Thermo- and Photoresponsive Behaviors of Dual-Stimuli-Responsive Organogels Consisting of Homopolymers of Coumarin-Containing Methacrylate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
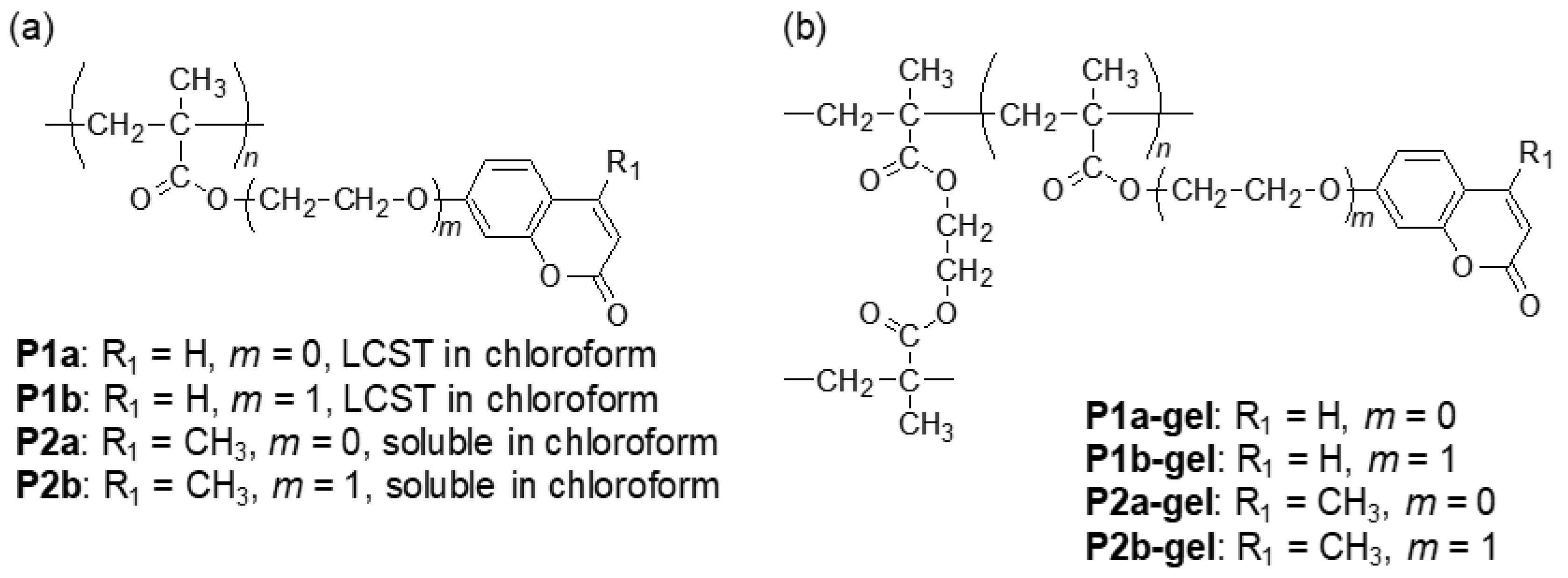
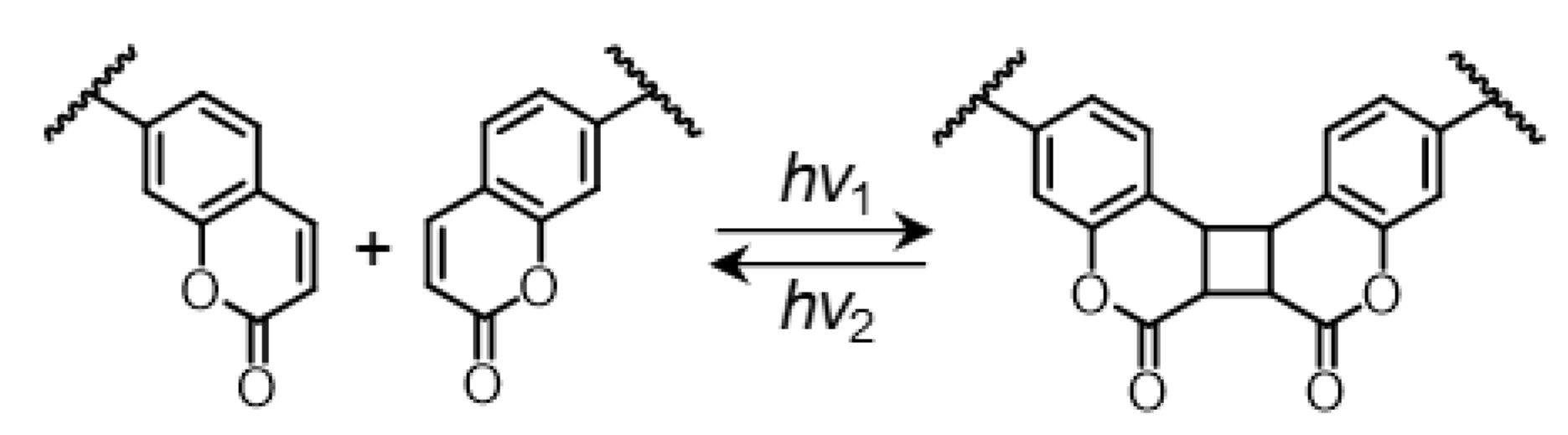
2.2. Synthesis of Coumarin-Containing Organogels
2.3. Observation of Organogels during Heating
2.4. Determination of Gel Fraction and Degree of Swelling
2.5. Photopatterning
3. Results and Discussion
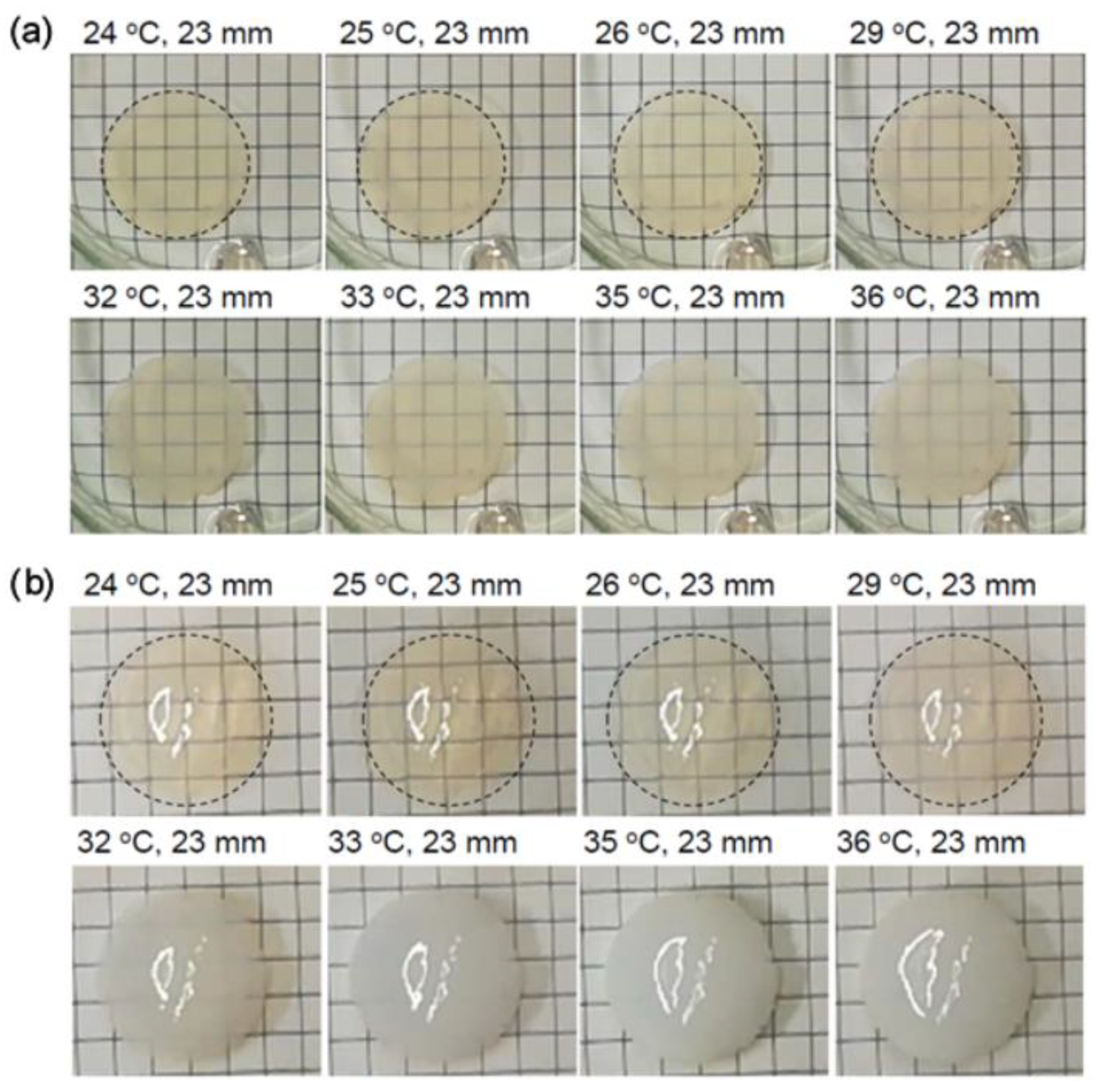
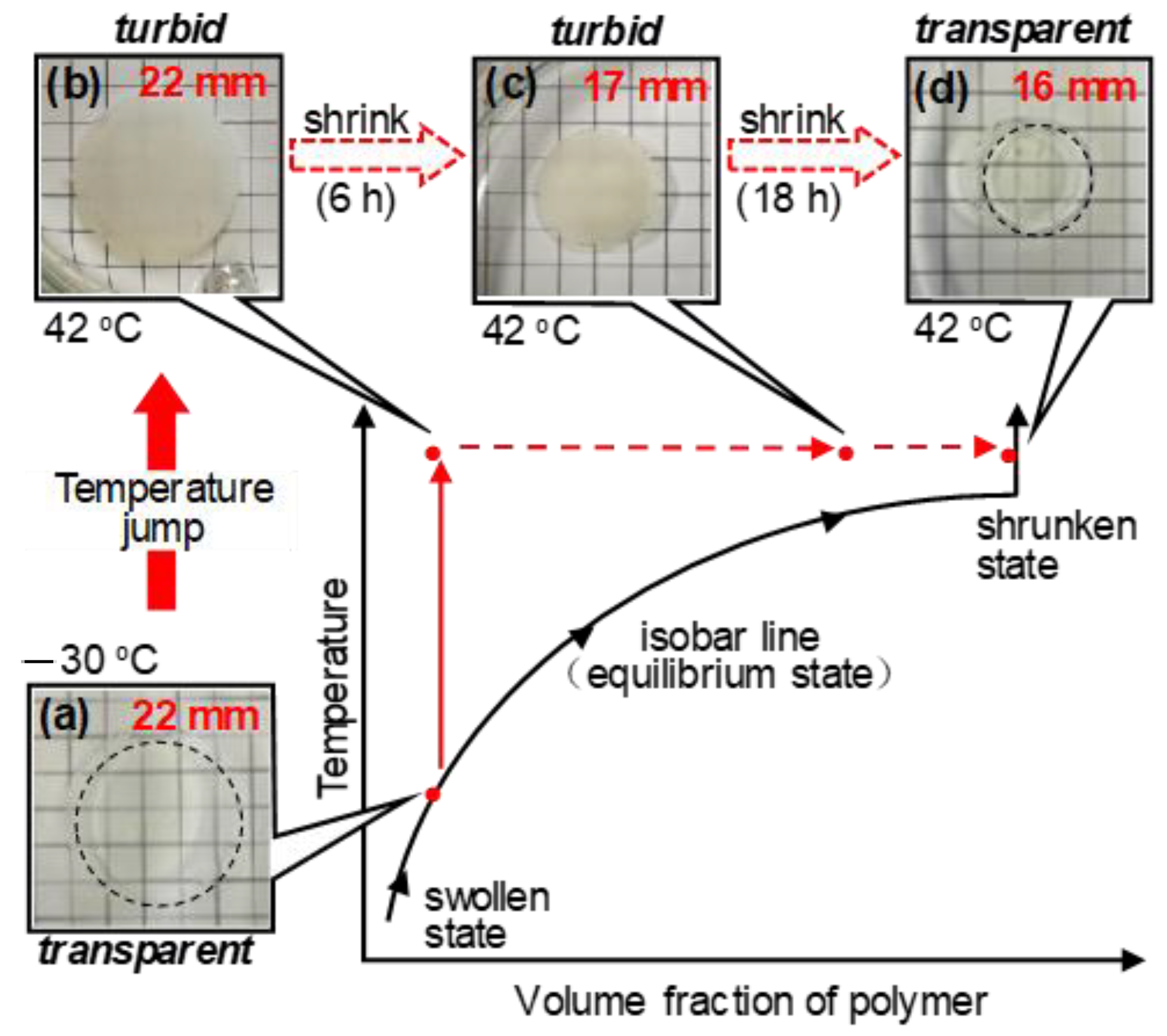
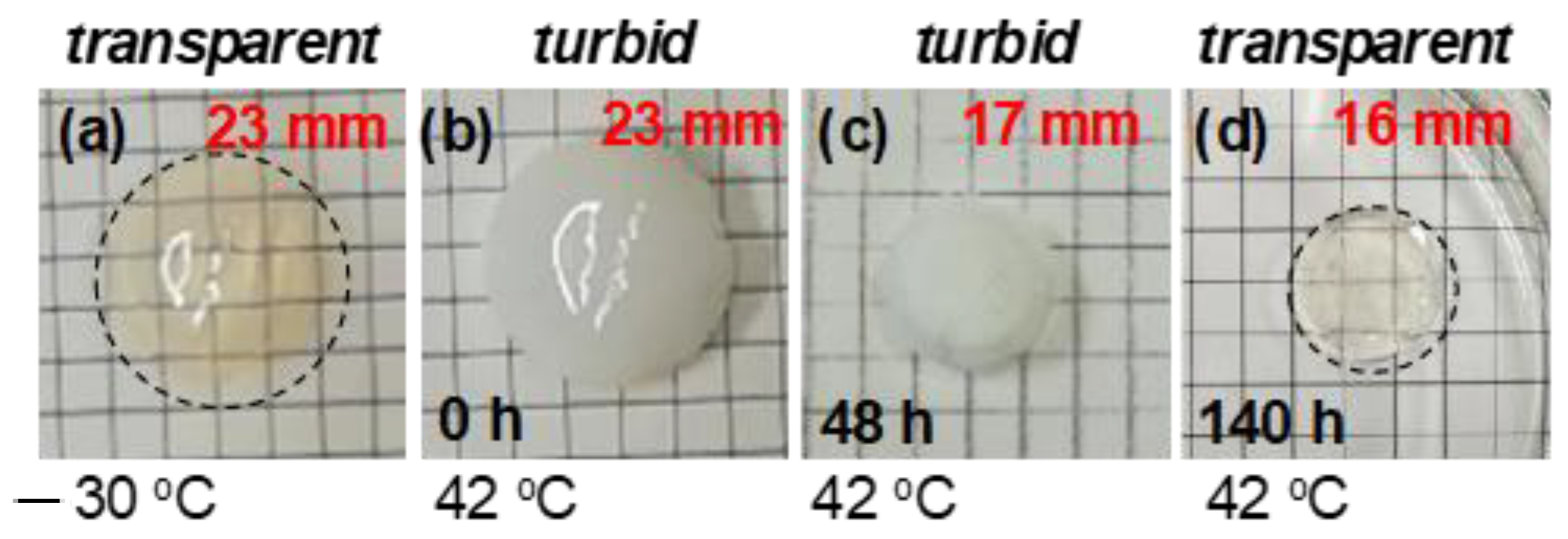
3.1. Thermoresponsive Behavior of Coumarin-Containing Organogels
3.2. Deswelling Behavior during the Isothermal Process
3.3. Reversibility of Thermoresponsive Cehavior of Coumarin-Containing Organogels
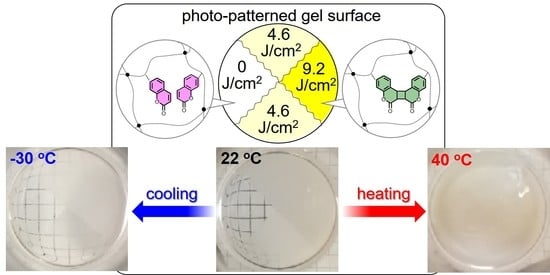
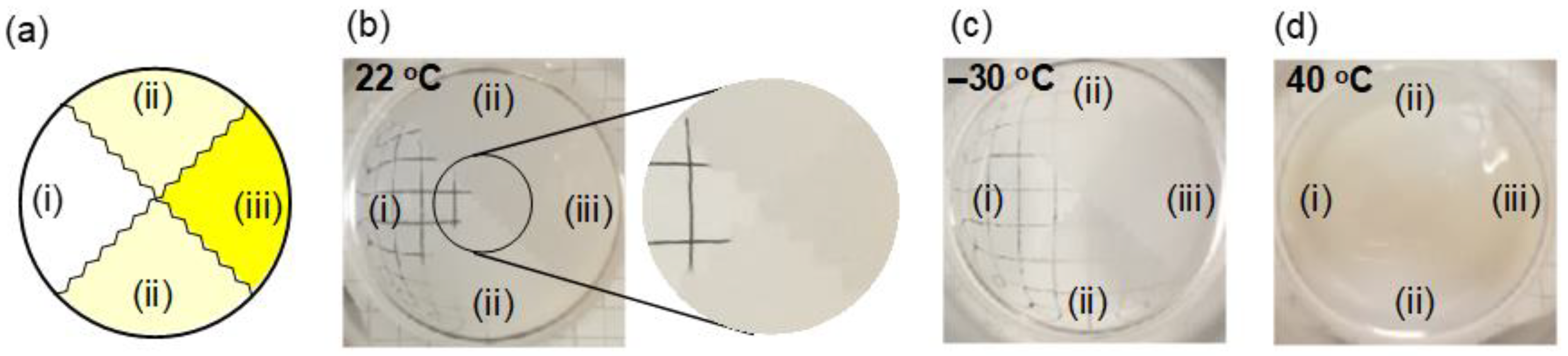
3.4. Photopatterning of Thermoresponsive Sites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ahn, S.-K.; Kasi, R.M.; Kim, S.-C.; Sharma, N.; Zhou, Y. Stimuli-responsive polymer gels. Soft Matter 2008, 4, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Pasparakis, G.; Vamvakaki, M. Multiresponsive polymers: Nano-sezed assemblies, stimuli-sensitive gels and smart surfaces. Polym. Chem. 2011, 2, 1234–1248. [Google Scholar] [CrossRef]
- Cao, Z.Q.; Wang, G.J. Multi-Stimuli-Responsive PolymerMaterials: Particles, Films, and Bulk Gels. Chem. Rec. 2016, 16, 1398–1435. [Google Scholar] [CrossRef] [PubMed]
- Brazel, C.S.; Peppas, N.A. Synthesis and Characterization of Thermo-and Chemomechanically Responsive Poly (N-isopropylacrylamide-co-methacrylic acid) Hydrogels. Macromolecules 1995, 28, 8016–8020. [Google Scholar] [CrossRef]
- Luo, Q.; Guan, Y.; Zhang, Y.; Siddiq, M. Lead-Sensitive PNIPAM Microgels Modified with Crown Ether Groups. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 4120–4127. [Google Scholar] [CrossRef]
- Shi, K.; Liu, Z.; Wei, Y.-Y.; Wang, W.; Ju, X.-J.; Xie, R.; Chu, L.-Y. Near-Infrared Light-Responsive Poly (N-isopropylacrylamide)/Graphene Oxide Nanocomposite Hydrogels with Ultrahigh Tensibility. ACS Appl. Mater. Interfaces 2015, 7, 27289–27298. [Google Scholar] [CrossRef]
- Rubio-Retama, J.; Zafeiropoulos, N.E.; Serafinelli, C.; Rojas-Reyna, R.; Voit, B.; Cabarcos, E.L.; Stamm, M. Synthesis and Characterization of Thermosensitive PNIPAM Microgels Covered with Superparamagnetic γ-Fe2O3 Nanoparticles. Langmuir 2007, 23, 10280–10285. [Google Scholar] [CrossRef]
- Zhang, K.; Feng, X.; Ye, C.; Hempenius, M.A.; Vancso, J. Hydrogels with a Memory: Dual-Responsive, Organometallic Poly(ionic liquid)s with Hysteretic Volume-Phase Transition. J. Am. Chem. Soc. 2017, 139, 10029–10035. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Zhang, M.; Feng, X.; Hempenius, M.A.; Vancso, J. Switching Light Transmittance by Responsive Organometallic Poly(ionic liquid)s: Control by Cross Talk of Thermal and Redox Stimuli. Adv. Funct. Mater. 2017, 27, 201770247. [Google Scholar] [CrossRef]
- Gonza’lez, N.; Elvira, C.; Roma’n, J.S. Novel dual-stimuli-responsive polymers derived from ethylpyrrolidine. Macromolecules 2005, 38, 9298–9303. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, N.; Fu, C.; Li, K.; Tao, L.; Feng, L.; Wei, Y. Thermo and pH dual-responsive materials for controllable oil/water separation. ACS Appl. Mater. Interfaces 2014, 6, 2026–2030. [Google Scholar] [CrossRef]
- Sato, E.; Masuda, Y.; Kadota, J.; Nishiyama, T.; Horibe, H. Dual Stimuli-Responsive Homopolymers: Thermo- and Photo-responsive Properties of Coumarin-Containing Polymers in Organic Solvents. Eur. Polym. J. 2015, 69, 605–615. [Google Scholar] [CrossRef] [Green Version]
- Trenor, S.R.; Shultz, A.R.; Love, B.J.; Long, T.E. Coumarins in Polymers: From Light Harvesting to Photo-Cross-Linkable Tissue Scaffolds. Chem. Rev. 2004, 104, 3059–3077. [Google Scholar] [CrossRef] [PubMed]
- Sato, E.; Nakanishi, R.; Nishiyama, T.; Horibe, H. Coumarin-containing Polymers as Thermo- and Photo-responsive Polymers: Copolymerization with Solvophilic or Solvophobic Comonomers to Control Thermoresponsive Behavior. Chem. Lett. 2017, 46, 108–110. [Google Scholar] [CrossRef] [Green Version]
- Sato, E. Coumarin Derivative Polymers for Thermo- and Light- Responsive Monofunctional Polymers: Effect of Additives on Thermo-Responsivity. AIP Conf. Proc. 2018, 2040, 020006. [Google Scholar]
- Hirokawa, Y.; Tanaka, Y. Volume Phase Transition in a Nonionic Gel. J. Chem. Phys. 1984, 81, 6379–6380. [Google Scholar] [CrossRef]
- Hoffman, A.S.; Afrassiabi, A.; Dong, L.C. Thermally Reversible Hydrogels: II. Delivery and Selective Removal of Substances from Aqueous Solutions. J. Control. Release 1986, 4, 213–222. [Google Scholar] [CrossRef]
- Shibayama, M. Spatial inhomogeneity and dynamic fluctuations of polymer gels. Macromol. Chem. Phys. 1998, 199, 1–30. [Google Scholar] [CrossRef]
- Lyon, L.A.; Debord, J.D.; Debord, S.B.; Jones, C.D.; McGrath, J.G.; Serpe, M.J. Microgel Colloidal Crystals. J. Phys. Chem. B 2004, 108, 19099–19108. [Google Scholar] [CrossRef]
- Yoshida, R. Self-Oscillating Gels Driven by the Belousov-Zhabotinsky Reaction as Novel Smart Materials. Adv. Mater. 2010, 22, 3463–3483. [Google Scholar] [CrossRef]
- Illeperuma, W.R.K.; Sun, J.-Y.; Suo, Z.; Vlassak, J.J. Force and stroke of a hydrogel actuator. Soft Matter 2012, 9, 8504–8511. [Google Scholar] [CrossRef] [Green Version]
- Harmon, M.E.; Tang, M.; Frank, C.W. A microfluidic actuator based on thermoresponsive hydrogels. Polymer 2003, 44, 4547–4556. [Google Scholar] [CrossRef]
- Depa, K.; Strachota, A.; Slouf, M.; Brus, J.; Cimrova, V. Synthesis of Conductive Doubly Filled Poly (N-isopropylacrylamide)-polyaniline-SiO2 Hydrogels. Sens. Actuators B 2017, 244, 616–634. [Google Scholar] [CrossRef]
- Gutowska, A.; Bae, Y.H.; Feijen, J.; Kim, S.W. Heparin release from thermosensitive hydrogels. J. Control. Release 1992, 22, 95–104. [Google Scholar] [CrossRef] [Green Version]
- Sato, E.; Nagai, S.; Matsumoto, A. Reversible thickness control of polymer thin films containing photoreactive coumarin derivative units. Prog. Org. Coat. 2013, 76, 1747–1751. [Google Scholar] [CrossRef]
- Jackson, P.O.; O’Neill, M. An Investigation of the Role of Cross-Linking and Photodegradation of Side-Chain Coumarin Polymers in the Photoalignment of Liquid Crystals. Chem. Mater. 2001, 13, 694–703. [Google Scholar] [CrossRef]
- Kaneko, Y.; Sakai, K.; Kikuchi, A.; Yoshida, R.; Sakurai, Y.; Okano, T. Influence of freely mobile grafted chain length on dynamic properties of comb-type grafted poly (N-isopropylacrylamide) hydrogels. Macromolecules 1995, 28, 7717–7723. [Google Scholar] [CrossRef]
- Park, T.G.; Hoffman, A.S. Deswelling Characteristics of Poly(N-isopropylacrylamide) Hydrogel. J. Appl. Polym. Sci. 1994, 52, 85–89. [Google Scholar] [CrossRef]
- Nomura, R.; Yamada, K.; Tabei, J.; Takakura, Y.; Takigawa, T.; Masuda, T. Stimuli-Responsive Organoles Based on Poly (N-propargylamide). Macromolecules 2003, 36, 6939–6941. [Google Scholar] [CrossRef]
- Ueki, T.; Watanabe, M. Lower Critical Solution Temperature Behavior of Linear Polymers in Ionic Liquids and the Corresponding Volume Phase Transition of Polymer Gels. Langmuir 2007, 23, 988–990. [Google Scholar] [CrossRef]
- Zhao, Y.; Tremblay, L.; Zhao, Y. Phototunable LCST of Water-Soluble Polymers: Exploring a Topological Effect. Macromolecules 2011, 44, 4007–4011. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okada, S.; Sato, E. Thermo- and Photoresponsive Behaviors of Dual-Stimuli-Responsive Organogels Consisting of Homopolymers of Coumarin-Containing Methacrylate. Polymers 2021, 13, 329. https://doi.org/10.3390/polym13030329
Okada S, Sato E. Thermo- and Photoresponsive Behaviors of Dual-Stimuli-Responsive Organogels Consisting of Homopolymers of Coumarin-Containing Methacrylate. Polymers. 2021; 13(3):329. https://doi.org/10.3390/polym13030329
Chicago/Turabian StyleOkada, Seidai, and Eriko Sato. 2021. "Thermo- and Photoresponsive Behaviors of Dual-Stimuli-Responsive Organogels Consisting of Homopolymers of Coumarin-Containing Methacrylate" Polymers 13, no. 3: 329. https://doi.org/10.3390/polym13030329