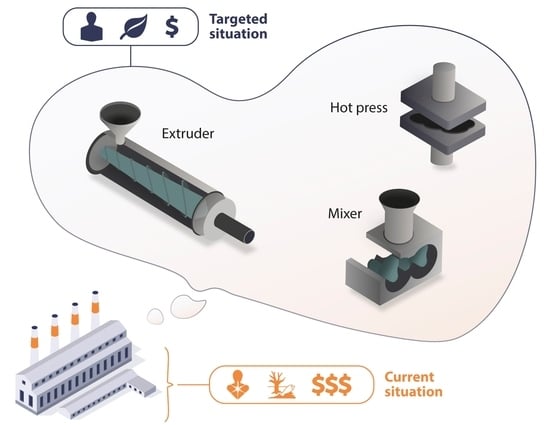
Challenges in Solvent-Free Methods for Manufacturing Electrodes and Electrolytes for Lithium-Based Batteries
Abstract
:1. Introduction
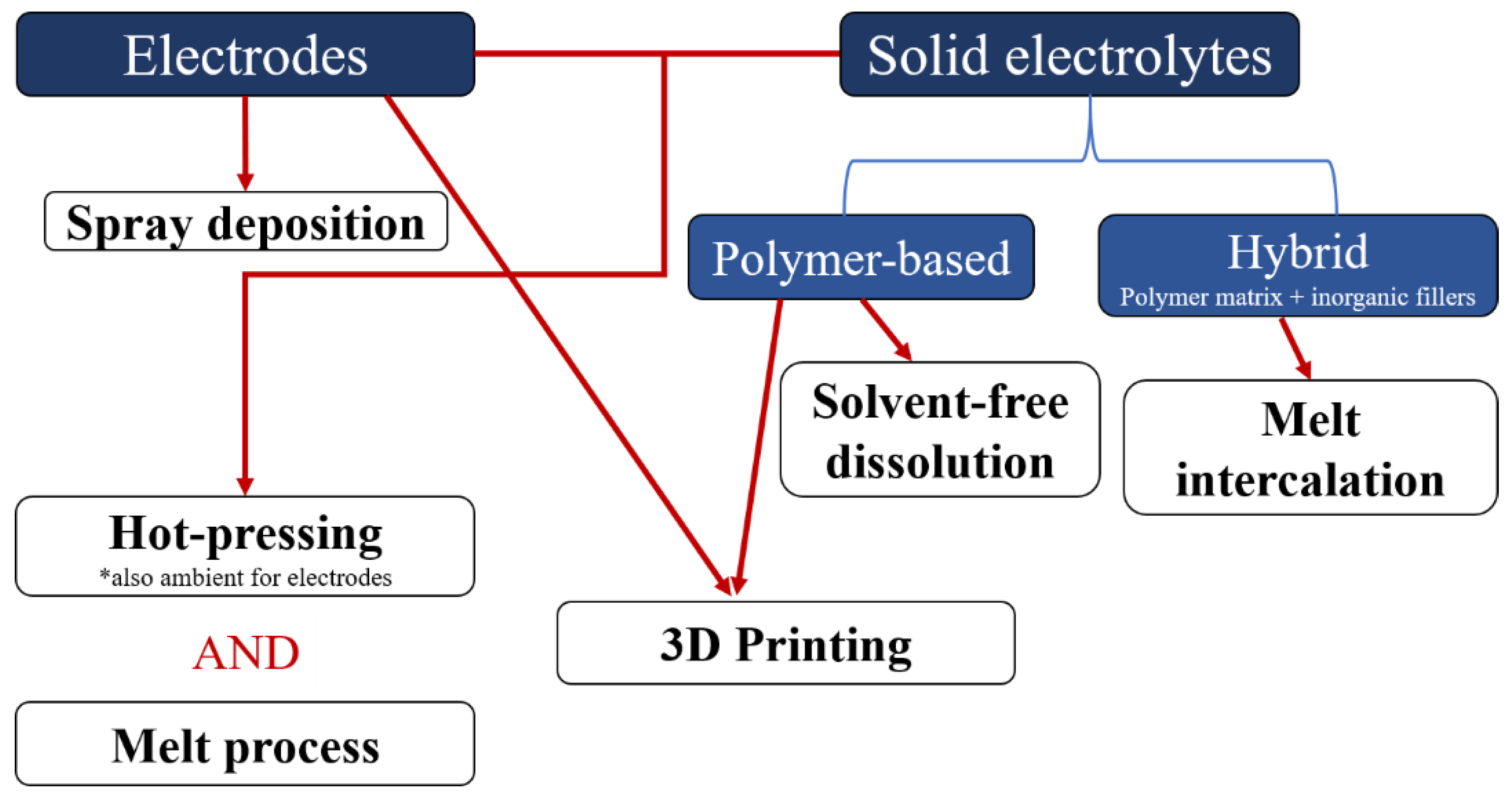
2. Dry Processes for Polymer Electrolytes
2.1. Solid Polymer Electrolytes (SPE)
2.1.1. Solvent-Free Dissolution
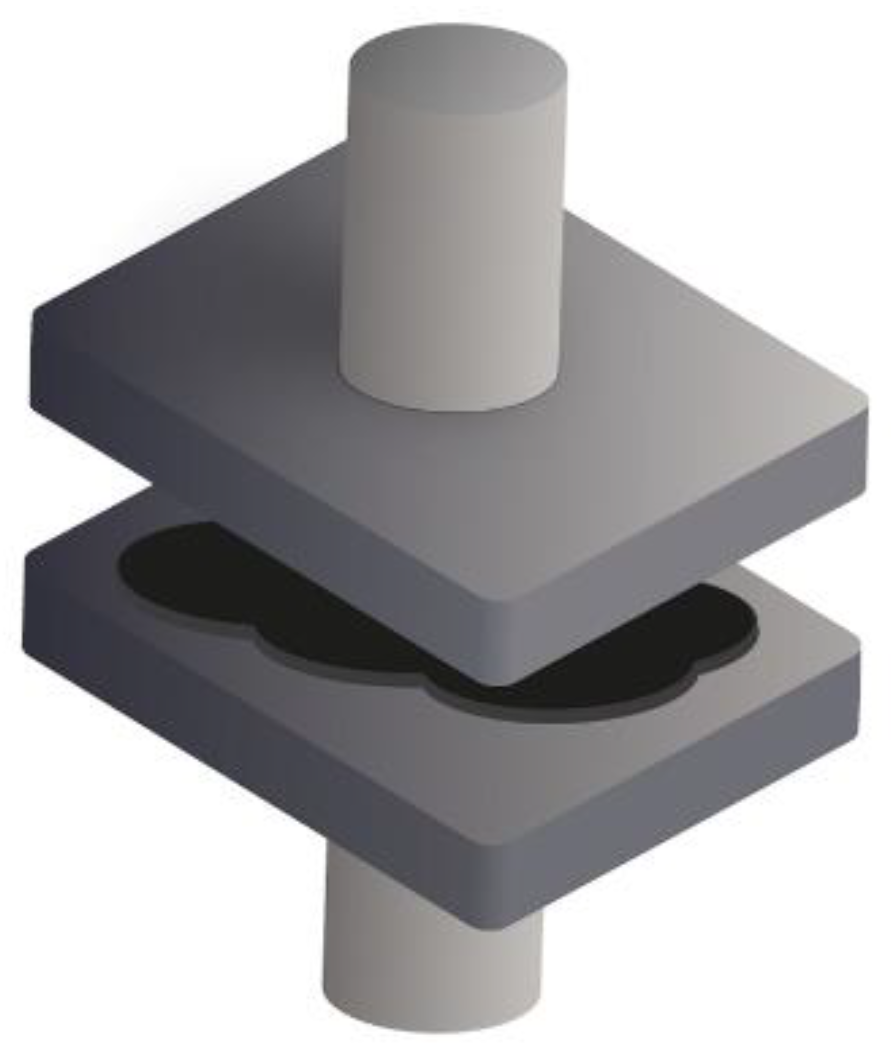
2.1.2. Hot-Pressing
2.1.3. 3D Printing
2.1.4. Melt Processing
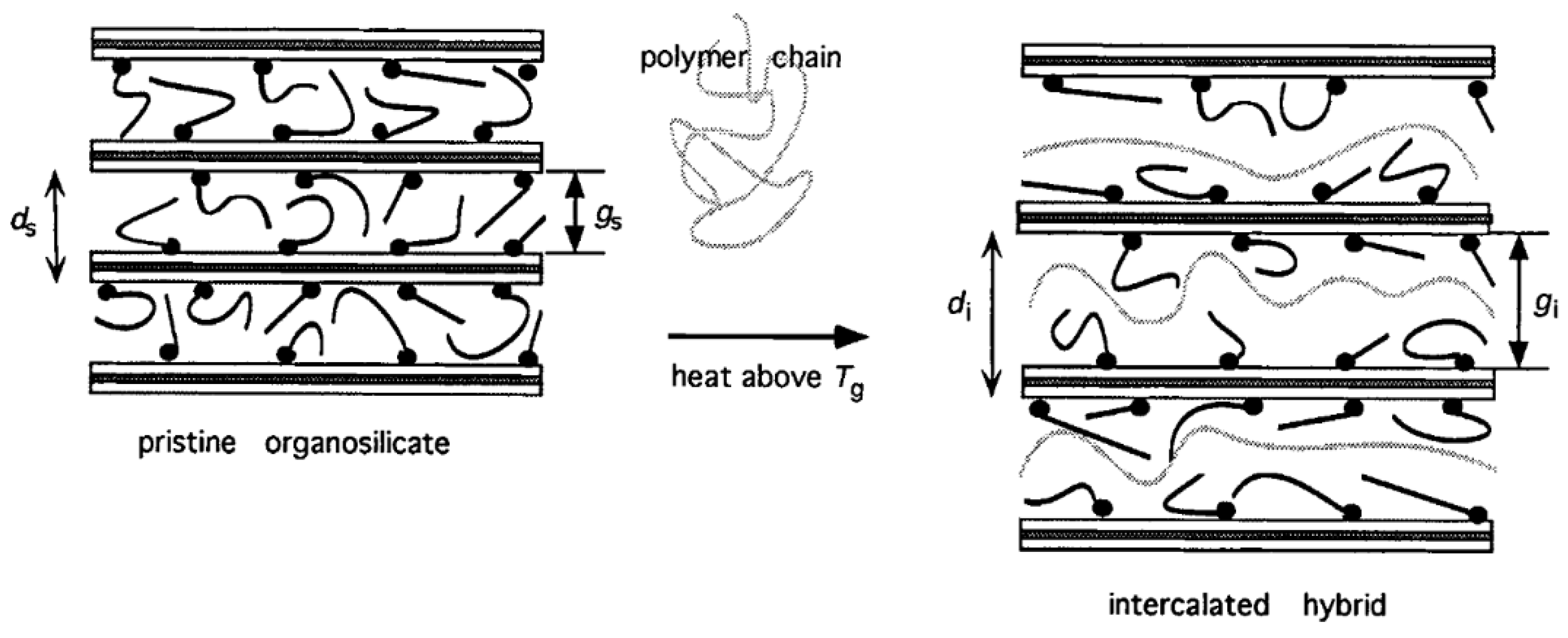
2.2. Hybrid Polymer Electrolytes
2.2.1. Melt Intercalation
2.2.2. Hot-Pressing
2.2.3. Melt Processing
Non-Conductive Fillers
Ionic Conductive Ceramics
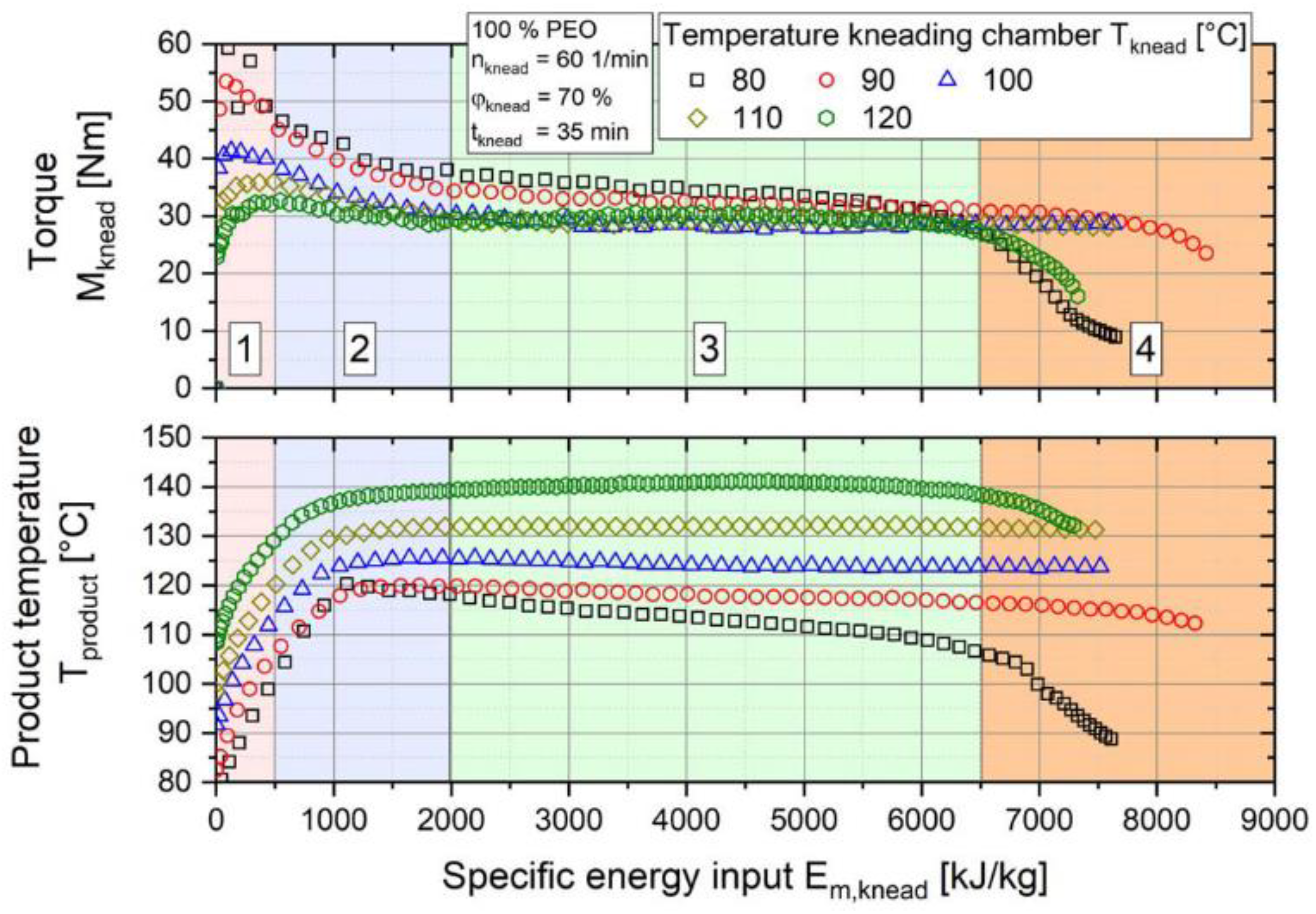
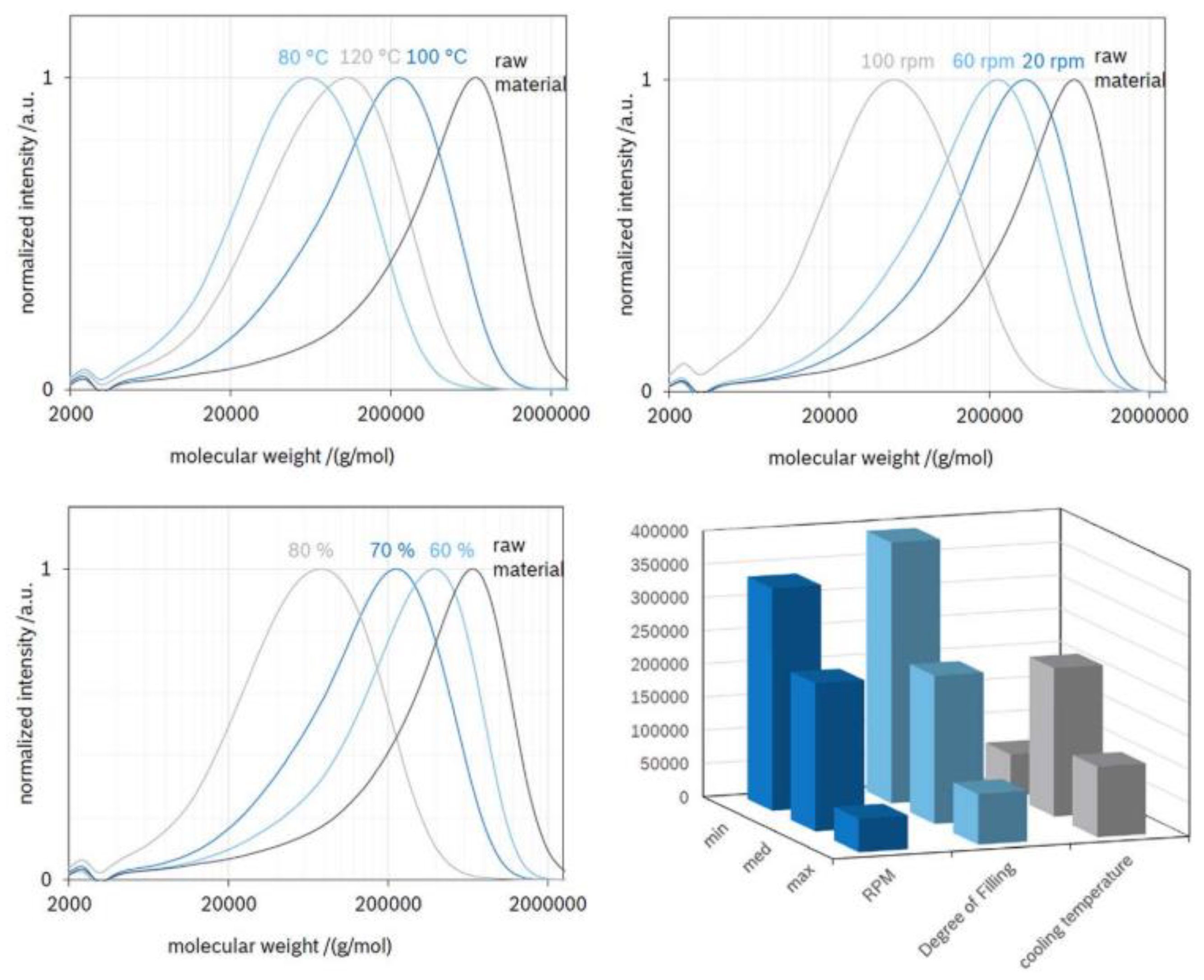
Extrusion Parameters
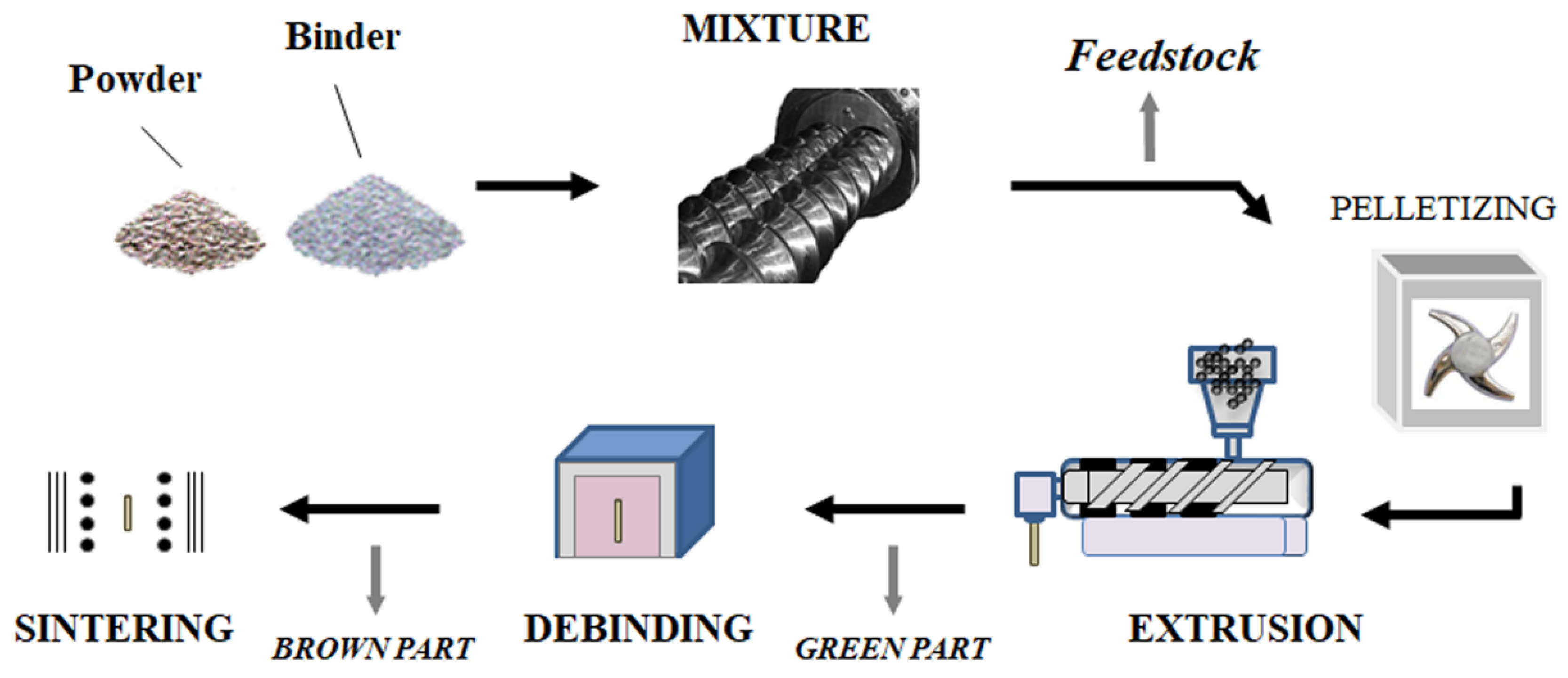
3. Dry Processes for Electrodes
3.1. Hot and Ambient Temperature Pressing
3.2. Spray Deposition
3.3. 3D Printing for Electrodes
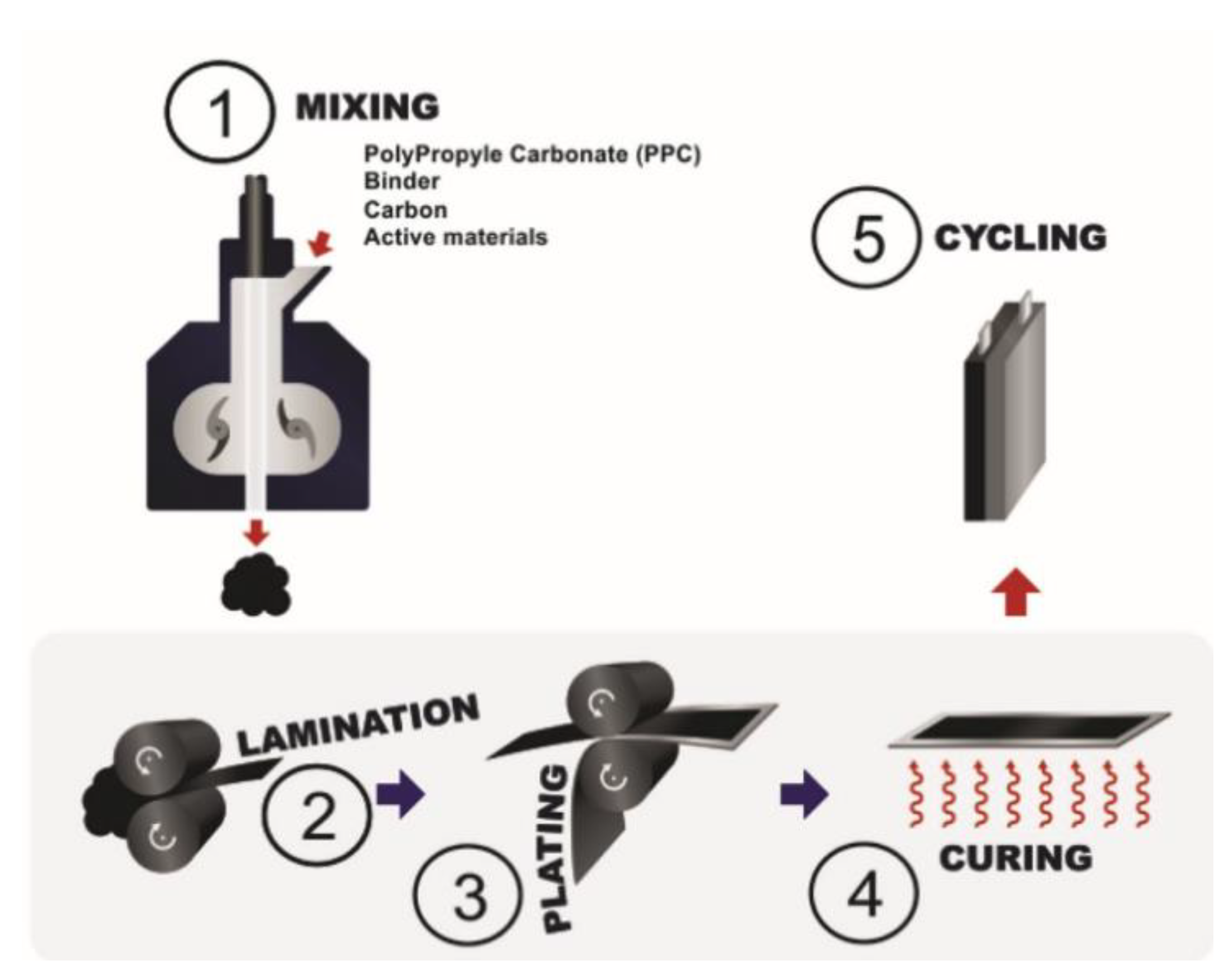
3.4. Melt Processing
3.4.1. Non-Porous Electrodes
3.4.2. Porous Electrodes
3.4.3. Extrusion Parameters for Electrode Preparation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hannan, M.A.; Hoque, M.M.; Hussain, A.; Yusof, Y.; Ker, P.J. State-of-the-art and energy management system of lithium-ion batteries in electric vehicle applications: Issues and recommendations. IEEE Access 2018, 6, 19362–19378. [Google Scholar] [CrossRef]
- Marks, T.; Trussler, S.; Smith, A.; Xiong, D.; Dahn, J. A guide to Li-ion coin-cell electrode making for academic researchers. J. Electrochem. Soc. 2010, 158, A51. [Google Scholar] [CrossRef]
- Fergus, J.W. Ceramic and polymeric solid electrolytes for lithium-ion batteries. J. Power Sources 2010, 195, 4554–4569. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, K.; Ding, F.; Liu, X. Recent advances in solid polymer electrolytes for lithium batteries. Nano Res. 2017, 10, 4139–4174. [Google Scholar] [CrossRef]
- Monroe, C.; Newman, J. The impact of elastic deformation on deposition kinetics at lithium/polymer interfaces. J. Electrochem. Soc. 2005, 152, A396–A404. [Google Scholar] [CrossRef]
- Wright, P.V. Electrical conductivity in ionic complexes of poly(ethylene oxide). Br. Polym. J. 1975, 7, 319–327. [Google Scholar] [CrossRef]
- Das, S.; Ghosh, A. Ion conduction and relaxation in PEO-LiTFSI-Al2O3 polymer nanocomposite electrolytes. J. Appl. Phys. 2015, 117, 174103. [Google Scholar] [CrossRef]
- Gong, L.; Nguyen, M.H.T.; Oh, E.-S. High polar polyacrylonitrile as a potential binder for negative electrodes in lithium ion batteries. Electrochem. Commun. 2013, 29, 45–47. [Google Scholar] [CrossRef]
- Mankovsky, D.; Lepage, D.; Lachal, M.; Caradant, L.; Aymé-Perrot, D.; Dollé, M. Water content in solid polymer electrolytes: The lost knowledge. Chem. Commun. 2020, 56, 10167–10170. [Google Scholar] [CrossRef]
- Zhou, C.; Bag, S.; Lv, B.; Thangadurai, V. Understanding the Role of Solvents on the Morphological Structure and Li-Ion Conductivity of Poly (vinylidene fluoride)-Based Polymer Electrolytes. J. Electrochem. Soc. 2020, 167, 070552. [Google Scholar] [CrossRef]
- Foran, G.; Mankovsky, D.; Verdier, N.; Lepage, D.; Prébé, A.; Aymé-Perrot, D.; Dollé, M. The Impact of Absorbed Solvent on the Performance of Solid Polymer Electrolytes for Use in Solid-State Lithium Batteries. iScience 2020, 101597. [Google Scholar] [CrossRef] [PubMed]
- González, F.; Garcia-Calvo, O.; Tiemblo, P.; García, N.; Fedeli, E.; Thieu, T.; Urdampilleta, I.; Kvasha, A. Synergy of Inorganic Fillers in Composite Thermoplastic Polymer/Ionic Liquid/LiTFSI Electrolytes. J. Electrochem. Soc. 2020, 167, 070519. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.; Pang, W.; Liang, P.; Jin, Z.; Grundish, N.; Li, Y.; Wang, C.-A. A dopamine modified Li6.4La3Zr1.4Ta0.6O12/PEO solid-state electrolyte: Enhanced thermal and electrochemical properties. J. Mater. Chem. A 2019, 7, 16425–16436. [Google Scholar] [CrossRef]
- Xi, J.; Qiu, X.; Ma, X.; Cui, M.; Yang, J.; Tang, X.; Zhu, W.; Chen, L. Composite polymer electrolyte doped with mesoporous silica SBA-15 for lithium polymer battery. Solid State Ionics 2005, 176, 1249–1260. [Google Scholar] [CrossRef]
- Armand, M.; Chabagno, J.; Duclot, M. Extended Abstracts. In Proceedings of the Second International Meeting on Solid Electrolytes, St. Andrews, Scotland, 20−22 September 1978. [Google Scholar]
- Barai, P.; Higa, K.; Srinivasan, V. Lithium dendrite growth mechanisms in polymer electrolytes and prevention strategies. Phys. Chem. Chem. Phys. 2017, 19, 20493–20505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armstrong, R.; Clarke, M. Lithium ion conducting polymeric electrolytes based on poly (ethylene adipate). Electrochim. acta 1984, 29, 1443–1446. [Google Scholar] [CrossRef]
- Harris, C.S.; Rukavina, T.G. Lithium ion conductors and proton conductors: Effects of plasticizers and hydration. Electrochim. Acta 1995, 40, 2315–2320. [Google Scholar] [CrossRef]
- Watanabe, M.; Rikukawa, M.; Sanui, K.; Ogata, N.; Kato, H.; Kobayashi, T.; Ohtaki, Z. Ionic conductivity of polymer complexes formed by poly(ethylene succinate) and lithium perchlorate. Macromolecules 1984, 17, 2902–2908. [Google Scholar] [CrossRef]
- Wang, B.; Lou, H.; Xu, H.; Zhao, J.; Wang, Q.; Shi, Q.; Deng, Y. High voltage, solvent-free solid polymer electrolyte based on a star-comb PDLLA–PEG copolymer for lithium ion batteries. RSC Adv. 2018, 8, 6373–6380. [Google Scholar] [CrossRef] [Green Version]
- Macfarlane, D.R.; Zhou, F.; Forsyth, M. Ion conductivity in amorphous polymer/salt mixtures. Solid State Ionics 1998, 113, 193–197. [Google Scholar] [CrossRef]
- Forsyth, M.; Jiazeng, S.; Macfarlane, D.R. Novel high salt content polymer electrolytes based on high Tg polymers. Electrochim. Acta 2000, 45, 1249–1254. [Google Scholar] [CrossRef]
- Kim, G.-T.; Appetecchi, G.B.; Alessandrini, F.; Passerini, S. Solvent-free, PYR1ATFSI ionic liquid-based ternary polymer electrolyte systems: I. Electrochemical characterization. J. Power Sources 2007, 171, 861–869. [Google Scholar] [CrossRef]
- Maurel, A.; Armand, M.; Grugeon, S.; Fleutot, B.; Davoisne, C.; Tortajada, H.; Courty, M.; Panier, S.; Dupont, L. Poly(Ethylene Oxide)− LiTFSI Solid Polymer Electrolyte Filaments for Fused Deposition Modeling Three-Dimensional Printing. J. Electrochem. Soc. 2020, 167, 070536. [Google Scholar] [CrossRef]
- Gray, F.; Vincent, C.; Kent, M. Dielectric studies of poly (ethylene oxide)-based polymer electrolytes using time-domain spectroscopy. Solid State Ionics 1988, 28, 936–940. [Google Scholar] [CrossRef]
- Cook, J.A.; Park, G.B.; McLoughlin, R.H. Materials for Electrical Devices. EP0145498A2, 21 November 1989. [Google Scholar]
- Duval, M. Process of Coating by Melt Extrusion a Solid Polymer Electrolyte on Positive Electrode of Lithium Battery. US5348824A, 25 October 1993. [Google Scholar]
- Ma, X.; Yu, J.; He, K. Thermoplastic starch plasticized by glycerol as solid polymer electrolytes. Macromol. Mater. Eng. 2006, 291, 1407–1413. [Google Scholar] [CrossRef]
- Ma, X.; Yu, J.; He, K.; Wang, N. The effects of different plasticizers on the properties of thermoplastic starch as solid polymer electrolytes. Macromol. Mater. Eng. 2007, 292, 503–510. [Google Scholar] [CrossRef]
- Caradant, L.; Lepage, D.; Nicolle, P.; Prébé, A.; Aymé-Perrot, D.; Dollé, M. Effect of Li+ Affinity on Ionic Conductivities in Melt-Blended Nitrile Rubber/Polyether. ACS Appl. Polym. Mater. 2020, 2, 4943–4951. [Google Scholar] [CrossRef]
- Croce, F.; Appetecchi, G.; Persi, L.; Scrosati, B. Nanocomposite polymer electrolytes for lithium batteries. Nature 1998, 394, 456–458. [Google Scholar] [CrossRef]
- Vaia, R.A.; Vasudevan, S.; Krawiec, W.; Scanlon, L.G.; Giannelis, E.P. New polymer electrolyte nanocomposites: Melt intercalation of poly(ethylene oxide) in mica-type silicates. Adv. Mater. 1995, 7, 154–156. [Google Scholar] [CrossRef]
- Chen, W.; Xu, Q.; Yuan, R. The influence of polymer state on the electrical properties of polymer/layered-silicate nanocomposites. Compos. Sci. Technol. 2001, 61, 935–939. [Google Scholar] [CrossRef]
- Ray, S.S.; Okamoto, M. Polymer/layered silicate nanocomposites: A review from preparation to processing. Prog. Polym. Sci. 2003, 28, 1539–1641. [Google Scholar]
- Sikka, M.; Cerini, L.N.; Ghosh, S.S.; Winey, K.I. Melt intercalation of polystyrene in layered silicates. J. Polym. Sci. Part B Polym. Phys. 1996, 34, 1443–1449. [Google Scholar] [CrossRef]
- Appetecchi, G.B.; Croce, F.; Hassoun, J.; Scrosati, B.; Salomon, M.; Cassel, F. Hot-pressed, dry, composite, PEO-based electrolyte membranes: I. Ionic conductivity characterization. J. Power Sources 2003, 114, 105–112. [Google Scholar] [CrossRef]
- Shin, J.-H.; Passerini, S. Effect of fillers on the electrochemical and interfacial properties of PEO–LiN(SO2CF2CF3)2 polymer electrolytes. Electrochim. Acta 2004, 49, 1605–1612. [Google Scholar] [CrossRef]
- Keller, M.; Appetecchi, G.B.; Kim, G.-T.; Sharova, V.; Schneider, M.; Schuhmacher, J.; Roters, A.; Passerini, S. Electrochemical performance of a solvent-free hybrid ceramic-polymer electrolyte based on Li7La3Zr2O12 in P(EO)15LiTFSI. J. Power Sources 2017, 353, 287–297. [Google Scholar] [CrossRef]
- Thiry, J.; Krier, F.; Evrard, B. A review of pharmaceutical extrusion: Critical process parameters and scaling-up. Int. J. Pharm. 2015, 479, 227–240. [Google Scholar] [CrossRef]
- Brochu, F.; Duval, M. Additives for Extruding Polymer Electrolytes; Hydro-Quebec: Quebec, QC, Canada, 1996. [Google Scholar]
- Loyens, W.; Maurer, F.H.; Jannasch, P. Melt-compounded salt-containing poly(ethylene oxide)/clay nanocomposites for polymer electrolyte membranes. Polymers 2005, 46, 7334–7345. [Google Scholar] [CrossRef]
- Mejía, A.; García, N.; Guzmán, J.; Tiemblo, P. Extrusion processed polymer electrolytes based on poly(ethylene oxide) and modified sepiolite nanofibers: Effect of composition and filler nature on rheology and conductivity. Electrochim. Acta 2014, 137, 526–534. [Google Scholar] [CrossRef]
- Mejía, A.; García, N.; Guzmán, J.; Tiemblo, P. Thermoplastic and solid-like electrolytes with liquid-like ionic conductivity based on poly (ethylene oxide) nanocomposites. Solid State Ionics 2014, 261, 74–80. [Google Scholar] [CrossRef] [Green Version]
- Mejía, A.; Devaraj, S.; Guzmán, J.; del Amo, J.M.L.; García, N.; Rojo, T.; Armand, M.; Tiemblo, P. Scalable plasticized polymer electrolytes reinforced with surface-modified sepiolite fillers–A feasibility study in lithium metal polymer batteries. J. Power Sources 2016, 306, 772–778. [Google Scholar] [CrossRef]
- Mejía, A.; Benito, E.; Guzman, J.; Garrido, L.; García, N.; Hoyos, M.; Tiemblo, P. Polymer/ionic liquid thermoplastic electrolytes for energy storage processed by solvent free procedures. ACS Sustain. Chem. Eng. 2016, 4, 2114–2121. [Google Scholar] [CrossRef]
- González, F.; Gregorio, V.; Rubio, A.; Garrido, L.; García, N.; Tiemblo, P. Ionic liquid-based thermoplastic solid electrolytes processed by solvent-free procedures. Polymers 2018, 10, 124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González, F.; Tiemblo, P.; García, N.; Garcia-Calvo, O.; Fedeli, E.; Kvasha, A.; Urdampilleta, I. High performance polymer/ionic liquid thermoplastic solid electrolyte prepared by solvent free processing for solid state lithium metal batteries. Membranes 2018, 8, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gregorio, V.; García, N.; Tiemblo, P. Solvent-Free and Scalable Procedure to Prepare PYR13TFSI/LiTFSI/PVDF–HFP Thermoplastic Electrolytes with Controlled Phase Separation and Enhanced Li-Ion Diffusion. Membranes 2019, 9, 50. [Google Scholar] [CrossRef] [Green Version]
- Miguel, Á.; González, F.; Gregorio, V.; García, N.; Tiemblo, P. Solvent-Free Procedure for the Preparation under Controlled Atmosphere Conditions of Phase-Segregated Thermoplastic Polymer Electrolytes. Polymers 2019, 11, 406. [Google Scholar] [CrossRef] [Green Version]
- Froboese, L.; van der Sichel, J.F.; Loellhoeffel, T.; Helmers, L.; Kwade, A. Enhancing the Lithium Ion Conductivity of an All Solid-State Electrolyte via Dry and Solvent-Free Scalable Series Production Processes. J. Electrochem. Soc. 2020, 167, 020558. [Google Scholar] [CrossRef]
- Sharafi, A.; Yu, S.; Naguib, M.; Lee, M.; Ma, C.; Meyer, H.M.; Nanda, J.; Chi, M.; Siegel, D.J.; Sakamoto, J. Impact of air exposure and surface chemistry on Li–Li7La3Zr2O12 interfacial resistance. J. Mater. Chem. A 2017, 5, 13475–13487. [Google Scholar] [CrossRef]
- Huang, Z.; Tong, R.; Zhang, J.; Chen, L.; Wang, C.-A. Blending Poly(ethylene oxide) and Li6.4La3Zr1.4Ta0.6O12 by Haake Rheomixer without any solvent: A low-cost manufacture method for mass production of composite polymer electrolyte. J. Power Sources 2020, 451, 227797. [Google Scholar] [CrossRef]
- Malik, P.; Castro, M.; Carrot, C. Thermal degradation during melt processing of poly(ethylene oxide), poly(vinylidenefluoride-co-hexafluoropropylene) and their blends in the presence of additives, for conducting applications. Polym. Degrad. Stab. 2006, 91, 634–640. [Google Scholar] [CrossRef]
- Bresser, D.; Buchholz, D.; Moretti, A.; Varzi, A.; Passerini, S. Alternative binders for sustainable electrochemical energy storage–the transition to aqueous electrode processing and bio-derived polymers. Energy Environ. Sci. 2018, 11, 3096–3127. [Google Scholar] [CrossRef] [Green Version]
- Kirsch, D.J.; Lacey, S.D.; Kuang, Y.; Pastel, G.; Xie, H.; Connell, J.W.; Lin, Y.; Hu, L. Scalable dry processing of binder-free lithium-ion battery electrodes enabled by holey graphene. ACS Appl. Energy Mater. 2019, 2, 2990–2997. [Google Scholar] [CrossRef]
- Yubuchi, S.; Ito, Y.; Matsuyama, T.; Hayashi, A.; Tatsumisago, M. 5V class LiNi0.5Mn1.5O4 positive electrode coated with Li3PO4 thin film for all-solid-state batteries using sulfide solid electrolyte. Solid State Ionics 2016, 285, 79–82. [Google Scholar] [CrossRef]
- Otoyama, M.; Ito, Y.; Hayashi, A.; Tatsumisago, M. Raman imaging for LiCoO2 composite positive electrodes in all-solid-state lithium batteries using Li2S–P2S5 solid electrolytes. J. Power Sources 2016, 302, 419–425. [Google Scholar] [CrossRef] [Green Version]
- Ito, Y.; Yamakawa, S.; Hayashi, A.; Tatsumisago, M. Effects of the microstructure of solid-electrolyte-coated LiCoO2 on its discharge properties in all-solid-state lithium batteries. J. Mater. Chem. A 2017, 5, 10658–10668. [Google Scholar] [CrossRef]
- Yubuchi, S.; Nakamura, W.; Bibienne, T.; Rousselot, S.; Taylor, L.W.; Pasquali, M.; Dollé, M.; Sakuda, A.; Hayashi, A.; Tatsumisago, M. All-solid-state cells with Li4Ti5O12/carbon nanotube composite electrodes prepared by infiltration with argyrodite sulfide-based solid electrolytes via liquid-phase processing. J. Power Sources 2019, 417, 125–131. [Google Scholar] [CrossRef]
- Auvergniot, J.; Cassel, A.; Ledeuil, J.-B.; Viallet, V.; Seznec, V.; Dedryvère, R. Interface stability of argyrodite Li6PS5Cl toward LiCoO2, LiNi1/3Co1/3Mn1/3O2, and LiMn2O4 in bulk all-solid-state batteries. Chem. Mater. 2017, 29, 3883–3890. [Google Scholar] [CrossRef]
- Boulineau, S.; Tarascon, J.-M.; Leriche, J.-B.; Viallet, V. Electrochemical properties of all-solid-state lithium secondary batteries using Li-argyrodite Li6PS5Cl as solid electrolyte. Solid State Ionics 2013, 242, 45–48. [Google Scholar] [CrossRef]
- Hippauf, F.; Schumm, B.; Doerfler, S.; Althues, H.; Fujiki, S.; Shiratsushi, T.; Tsujimura, T.; Aihara, Y.; Kaskel, S. Overcoming binder limitations of sheet-type solid-state cathodes using a solvent-free dry-film approach. Energy Storage Mater. 2019, 21, 390–398. [Google Scholar] [CrossRef]
- Mitchell, P.; Zhong, L.; Xi, X.; Zou, B. Dry Particle Based Adhesive and Dry Film and Methods of Making Same. U.S. Patent 7508651, 24 March 2009. [Google Scholar]
- Ludwig, B.; Zheng, Z.; Shou, W.; Wang, Y.; Pan, H. Solvent-Free Manufacturing of Electrodes for Lithium-ion Batteries. Sci. Rep. 2016, 6, 23150. [Google Scholar] [CrossRef] [Green Version]
- Al-Shroofy, M.; Zhang, Q.; Xu, J.; Chen, T.; Kaur, A.P.; Cheng, Y.-T. Solvent-free dry powder coating process for low-cost manufacturing of LiNi1/3Mn1/3Co1/3O2 cathodes in lithium-ion batteries. J. Power Sources 2017, 352, 187–193. [Google Scholar] [CrossRef] [Green Version]
- Zhu, C.; Fu, Y.; Yu, Y. Designed Nanoarchitectures by Electrostatic Spray Deposition for Energy Storage. Adv. Mater. 2019, 31, 1803408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Hu, J.; Wang, Y.; Cheng, Y.-T. The Influence of Polyvinylidene Fluoride (PVDF) Binder Properties on LiNi0.33Co0.33Mn0.33O2 (NMC) Electrodes Made by a Dry-Powder-Coating Process. J. Electrochem. Soc. 2019, 166, A2151–A2157. [Google Scholar] [CrossRef]
- Liu, J.; Ludwig, B.; Liu, Y.; Zheng, Z.; Wang, F.; Tang, M.; Wang, J.; Wang, J.; Pan, H.; Wang, Y. Scalable Dry Printing Manufacturing to Enable Long-Life and High Energy Lithium-Ion Batteries. Adv. Mater. Technol. 2017, 2, 1700106. [Google Scholar] [CrossRef]
- Schälicke, G.; Landwehr, I.; Dinter, A.; Pettinger, K.-H.; Haselrieder, W.; Kwade, A. Solvent-Free Manufacturing of Electrodes for Lithium-Ion Batteries via Electrostatic Coating. Energy Technol. 2020, 8, 1900309. [Google Scholar] [CrossRef]
- Park, D.-W.; Cañas, N.A.; Wagner, N.; Friedrich, K.A. Novel solvent-free direct coating process for battery electrodes and their electrochemical performance. J. Power Sources 2016, 306, 758–763. [Google Scholar] [CrossRef] [Green Version]
- Sun, K.; Wei, T.S.; Ahn, B.Y.; Seo, J.Y.; Dillon, S.J.; Lewis, J.A. 3D printing of interdigitated Li-Ion microbattery architectures. Adv. Mater. 2013, 25, 4539–4543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, K.; Wang, Y.; Yan, C.; Yao, Y.; Chen, Y.; Dai, J.; Lacey, S.; Wang, Y.; Wan, J.; Li, T. Graphene oxide-based electrode inks for 3D-printed lithium-ion batteries. Adv. Mater. 2016, 28, 2587–2594. [Google Scholar] [CrossRef] [PubMed]
- Maurel, A.; Grugeon, S.; Armand, M.; Fleutot, B.; Courty, M.; Prashantha, K.; Davoisne, C.; Tortajada, H.; Panier, S.; Dupont, L. Overview on Lithium-Ion Battery 3D-Printing By Means of Material Extrusion. ECS Trans. 2020, 98, 3. [Google Scholar] [CrossRef]
- Foster, C.W.; Down, M.P.; Zhang, Y.; Ji, X.; Rowley-Neale, S.J.; Smith, G.C.; Kelly, P.J.; Banks, C.E. 3D printed graphene based energy storage devices. Sci. Rep. 2017, 7, 42233. [Google Scholar] [CrossRef]
- Ragones, H.; Menkin, S.; Kamir, Y.; Gladkikh, A.; Mukra, T.; Kosa, G.; Golodnitsky, D. Towards smart free form-factor 3D printable batteries. Sustain. Energy Fuels 2018, 2, 1542–1549. [Google Scholar] [CrossRef] [Green Version]
- Reyes, C.; Somogyi, R.; Niu, S.; Cruz, M.A.; Yang, F.; Catenacci, M.J.; Rhodes, C.P.; Wiley, B.J. Three-dimensional printing of a complete lithium ion battery with fused filament fabrication. ACS Appl. Energy Mater. 2018, 1, 5268–5279. [Google Scholar] [CrossRef]
- Maurel, A.; Courty, M.; Fleutot, B.; Tortajada, H.; Prashantha, K.; Armand, M.; Grugeon, S.; Panier, S.; Dupont, L. Highly loaded graphite–polylactic acid composite-based filaments for lithium-ion battery three-dimensional printing. Chem. Mater. 2018, 30, 7484–7493. [Google Scholar] [CrossRef]
- Maurel, A.; Grugeon, S.; Fleutot, B.; Courty, M.; Prashantha, K.; Tortajada, H.; Armand, M.; Panier, S.; Dupont, L. Three-Dimensional Printing of a LiFePO4/Graphite Battery Cell via Fused Deposition Modeling. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, J.L. Apparatus and Method for Extruding Shear Thinning Material. US5316556A, 2 February 1994. [Google Scholar]
- Gueguen, M.; Billion, M.; Majastre, H. Method of Manufacturing a Multilayer Electrochemical Assembly Comprising an Electrolyte between Two Electrodes, and an Assembly Made Thereby. US5593462A, 10 September 1997. [Google Scholar]
- Solutions, B. LMP® Batteries: The Unique All-Solid-State Technologie. Available online: https://www.blue-solutions.com/en/blue-solutions/technology/batteries-lmp/ (accessed on 16 November 2020).
- Chern, T.S.-H.; Keller, D.G.; MacFadden, K.O. Continuous Process to Produce Lithium-Polymer Batteries. US5,749,927A, 12 May 1998. [Google Scholar]
- Keller, D.G.; Giovannoni, R.T.; MacFadden, K.O. Extrusion of Electrode Material by Liquid Injection into Extruder Barrel. US5725822A, 1 January 1998. [Google Scholar]
- Lavoie, P.-A.; Laliberte, R.; Besner, S.; Gagnon, Y.; Simoneau, M.; Vallee, A.; Duval, M.; Brochu, F. Positive Electrode Films for Alkali Metal Polymer Batteries and Method for Making Same. U.S. Patent 7700018, 20 April 2004. [Google Scholar]
- Haarmann, M.; Haselrieder, W.; Kwade, A. Extrusion-Based Processing of Cathodes: Influence of Solid Content on Suspension and Electrode Properties. Energy Technol. 2019, 8, 1801169. [Google Scholar] [CrossRef]
- Sotomayor, M.E.; de la Torre-Gamarra, C.; Levenfeld, B.; Sanchez, J.-Y.; Varez, A.; Kim, G.-T.; Varzi, A.; Passerini, S. Ultra-thick battery electrodes for high gravimetric and volumetric energy density Li-ion batteries. J. Power Sources 2019, 437, 226923. [Google Scholar] [CrossRef]
- El Khakani, S.; Verdier, N.; Lepage, D.; Prébé, A.; Aymé-Perrot, D.; Rochefort, D.; Dollé, M. Melt-processed electrode for lithium ion battery. J. Power Sources 2020, 454, 227884. [Google Scholar] [CrossRef]
- Dreger, H.; Bockholt, H.; Haselrieder, W.; Kwade, A. Discontinuous and continuous processing of low-solvent battery slurries for lithium nickel cobalt manganese oxide electrodes. J. Electron. Mater. 2015, 44, 4434–4443. [Google Scholar] [CrossRef]
- Seeba, J.; Reuber, S.; Heubner, C.; Müller-Köhn, A.; Wolter, M.; Michaelis, A. Extrusion-Based Fabrication of Electrodes for High-Energy Li-Ion Batteries. Chem. Eng. J. 2020, 5, 574–581. [Google Scholar]
- Jardiel, T.; Sotomayor, M.E.; Levenfeld, B.; Várez, A. Optimization of the Processing of 8-YSZ Powder by Powder Injection Molding for SOFC Electrolytes. Int. J. Appl. Ceram. Technol. 2008, 5, 574–581. [Google Scholar] [CrossRef]
- De la Torre-Gamarra, C.; Sotomayor, M.E.; Sanchez, J.-Y.; Levenfeld, B.; Várez, A.; Laïk, B.; Pereira-Ramos, J.-P. High mass loading additive-free LiFePO4 cathodes with 500 μm thickness for high areal capacity Li-ion batteries. J. Power Sources 2020, 458, 228033. [Google Scholar] [CrossRef]
- Voillequin, B.; Ayme-Perrot, D.; Dufour, B.; Sonntag, P. Anode for a Cell of a Lithium-Ion Battery, Its Manufacturing Process and the Battery Incorporating It. US20150340684A1, 30 July 2015. [Google Scholar]
- Voillequin, B.; Ayme-Perrot, D.; Dufour, B.; Sonntag, P. Cathode for a Cell of a Lithium-Ion Battery, Its Manufacturing Process and the Battery Incorporating It. US9484571B2, 7 October 2016. [Google Scholar]
- Astafyeva, K.; Dousset, C.; Bureau, Y.; Stalmach, S.L.; Dufour, B. High Energy Li− Ion Electrodes Prepared via a Solventless Melt Process. Batter. Supercaps 2020, 3, 341–343. [Google Scholar] [CrossRef]
- Dreger, H.; Haselrieder, W.; Kwade, A. Influence of dispersing by extrusion and calendering on the performance of lithium-ion battery electrodes. J. Energy Storage 2019, 21, 231–240. [Google Scholar] [CrossRef]
- Seh, Z.W.; Sun, Y.; Zhang, Q.; Cui, Y. Designing high-energy lithium–sulfur batteries. Chem. Soc. Rev. 2016, 45, 5605–5634. [Google Scholar] [CrossRef] [PubMed]
- Aykol, M.; Herring, P.; Anapolsky, A. Machine learning for continuous innovation in battery technologies. Nat. Rev. Mater. 2020, 5, 725–727. [Google Scholar] [CrossRef]


| Polymer | Salt | Filler | Additives | References |
|---|---|---|---|---|
| PEO | NaClO4 | Silica | - | [41] |
| PEO | LiTFSI | Silica | - | [50] |
| PEO | LiTf | Surface modified sepiolite | EC or EC:PC | [42,43,44] |
| PEO | LiTf | Surface modified sepiolite | EMITFSI, EMIFSI, PMPFSI, PMPTFSI (PYR13TFSI), BMBFSI, BMBFSI (PYR14TFSI) | [45,46,47,49] |
| PVdF-HFP | LiTf | Surface modified sepiolite | PYR13TFSI | [48] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verdier, N.; Foran, G.; Lepage, D.; Prébé, A.; Aymé-Perrot, D.; Dollé, M. Challenges in Solvent-Free Methods for Manufacturing Electrodes and Electrolytes for Lithium-Based Batteries. Polymers 2021, 13, 323. https://doi.org/10.3390/polym13030323
Verdier N, Foran G, Lepage D, Prébé A, Aymé-Perrot D, Dollé M. Challenges in Solvent-Free Methods for Manufacturing Electrodes and Electrolytes for Lithium-Based Batteries. Polymers. 2021; 13(3):323. https://doi.org/10.3390/polym13030323
Chicago/Turabian StyleVerdier, Nina, Gabrielle Foran, David Lepage, Arnaud Prébé, David Aymé-Perrot, and Mickaël Dollé. 2021. "Challenges in Solvent-Free Methods for Manufacturing Electrodes and Electrolytes for Lithium-Based Batteries" Polymers 13, no. 3: 323. https://doi.org/10.3390/polym13030323
APA StyleVerdier, N., Foran, G., Lepage, D., Prébé, A., Aymé-Perrot, D., & Dollé, M. (2021). Challenges in Solvent-Free Methods for Manufacturing Electrodes and Electrolytes for Lithium-Based Batteries. Polymers, 13(3), 323. https://doi.org/10.3390/polym13030323