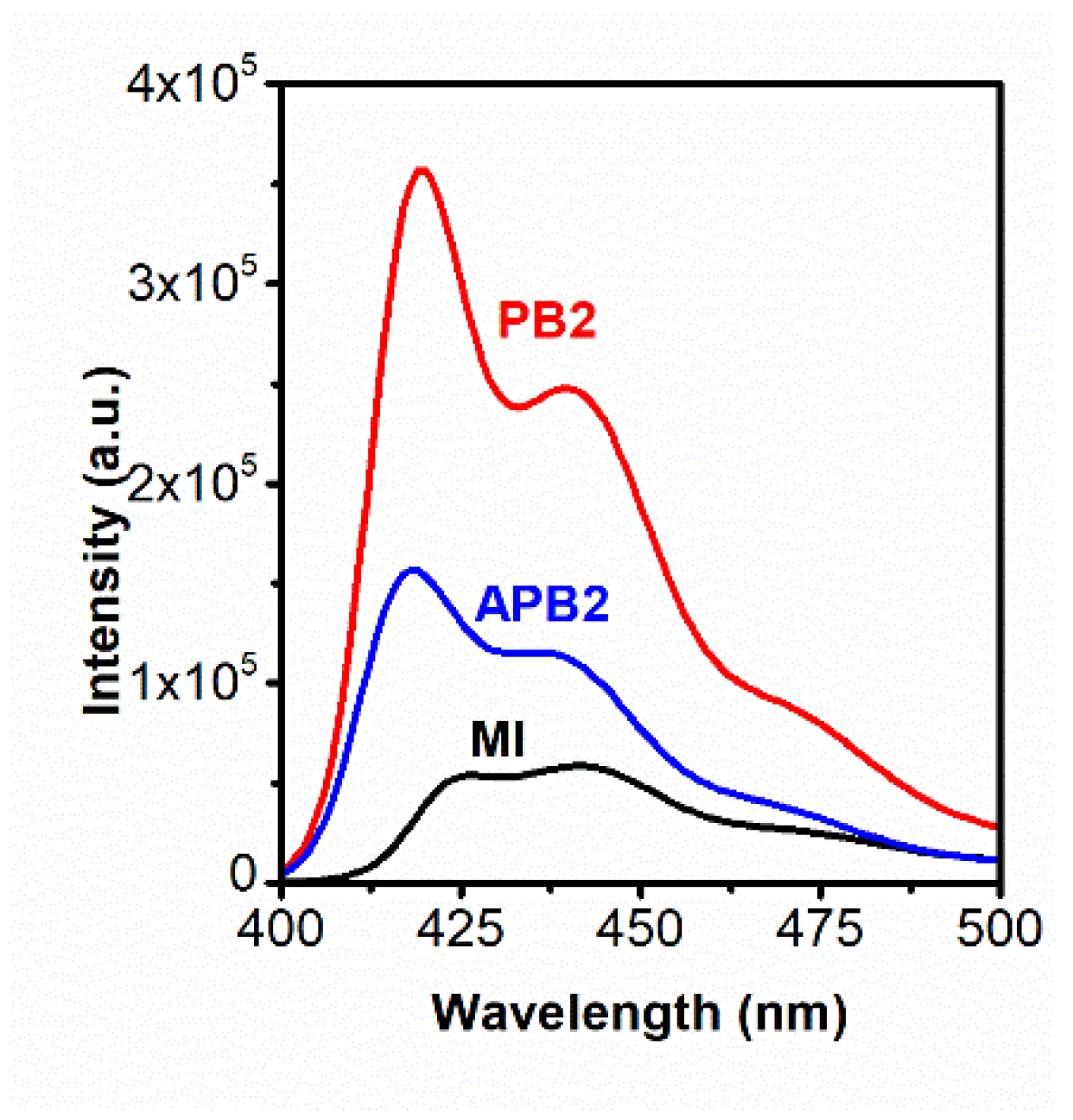
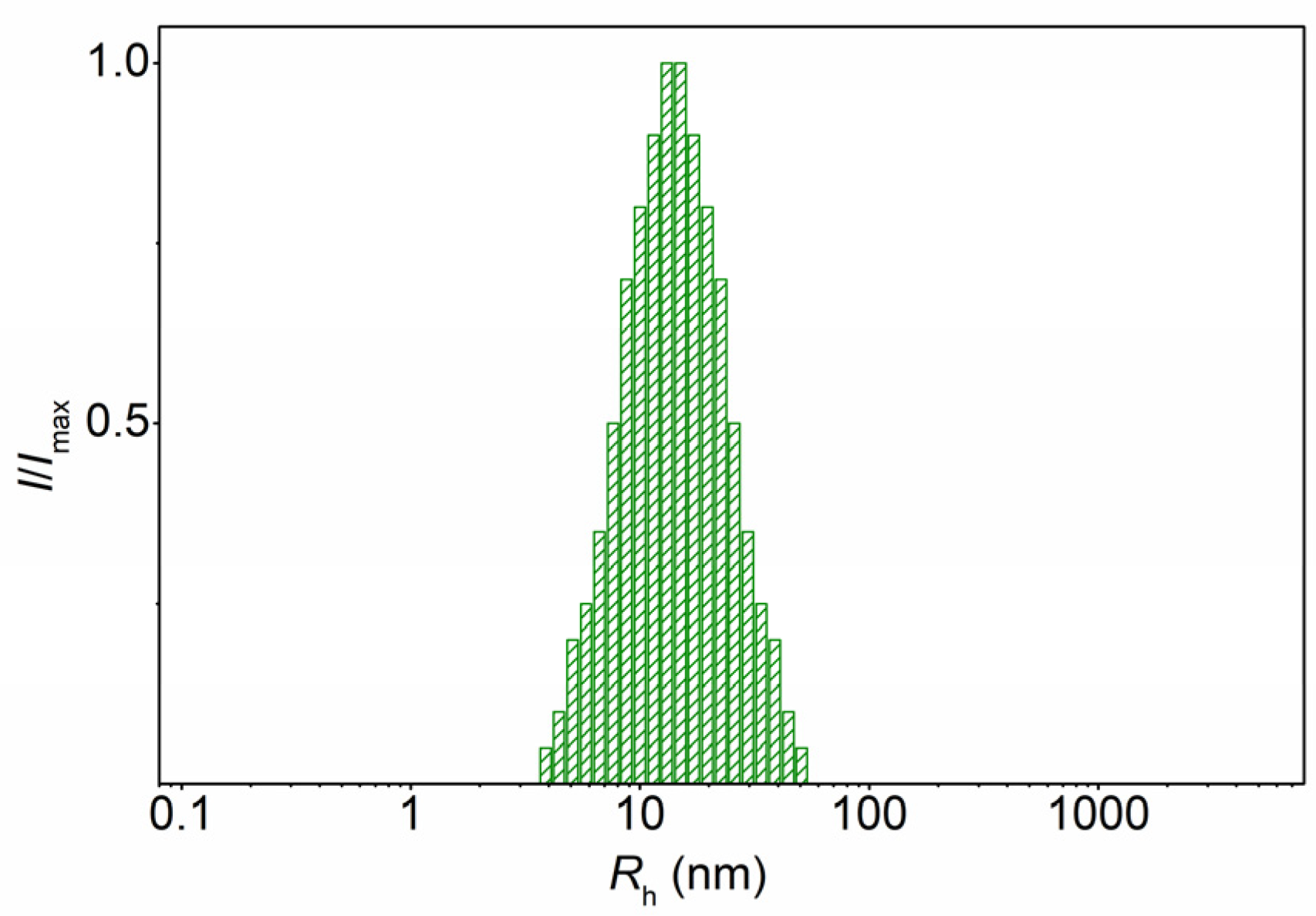

| Solvent | Solvent Characteristics | Solubility of the Structural Elements of Molecular Brushes | ||||
|---|---|---|---|---|---|---|
| ρ, g·cm−3 | η0, cP | n0 | PF | PtBMA | PMAA | |
| Chloroform | 1.49 | 0.57 | 1.446 | + | + | + |
| Ethanol | 0.79 | 1.08 | 1.359 | +/− | − | + |
| THF | 0.89 | 0.46 | 1.405 | + | + | − |
| Sample | MI:TBMA | τ 4, h | A5, % | Mn7 × 10–3, g·mol–1 | Mw/Mn7 | Tg8, °C |
|---|---|---|---|---|---|---|
| PB1 | 1:50 1 | 24 | 85 6 | 170 | 2.4 | 116.2 |
| PB2 | 1:50 1 | 4.0 | 67 | 140 | 2.1 | 113.9 |
| PB3 | 1:100 1 | 1.0 | 21 | 202 | 2.0 | 116.2 |
| PB4 | 1:100 1,2 | 1.33 | 37 | 178 | 2.4 | 116.2 |
| PB5 | 1:200 3 | 2.2 | 53 | 260 | 1.9 | 118.1 |
| PB6 | 1:200 3 | 2.8 | 68 | 290 | 1.8 | 118.4 |
| PB7 | 1:200 3 | 3.5 | 71 | 410 | 1.7 | 118.9 |
| PB8 | 1:200 3 | 4.2 | 76 | 510 | 1.8 | 119.5 |
| MI | 21.0 | 1.5 | - |
| Sample | Mncal × 10−3, g·mol−1 | Mwcal × 10–3, g·mol−1 | Mwexp × 10–3, g·mol−1 | Mwexp/Mwcal | Ls.ch./ΔL | [η], cm3g−1 | Rh-D, nm | A, nm | A0 × 1010, erg·K−1mol−1/3 | d, nm | QY |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PB1 | 147 | 353 | 320 | 0.91 | 9.5 | 27 | 10 | 17 | 3.2 | 11 | 0.75 |
| PB2 | 147 | 353 | 330 | 0.93 | 9.8 | 24 | 21 | 16 | 1.1 | 10 | 0.77 |
| PB3 | 276 | 579 | 282 | 0.49 | 8.2 | 24 | 13 | 18 | 2.3 | 9 | 0.64 |
| PB4 | 276 | 552 | 369 | 0.67 | 11.1 | 26 | 16 | 17 | 1.9 | 10 | 0.72 |
| PB5 | 534 | 1014 | 375 | 0.37 | 11.3 | 30 | 17 | 18 | 2.2 | 11 | 0.73 |
| PB6 | 534 | 1281 | 353 | 0.28 | 10.6 | 50 | 24 | 21 | 2.4 | 15 | - |
| PB7 | 534 | 1281 | 579 | 0.45 | 18 | 41 | 30 | 21 | 1.3 | 14 | - |
| PB8 | 534 | 1281 | 552 | 0.43 | 17 | 50 | 32 | 22 | 1.4 | 16 | 0.77 |
| Sample | Mw × 10−3, g·mol−1 | Rh-m, nm | Rg, nm | Rg/Rh | [η], cm3g−1 | Rh-m, nm in Water | QY |
|---|---|---|---|---|---|---|---|
| APB1 | 479 | 16 | 37 | 2.3 | 33 | 17 | 0.68 |
| APB2 | 500 | 20 | 44 | 2.2 | 35 | 22 | 0.64 |
| APB3 | 715 | 14 | 40 | 2.9 | 31 | 15 | 0.71 |
| APB4 | 368 | 12 | 37 | 3.1 | 35 | 16 | - |
| APB5 | 700 | 19 | 73 | 3.8 | 35 | 20 | 0.72 |
| APB6 | 1500 | 24 | 94 | 3.9 | 50 | 22 | - |
| APB7 | 4000 | 31 | 129 | 4.1 | 49 | 32 | - |
| APB8 | 720 | 24 | 41 | 1.7 | 61 | 30 | 0.69 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simonova, M.; Ilgach, D.; Kaskevich, K.; Nepomnyashaya, M.; Litvinova, L.; Filippov, A.; Yakimansky, A. Novel Amphiphilic Polyfluorene-Graft-(Polymethacrylic Acid) Brushes: Synthesis, Conformation, and Self-Assembly. Polymers 2021, 13, 4429. https://doi.org/10.3390/polym13244429
Simonova M, Ilgach D, Kaskevich K, Nepomnyashaya M, Litvinova L, Filippov A, Yakimansky A. Novel Amphiphilic Polyfluorene-Graft-(Polymethacrylic Acid) Brushes: Synthesis, Conformation, and Self-Assembly. Polymers. 2021; 13(24):4429. https://doi.org/10.3390/polym13244429
Chicago/Turabian StyleSimonova, Maria, Dmitry Ilgach, Ksenia Kaskevich, Maria Nepomnyashaya, Larisa Litvinova, Alexander Filippov, and Alexander Yakimansky. 2021. "Novel Amphiphilic Polyfluorene-Graft-(Polymethacrylic Acid) Brushes: Synthesis, Conformation, and Self-Assembly" Polymers 13, no. 24: 4429. https://doi.org/10.3390/polym13244429
APA StyleSimonova, M., Ilgach, D., Kaskevich, K., Nepomnyashaya, M., Litvinova, L., Filippov, A., & Yakimansky, A. (2021). Novel Amphiphilic Polyfluorene-Graft-(Polymethacrylic Acid) Brushes: Synthesis, Conformation, and Self-Assembly. Polymers, 13(24), 4429. https://doi.org/10.3390/polym13244429