Abstract
We synthesized medium-band-gap donor-acceptor (D-A) -type conjugated polymers (PBTZCZ-L and PBTZCZ-H) consisting of a benzotriazole building block as an acceptor and a carbazole unit as a donor. In comparison with the polymers, a small conjugated molecule (BTZCZ-2) was developed, and its structural, thermal, optical, and photovoltaic properties were investigated. The power conversion efficiency (PCE) of the BTZCZ-2-based solar cell devices was less than 0.5%, considerably lower than those of polymer-based devices with conventional device structures. However, inverted solar cell devices configured with glass/ITO/ZnO:PEIE/BTZCZ-2:PC71BM/MoO3/Ag showed a tremendously improved efficiency (PCE: 5.05%, Jsc: 9.95 mA/cm2, Voc: 0.89 V, and FF: 57.0%). We believe that this is attributed to high energy transfer and excellent film morphologies.
1. Introduction
Recently, π-conjugated small molecules and polymers have attracted considerable attention owing to their light weight, good mechanical flexibility, and compatibility with roll-to-roll printing processes that are potentially applicable to solution-fabricated electronic and optoelectronic devices [1,2,3,4,5]. In contrast to polymers, the production of π-conjugated small molecules can be easily scaled-up and high-purity molecules can be obtained [6,7,8]. In addition, they exhibit good crystallinity and a well-defined chemical structure [9]. Using suitable design strategies, π-conjugated small molecules can be adapted to control molecular orbital energy levels and optical transitions, thus achieving desired properties for specific device functions [10]. For example, π-conjugated small molecules with acceptor-donor-acceptor (A-D-A) structures are promising materials for bulk heterojunction organic photovoltaics (OPVs). The power conversion efficiency (PCE) of 11.7% has been obtained for such small molecules by optimizing their molecular structure and device fabrication conditions using an open-circuit voltage (Voc) of 0.95 V, short-circuit current (Jsc) of 15.7 mA/cm−2, and fill factor (FF) of 76% [11]. It is known that π-conjugated small molecules possessing a medium optical energy bandgap (Eg) exhibit high PCEs owing to the complementary broad absorption of fullerene acceptors (PC71BM) [12]. Recently, Zhu et al. reported a remarkable PCE of 13.7% of a binary π-conjugated small molecular system of BSFTR as a donor and Y6 as an acceptor [13].
The units of benzotriazole (BTZ) and its derivatives are relatively weak electron acceptors with medium-level Eg compared to those of benzothiadiazole, diketopyrrolopyrrole, and quinoxaline building blocks [14,15]. BTZ molecules can act as efficient light absorbing materials in solar cell devices because their molecular structure can prevent aggregation, despite the disadvantage of a wide band gap [16]. In addition, the solubility of BTZ molecules can be improved by directly linking alkyl chains on nitrogen atoms while maintaining a planar conformation [17]. When electron-withdrawing fluorine atoms are introduced into BTZ building blocks, their derivatives can be utilized as efficient acceptor alternatives to fullerenes [18,19,20]. We recently reported BTZ-based polymers with a medium Eg of 2.04 eV, highest occupied molecular orbital of −5.54 eV, and lowest unoccupied molecular orbital of −3.50 eV. The highest PCE of 3.81% was achieved with a Voc of 0.88 V, Jsc of 7.69 mA/cm2, and FF of 55.9% when used in inverted solar cells with a ZnO interlayer [21].
In this work, newly developed π-conjugated small molecules (BTZCZ-2) and polymers containing BTZ building blocks with carbazole units as donor materials were synthesized and characterized for photovoltaic applications. Polymerization was carefully controlled to obtain a low-molecular-weight polymer (PBTZCZ-L) and a high-molecular-weight polymer (PBTZCZ-H). The effects of molecular weights on OPV performance were investigated with respect to the structural characteristics. Among the three materials, the PBTZCZ-H-based device exhibited the best device performance, with Jsc of 11.5 mA/cm2, Voc of 0.74 V, FF of 0.60, and PCE of 5.13%. Surprisingly, the BTZCZ-2 based device, which shows a wider bandwidth compared to those of the BTZ polymers, possessed a high PCE of 5.05% with Jsc of 9.95 mA/cm2, Voc of 0.89 V, and FF of 0.57.
2. Experimental Details
Materials and characterization 4-(5-Bromothiophen-2-yl)-2-octyl-7-(thiophene-2-yl)-2H-benzo[d][1,2,3]triazole (1) [22], 4,7-bis(5-bromothiophen-2-yl)-2-octyl-2H-benzo[d][1,2,3] triazole (2) [23], and 9-(heptadecan-9-yl)-2,7-bis(4,4,5,5,-tetramethyl-1,3,2-dioxaborolan-2-yl)-9H-carbazole (3) [24] were synthesized according to the literature. For structural characterization, a Varian 300 spectrometer and Perkin-Elmer spectrophotometer were used to record 1H and 13C NMR spectra (300 MHz) and UV/vis spectra, respectively. Differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) were performed using TA Instruments Q50 at a ramping rate of 10 °C/min under N2 atmosphere. Molecular weight and polydispersity index (PDI) were measured using a Waters 1515 gel permeation chromatograph (GPC) with a refractive index detector at room temperature (RT) with chlorobenzene as the eluent and polystyrene as the standard. Atomic force microscopy (AFM) was performed using a Digital Instruments Nanoscope IV operated in tapping mode (~350 kHz frequency, Si tip).
BTZCZ-2 Compounds 1 (1.186 g, 2.5 mmol) and 3 (0.657 g, 1.0 mmol), (PPh3)4Pd(0) (2 mg, 0.0017 mmol), and a few drops of Aliquat 336 were dissolved in a mixture of toluene (30 mL) and aqueous 2 M Na2CO3 (4 mL). The solution was refluxed at 115 °C for 18 h under N2 atmosphere. The reaction mixture was cooled to RT, and the mixture was concentrated. The crude product was purified by column chromatography (silica gel, hexane/ethyl acetate = 1:5) to afford 0.966 g of BTZCZ-2 (81%). 1H NMR (CDCl3, ppm): ™ 8.20 (d, 4H), 7.81 (d, 2H), 7.73 (m, 6H), 7.60 (m, 4H), 7.41 (m, 4H), 4.53 (t, 2H), 4.65 (m, 2H), 1.70 (m, 10H), 1.28–1.33 (m, 106H), 0.92 (t, 15H). 13C NMR (CDCl3, ppm) ™ 148.9, 145.0, 137.1, 136.5, 128.6, 128.0, 126.4, 124.9, 122.6, 121.5, 119.1, 117.2, 111.6, 109.6, 68.1, 63.4, 35.3, 31.9, 29.6, 29.3, 27.0, 26.7, 25.7, 22.7, 14.1. Tg = 51 °C, Td = 365 °C.
PBTZCZ-L Compounds 2 (0.553 g, 1.0 mmol) and 3 (0.657 g, 1.0 mmol), (PPh3)4Pd(0) (2 mg, 0.0017 mmol), and a few drops of Aliquat 336 were dissolved in a mixture of toluene (20 mL) and aqueous 2 M Na2CO3 (2 mL). The solution was refluxed at 115 °C for 12 h under N2 atmosphere. To perform end-capping of the polymers, a small amount of bromobenzene was added first and stirred for 8 h to remove the boronic ester end group. Phenylboronic acid was then added, and the solution was stirred for another 8 h to remove the bromine end group. The reaction mixture was cooled to RT, and the mixture was slowly added to vigorously stirred methanol (100 mL) to obtain the polymer precipitate. The polymer was dissolved in chloroform and was passed through a short silica gel column. The filtrate was concentrated, re-precipitated in methanol, and finally purified by Soxhlet extraction using methanol and hexane. The hexane fraction was concentrated, filtered through a 0.45 μm Teflon filter, and precipitated in methanol to afford 0.463 g of PBTZCZ-L (57%). 1H NMR (CDCl3, ppm): ™ 8.22–8.17 (br, 4H), 7.90–7.53 (br, 8H), 4.90 (br, 2H), 4.68 (br, 1H), 2.50–1.80 (br, 6H), 1.50–0.78 (br, 45H). Mn = 3,500 g∙mol−1, PDI = 1.9. Tg = 98 °C, Td = 415 °C.
PBTZCZ-H In order to afford the high-molecular-weight polymer PBTZCZ-H, the reaction was allowed to proceed for a long duration of 72 h using a procedure similar to that described for the polymer PBTZCZ-L. Due to its lower solubility than that of PBTZCZ-L, it can be further purified by subsequent Soxhlet extraction using acetone, hexane, and chloroform. The chloroform fraction was concentrated, filtered through a 0.45 μm Teflon filter, and precipitated in methanol to afford 0.568 g of PBTZCZ-H (70%). ™ 8.22–8.17 (m, 4H), 7.90–7.53 (br, 8H), 4.90 (m, 2H), 4.68 (m, 1H), 2.50–1.80 (m, 6H), 1.50–0.78 (br, 45H). Mn = 26,700 g∙mol−1, PDI = 5.6. Tg = 126 °C, Td = 439 °C.
Fabrication and characterization of solar cells Photovoltaic properties were measured using the conventional device structure of indium-tin-oxide (ITO)/poly(3,4-ethylenedioxy-thiophene):polystyrene sulfonate (PEDOT:PSS)/active layer/LiF/Al and the inverted device structure of ITO/ZnO:polyethylenimine ethoxylated (PEIE)/active layer/MoO3/Ag. ITO substrates were cleaned with detergents, DI water, acetone, and isopropyl alcohol by sonication for 20 min each. The substrates were then treated with O2 plasma for 10 min. For the conventional device, a hole-transporting layer of PEDOT:PSS (Baytron PVP Al 4083) was spin-coated at 4000 rpm for 30 s and annealed at 120 °C for 1 h under N2 atmosphere. Active layers were deposited on the PEDOT:PSS layer by spin casting at 2000 rpm for 60 s from a 1,2-diclorobenzene solution containing 2.0 wt% of BTZ-based materials:PC71BM (1:2 w/w). The samples were annealed at 80 °C for 10 min prior to thermal evaporation of LiF (0.8 nm) and Al (100 nm). For the inverted devices, the ZnO solution was spin-coated at 4000 rpm onto ITO glass and annealed at 200 °C for 10 min in ambient atmosphere. After spin-coating the active layer, MoO3 (10 nm) and Ag (100 nm) were subsequently evaporated under high vacuum (<1 × 10−4 Pa). The dark and illuminated J-V characteristics of the solar cells were tested using a Keithley 2400 source meter and an Oriel white light source under AM 1.5, illuminated at 100 mW/cm2.
Synchrotron X-ray scattering measurements Synchrotron X-ray scattering measurements were carried out at the 3C beamline of the Pohang Accelerator Laboratory in Korea. The X-ray wavelength was 1.1651 Å. Two-dimensional X-ray patterns were recorded using a CCD (Rayonix 2D SX165) X-ray detector. The distance between the sample and the detector was 50 cm, and the exposure time was 20 s. The samples in the powder state were held for at least 10 min at the measurement temperature, and then X-ray scattering measurements were recorded.
3. Results and Discussion
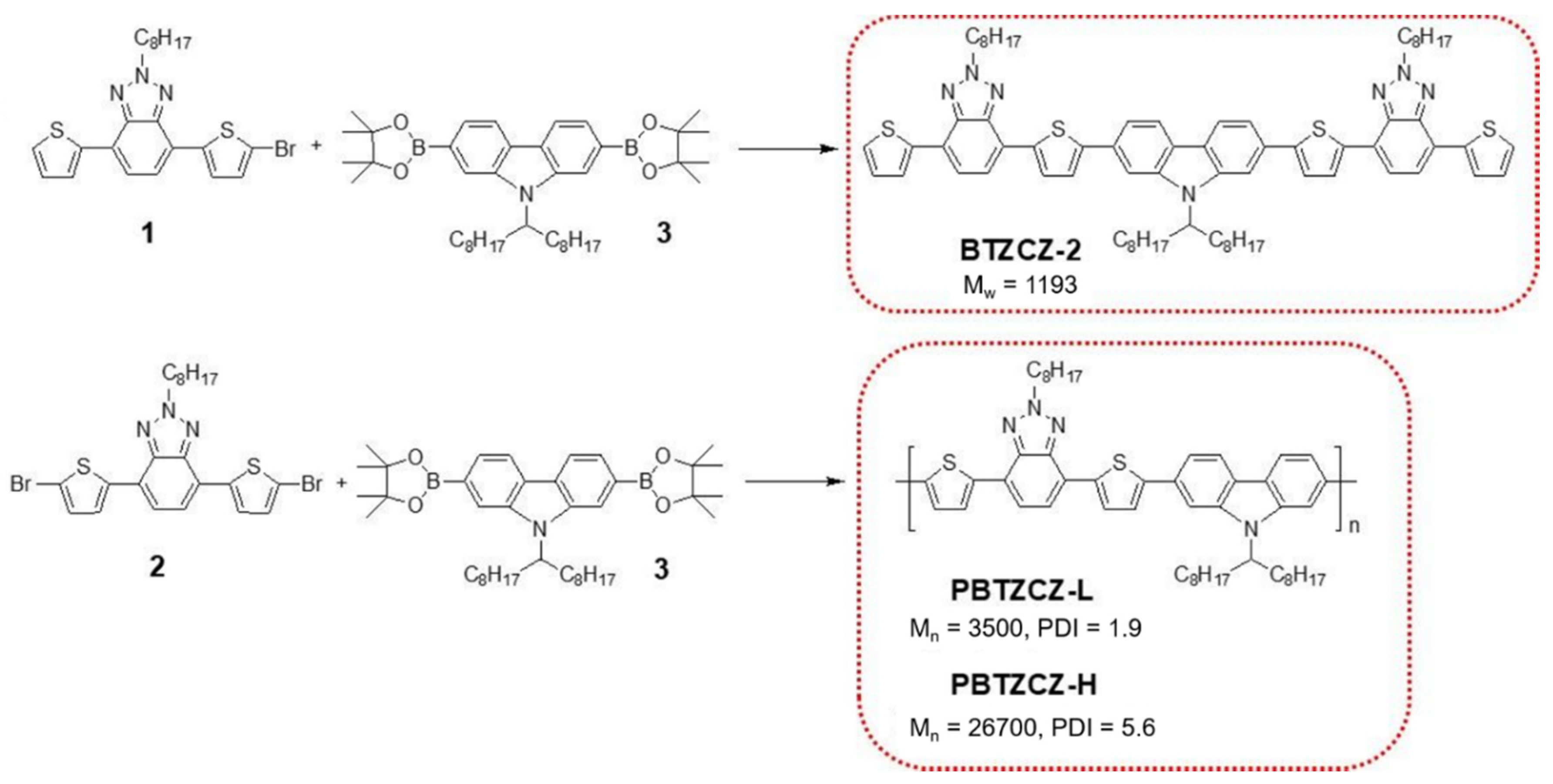
Scheme 1 shows the synthesis of BTZ-based small molecule (BTZCZ-2), low-molecular-weight oligomer (PBTZCZ-L), and high-molecular-weight polymer (PBTZCZ-H). The starting compounds were prepared as previously described [22,23,24], and their chemical structures were confirmed by 1H NMR and 13C NMR spectroscopy. BTZCZ-2, PBTZCZ-L, and PBTZCZ-H were prepared by Pd(0)-catalyzed Suzuki coupling reactions with carefully controlled molar ratios of the reactants. They were soluble in common organic solvents such as chloroform, toluene, chlorobenzene, and tetrahydrofuran owing to the long alkyl chain at the 2-position on the BTZ units. Notably, BTZCZ-2 and PBTZCZ-L were soluble even in acetone and hexane, whereas PBTZCZ-H, a high-molecular-weight polymer, was not. Therefore, after removing low-molecular-weight oligomers by subsequent Soxhlet extraction using acetone and hexane, we obtained PBTZCZ-H with a high number-average molecular weight (Mn). Table 1 lists the physical properties, including the molecular weights, of the polymers. The Mn values of PBTZCZ-L and PBTZCZ-H were 3500 and 26,700 g∙mol−1, respectively, and their PDIs were 1.9 and 5.6, respectively.
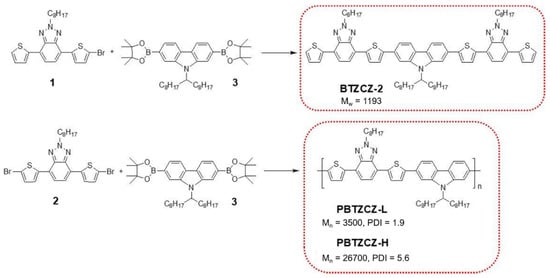
Scheme 1.
The synthesis of small molecule (BTZCZ-2), oligomer (PBTZCZ-L), and high-molecular-weight polymer (PBTZCZ-H) containing BTZ building blocks.

Table 1.
Physical properties of the BTZCZ-2, PBTZCZ-L, and PBTZCZ-H.
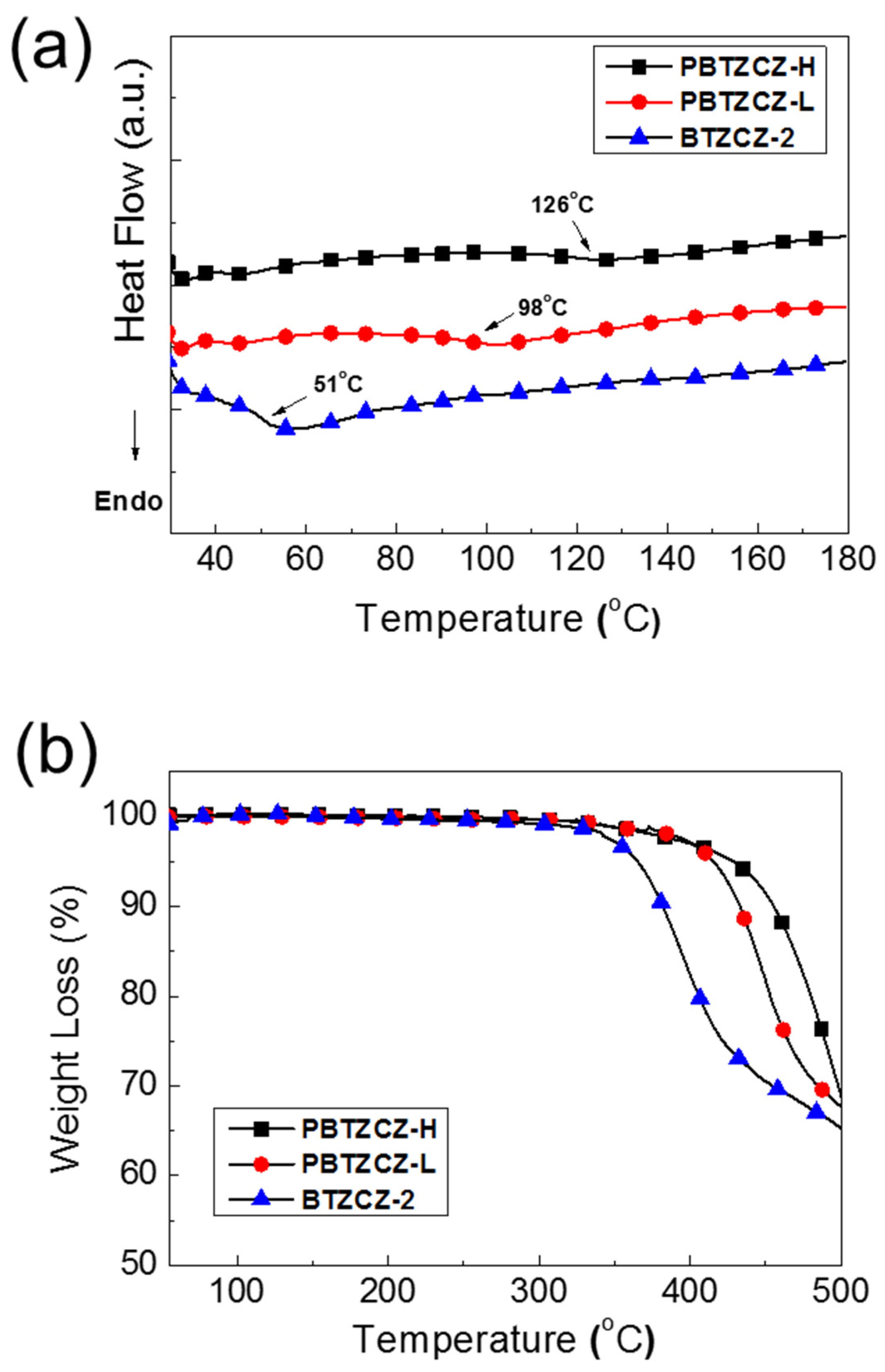
Figure 1 shows the DSC and TGA results of BTZCZ-2, PBTZCZ-L, and PBTZCZ-H at a ramping rate of 10 °C/min under N2 atmosphere. As seen in Figure 1a, the glass transition temperature (Tg) of the small molecule BTZCZ-2 was observed ~51 °C, but the melting temperature was not observed due to the flexible alkyl groups. The Tg values of PBTZCZ-L and PBTZCZ-H were 98 °C and 126 °C, respectively. They showed good thermal stability, indicating that the initial decomposition temperatures (5% weight loss, Td) were found to be 365 °C, 415 °C, and 439 °C for BTZCZ-2, PBTZCZ-L, and PBTZCZ-H, respectively, as shown in Figure 1b.
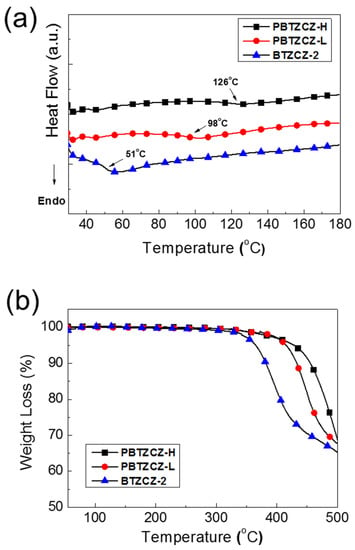
Figure 1.
(a) DSC and (b) TGA data for BTZCZ-2, PBTZCZ-L, and PBTZCZ-H samples showing thermal transition and thermal stability.
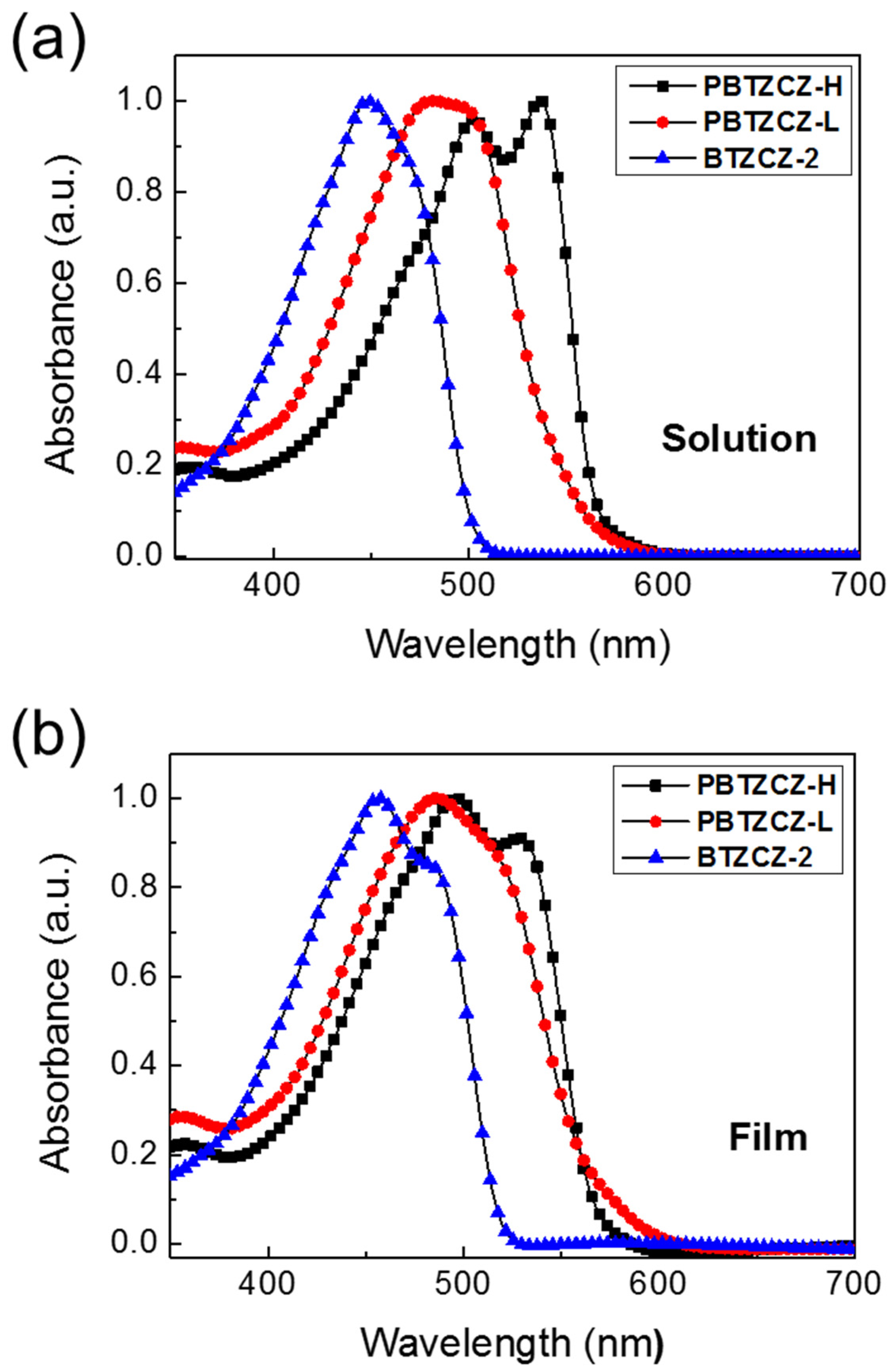
Figure 2 shows the UV/vis absorption spectra of BTZCZ-2, PBTZCZ-L, and PBTZCZ-H in solution and as thin solid films. In dilute chlorobenzene solution, the absorption maxima of BTZCZ-2, PBTZCZ-L, and PBTZCZ-H were located at 449, 481, and 536 nm, respectively, caused by the intramolecular charge transfer between the BTZ and carbazole units. PBTZCZ-H exhibited the broadest absorption bands between 400 and 600 nm, with two absorption maxima peaks at 502 nm and 536 nm. Compared to that of PBTZCZ-H as a solution, the absorption spectrum of PBTZCZ-H as a thin solid film remained almost unchanged. However, BTZCZ-2 and PBTZCZ-L as thin solid films exhibited a slight bathochromic shift (~5 nm) compared to their spectra recorded in the solution form, which might be due to the aggregation in the solid state.
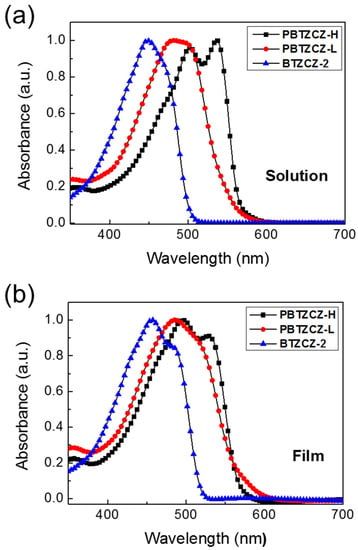
Figure 2.
UV/vis absorption spectra of BTZCZ-2, PBTZCZ-L, and PBTZCZ-H (a) in chlorobenzene solution and (b) as thin solid films.
Moreover, the cut-off absorption wavelength for PBTZCZ-L was estimated to be ~600 nm, which was higher than that of PBTZCZ-H (585 nm), even though the absorption maximum value for PBTZCZ-L was shown to be lower than that of PBTZCZ-H. These results suggest that small molecules and oligomers based on BTZ building blocks with carbazole units have better intermolecular interactions and aggregation in the solid state compared to those of high-molecular-weight polymers. The optical band gap (Eg) values, calculated from the cut-off absorption wavelength of the solid-state films, were 2.34 eV for BTZCZ-2, 2.11 eV for PBTZCZ-L, and 2.12 eV for PBTZCZ-H.
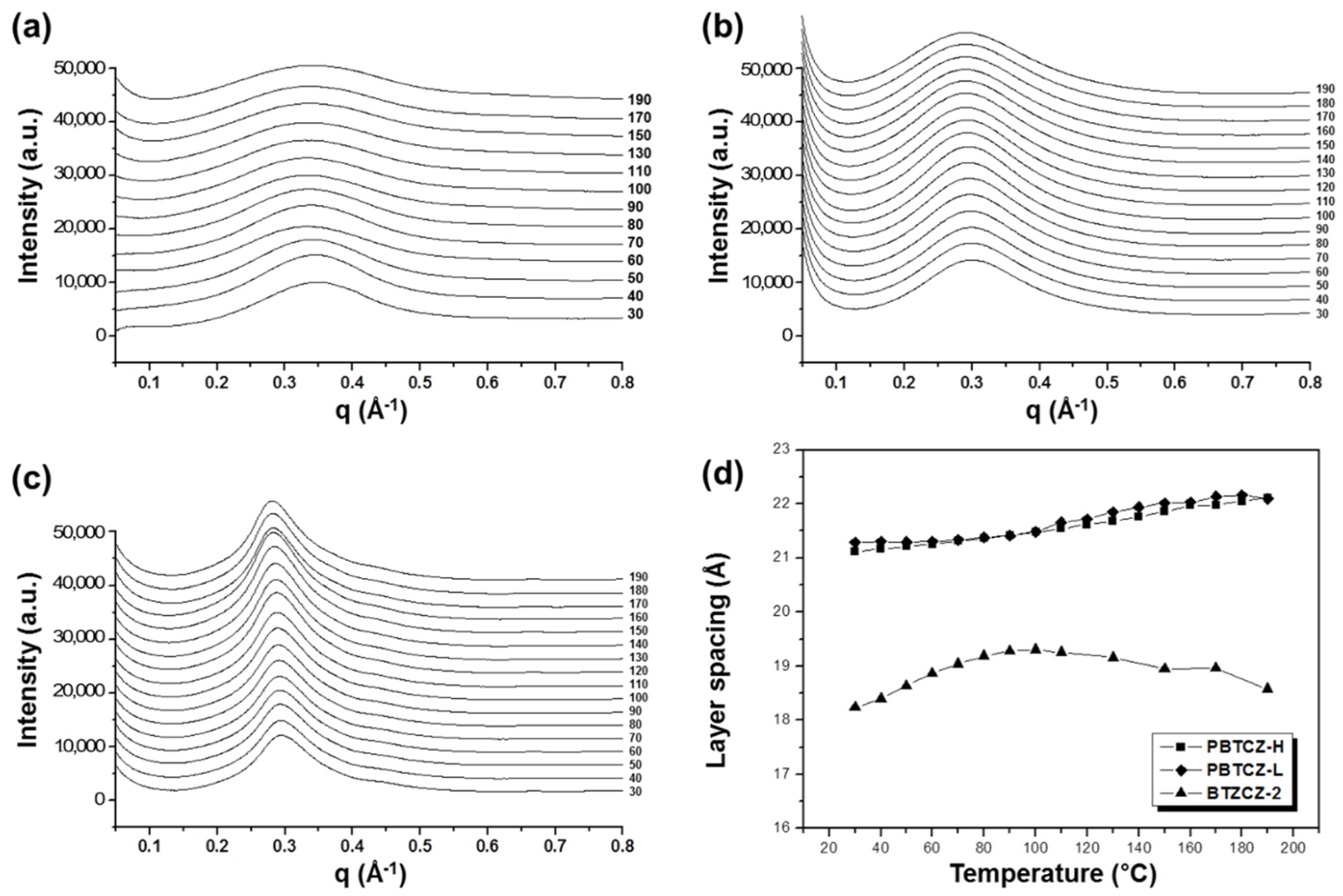
The X-ray scattering data for BTZCZ-2, PBTZCZ-L, and PBTZCZ-H during the heating process are shown in Figure 3. The structural changes occurring during the first heating, which were unstable during sample preparation, are not shown here. A chain rearrangement occurred during the second heating. It should be noted that the peak of PBTZCZ-H with a high molecular weight was located at the lowest q value. The q value is associated with the layer spacing between the polymer molecules. As shown in Figure 3d, the layer spacing of the polymers is dependent on the temperature change. The spacing for BTZCZ-2 was the shortest, ranging from 18 to 19 Å. It increased rapidly and then gradually decreased with increasing temperature. On the other hand, those for PBTZCZ-L and PBTZCZ-H only tended to increase up to 22 Å. This tendency is related to the intermolecular bonding forces at elevated temperatures. Because the intermolecular force is small in BTZCZ-2 despite the relatively narrow gap between the molecules (in comparison with that seen in other two specimens), the structure changed sensitively with temperature. The structures of the polymers changed smoothly owing to the intermolecular bonding of long chains with high molecular weights.
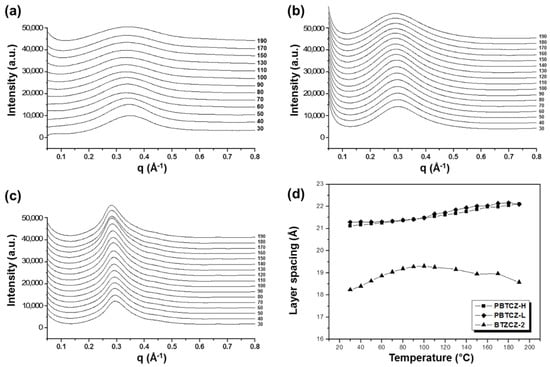
Figure 3.
X-ray scattering profiles for (a) BTZCZ-2, (b) PBTZCZ-L, and (c) PBTZCZ-H at 30–190 °C, and (d) dependence of layer spacing of the samples on increased temperature.
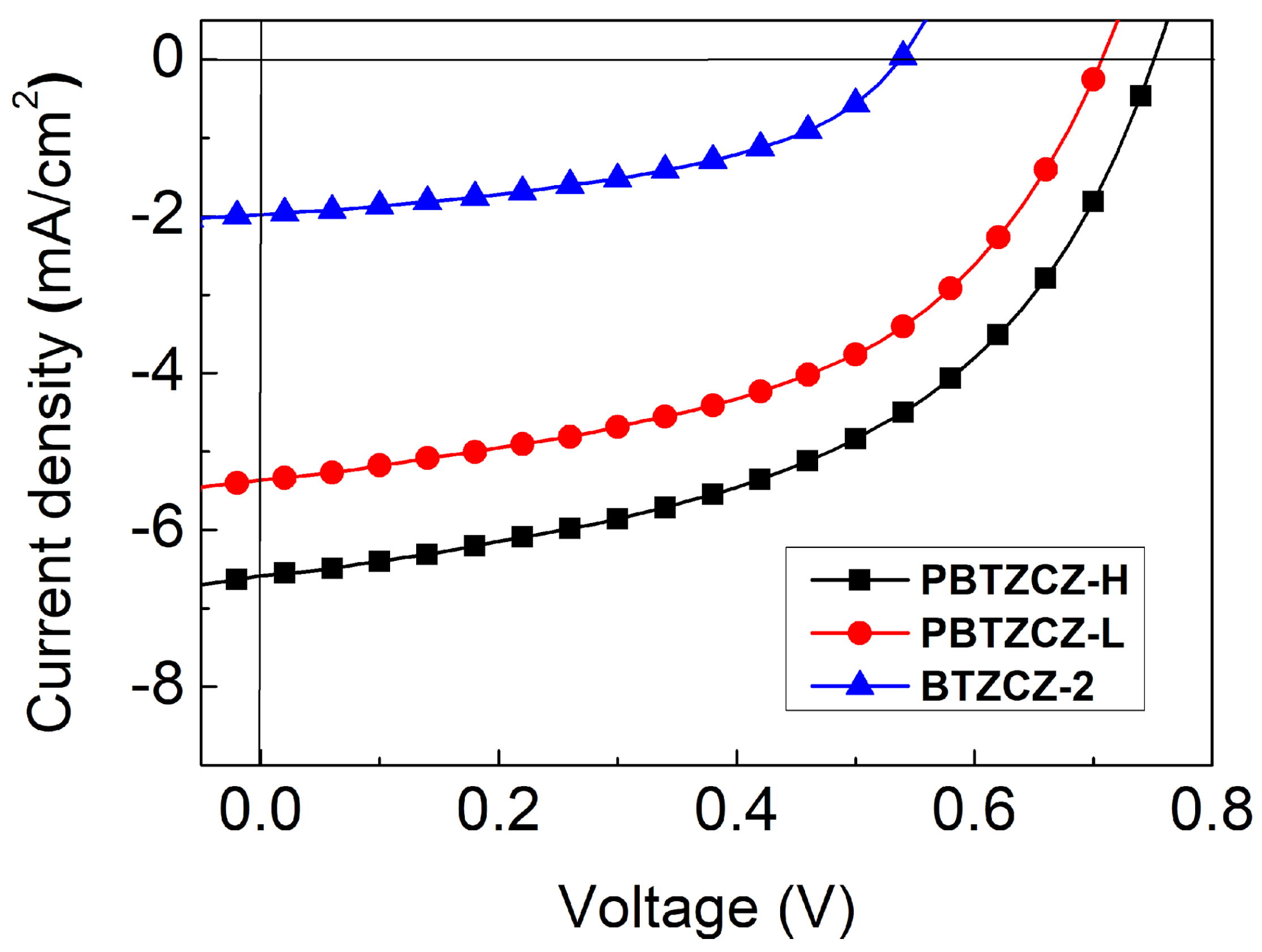
Photovoltaic properties were measured using the conventional (ITO/PEDOT: PSS/active layer/LiF/Al) and inverted (ITO/ZnO:PEIE(1:2)/active layer/MoO3/Ag) device structures. The active layers of the solar cells were fabricated by spin-casting a 1:2 (w/w) mixture of BTZ-based materials with PC71BM. After spin coating, the films were thermally annealed at 80 °C for 10 min. The corresponding J-V curves for conventional device structures under simulated AM 1.5G solar irradiation (100 mW/cm2) are shown in Figure 4, and the detailed device parameters are summarized in Table 2. The BTZCZ-2:PC71BM-based device showed low device efficiency, with PCE of 0.49%, Jsc of 1.97 mA/cm2, Voc of 0.54 V, and FF of 45.9%. Device made from an oligomer type of PBTZCZ-L showed better device performance: PCE = 1.87% (Jsc: 5.36 mA/cm2, Voc: 0.71 V, and FF: 49.5%), which was almost four times higher than that of the BTZCZ-2-based device. The enhanced PCE was attributed to the lower optical band gap and broader absorption spectrum
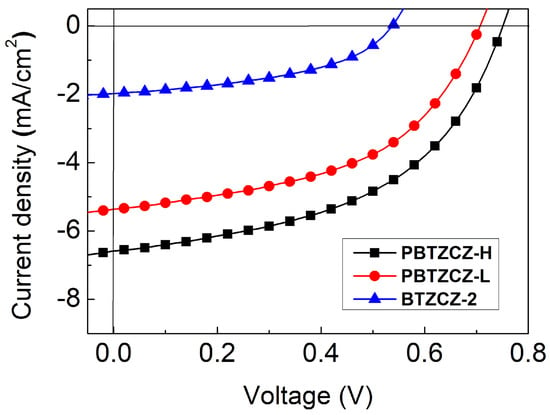
Figure 4.
J-V characteristics of the BTZCZ-2-, PBTZCZ-L-, and PBTZCZ-H-based solar cell devices.

Table 2.
Photovoltaic performances of the conventional and inverted devices based on the PBTZCZ-H, PBTZCZ-L, and BTZCZ-2 with PC71BM (1:2).
The best solar cell performance in the conventional device structures was obtained using PBTZCZ-H. Its PCE was 2.43% with Jsc of 6.58 mA/cm2, Voc of 0.74 V, and FF of 49.2%. While the Jsc of the PBTZCZ-H-based device significantly increased by over 20%, the Voc barely increased by less than 5% compared to that of the PBTZCZ-L-based device. These results indicate that PBTZCZ-L with Mn of 3500 g∙mol−1 already reached the effective conjugated length for the energy band gap, resulting in a Voc similar to that of the high-molecular-polymer PBTZCZ-H. On the other hand, the enhanced Jsc for the PBTZCZ-H-based device is explained by the efficient exciton dissociation and charge collection from the PBTZCZ-H and PC71BM domains, which increase with increasing molecular weight.
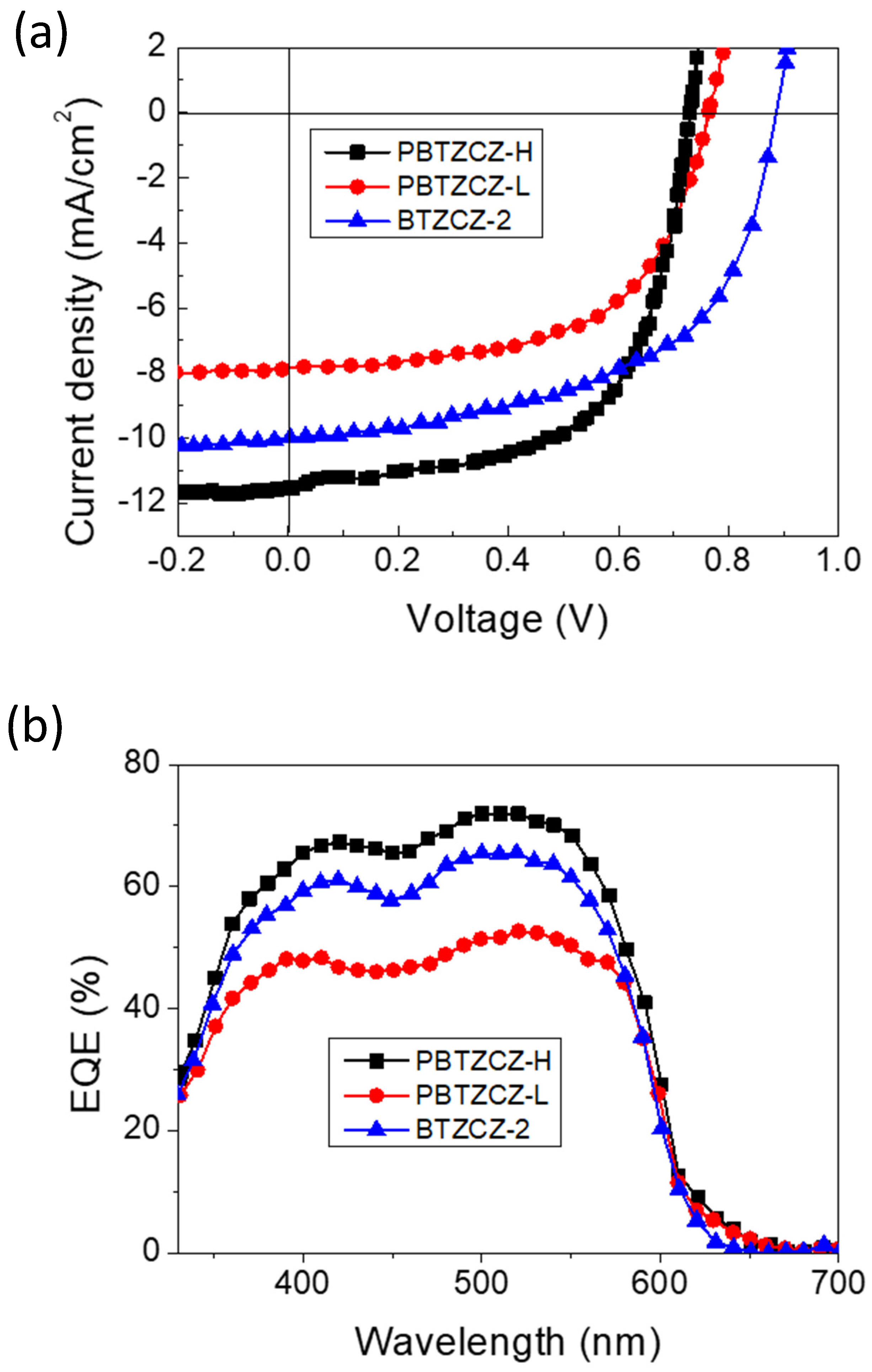
We found that these active materials showed improved device performance with inverted device structures with two different interfacial layers (ZnO only and a combination of ZnO and PEIE). It is known that the polyelectrolyte PEIE introduced on top of ZnO, which is used as the electron-collecting interlayer, can improve the electron extraction and transport efficiency at the cathode in inverted OPV devices [25,26]. PBTZCZ-H- and PBTZCZ-L-based devices with only ZnO layer showed PCEs of 3.81% and 3.53%, respectively, and high Jsc, Voc, and FF. Surprisingly, the device performance of BTZCZ-2 was similar to that of PBTZCZ-L and PBTZCZ-H; The PCE was 3.76% with Jsc of 7.71 mA/cm2, Voc of 0.87 V, and FF of 56.5%. Further enhancement of the photovoltaic performance was achieved by using a combination of ZnO and PEIE layers (1:2). The PBTZCZ-H-based device showed the highest PCE of 5.13% with Jsc of 11.5 mA/cm2, Voc of 0.74 V, and FF of 60.1%. The BTZCZ-2-based device showed a slightly lower PCE of 5.05%. This improvement is attributed to the reduced work function of the combined ZnO and PEIE (3.8 eV from 4.5 eV), resulting in an increase in the work function difference between the anode and cathode, thus leading to an increase in the Jsc, Voc, and FF. Figure 5a shows the J-V curves of BTZCZ-2-, PBTZCZ-L-, and PBTZCZ-H-based devices. The external quantum efficiency (EQE) spectra of these devices are shown in Figure 5b. The EQE values of BTZCZ-2- and PBTZCZ-H-based devices reached 70% over a wide absorption range.
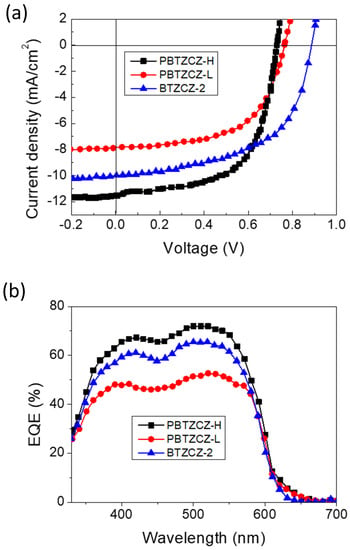
Figure 5.
(a) J-V characteristics and (b) External quantum efficiency characteristics of the BTZCZ-2-, PBTZCZ-L-, and PBTZCZ-H-based inverted OPV devices.
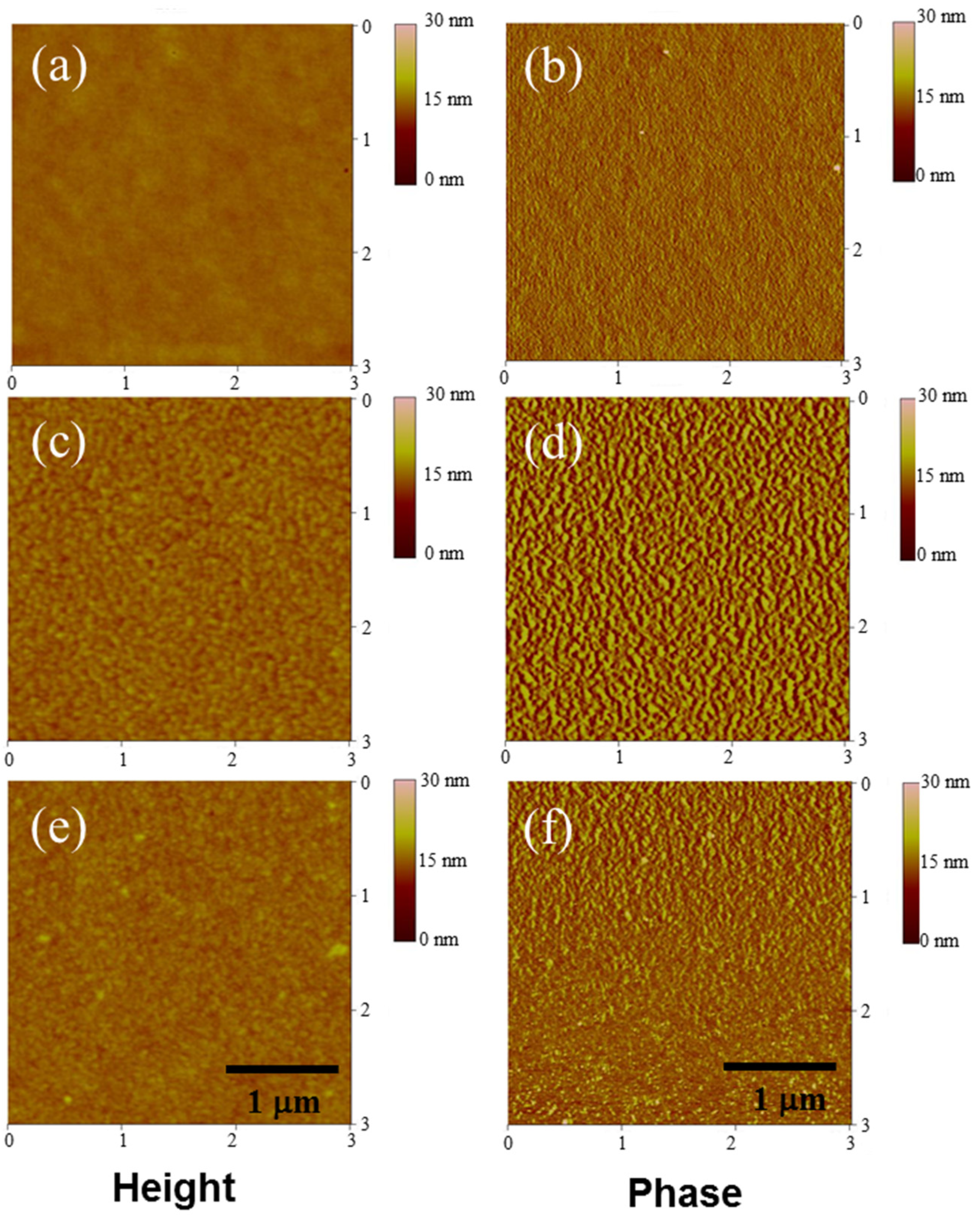
The high PCE and Voc of the BTZCZ-2-based device may originate from its superior film morphology compared to those of the PBTZCZ-H- and PBTZCZL-based devices. Polymer chains generally have stronger van der Waals interactions than do small molecules, causing more phase separation between the donor materials and the PC71BM. These different morphologies were characterized using tapping-mode AFM. Figure 6 shows the topographic height and phase images (3 × 3 μm). Notably, the small molecule-based BTZCZ-2:PC71BM film exhibited a more uniform surface than did the other two films. The root mean square (RMS) roughness values of BTZCZ-2:PC71BM, PBTZCZ-L:PC71BM, and PBTZCZ-H:PC71BM films were 0.36, 1.00, and 0.62 nm, respectively.
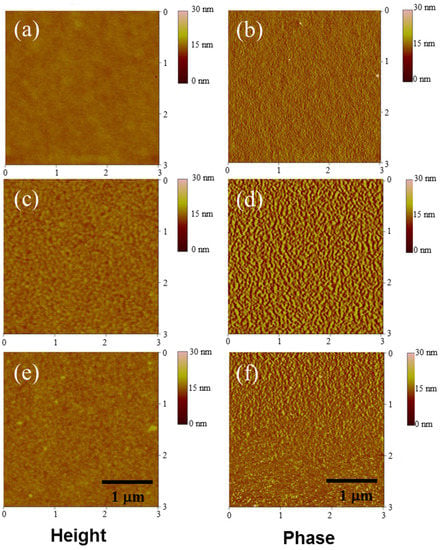
Figure 6.
AFM images of the three active layer films in a 3 × 3 μm scale. Height images (a,c,e) and phase images (b,d,f) of BTZCZ-2, PBTZCZ-L, PBTZCZ-H based films.
4. Conclusions
We successfully synthesized D-A π-conjugated small molecule, oligomer, and polymer consisting of a BTZ building block as an acceptor and a carbazole unit as a donor. All materials showed good solubility and high thermal stability. The high-molecular-weight polymer PBTZCZ-H exhibited an Mn value of 26,700 g∙mol−1 and a medium optical band gap of 2.12 eV. A solar cell device using PBTZCZ-H:PC71BM possessed a PCE of 2.43% with Jsc of 6.58 mA/cm2, Voc of 0.74 V, and FF of 49.2%. Further enhancement of the photovoltaic performance was achieved by using a combination of ZnO and PEIE layers (1:2). The PBTZCZ-H-based device showed the highest PCE of 5.13% at Jsc of 11.5 mA/cm2, Voc of 0.74 V, and FF of 60.1%. The BTZCZ-2-based inverted solar cell device displayed a PCE of 5.05%, which is considerably higher than those obtained for conventional solar cells.
Author Contributions
Investigation, J.E.L., Y.K., Y.H.N., N.S.B., J.W.J. and Y.C.; Supervision, N.C. and T.-D.K.; Writing—original draft, J.E.L., Y.K., Y.H.N., N.S.B., J.W.J. and Y.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Industrial Strategic Technology Development Program funded by the Ministry of Trade, Industry & Energy (10085643) and the National Research Foundation of Korea (NRF-2019R1F1A1063902). This work was supported by the Korea Institute of Energy Technology Evaluation and Planning (KETEP) and the Ministry of Trade, Industry & Energy (MOTIE) of the Republic of Korea under Grant No. 20184030202130. This work also supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning under Grant No. NRF-2019R1A4A1021237.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Forrest, S.R. The path to ubiquitous and low-cost organic electronic appliances on plastic. Nature 2004, 428, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Dou, L.; Liu, Y.; Hong, Z.; Li, G.; Yang, Y. Low-bandgap near-IR conjugated polymers/molecules for organic electronics. Chem. Rev. 2015, 115, 12633–12665. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.D.; Lee, K.S. D-π-A Conjugated Molecules for Optoelectronic Applications. Macromol. Rapid Commun. 2015, 36, 943–958. [Google Scholar] [CrossRef]
- Aizawa, N.; Pu, Y.J.; Watanabe, M.; Chiba, T.; Ideta, K.; Toyota, N.; Igarashi, M.; Suzuri, Y.; Sasabe, H.; Kido, J. Solution-processed multilayer small-molecule light-emitting devices with high-efficiency white-light emission. Nat. Commun. 2014, 5, 5756. [Google Scholar] [CrossRef] [PubMed]
- Murad, A.R.; Iraqi, A.; Aziz, S.B.; Abdullah, S.N.; Brza, M.A. Conducting Polymers for Optoelectronic Devices and Organic Solar Cells: A Review. Polymers 2020, 12, 2627. [Google Scholar] [CrossRef] [PubMed]
- Ala’a, F.E.; Sun, J.P.; Hill, I.G.; Welch, G.C. Recent advances of non-fullerene, small molecular acceptors for solution processed bulk heterojunction solar cells. J. Mater. Chem. A 2014, 2, 1201–1213. [Google Scholar]
- Kim, H.M.; Cho, B.R. Small-molecule two-photon probes for bioimaging applications. Chem. Rev. 2015, 115, 5014–5055. [Google Scholar] [CrossRef]
- Yook, K.S.; Lee, J.Y. Small molecule host materials for solution processed phosphorescent organic light-emitting diodes. Adv. Mater. 2014, 26, 4218–4233. [Google Scholar] [CrossRef] [PubMed]
- Roncali, J.; Leriche, P.; Blanchard, P. Molecular materials for organic photovoltaics: Small is beautiful. Adv. Mater. 2014, 26, 3821–3838. [Google Scholar] [CrossRef]
- Lin, Y.; Li, Y.; Zhan, X. Small molecule semiconductors for high-efficiency organic photovoltaics. Chem. Soc. Rev. 2012, 41, 4245–4272. [Google Scholar] [CrossRef]
- Deng, D.; Zhang, Y.; Zhang, J.; Wang, Z.; Zhu, L.; Fang, J.; Xia, B.; Wang, Z.; Lu, K.; Ma, W.; et al. Fluorination-enabled optimal morphology leads to over 11% efficiency for inverted small-molecule organic solar cells. Nat. Commun. 2016, 7, 1–9. [Google Scholar] [CrossRef]
- Li, M.; Gao, K.; Wan, X.; Zhang, Q.; Kan, B.; Xia, R.; Liu, F.; Yang, X.; Feng, H.; Ni, W.; et al. Solution-processed organic tandem solar cells with power conversion efficiencies > 12%. Nat. Photonics 2017, 11, 85–90. [Google Scholar] [CrossRef]
- Yue, Q.; Wu, H.; Zhou, Z.; Zhang, M.; Liu, F.; Zhu, X. 13.7% Efficiency Small-Molecule Solar Cells Enabled by a Combination of Material and Morphology Optimization. Adv. Mater. 2019, 31, 1904283. [Google Scholar] [CrossRef]
- Pola, M.K.; Boopathi, K.M.; Padhy, H.; Raghunath, P.; Singh, A.; Lin, M.C.; Chu, C.W.; Lin, H.C. Synthesis of fluorinated benzotriazole (BTZ)-and benzodithiophene (BDT)-based low-bandgap conjugated polymers for solar cell applications. Dyes Pigments 2017, 139, 349–360. [Google Scholar] [CrossRef]
- Unay, H.; dos Benatto, G.A.R.; Beliatis, M.J.; Gevorgyan, S.A.; Kavak, P.; Memiş, S.; Cirpan, A.; Toppare, L.; Parlak, E.A.; Krebs, F.C. High stability of benzotriazole and benzodithiophene containing medium band-gap polymer solar cell. Sol. Energy Mater. Sol. Cells 2018, 174, 433–444. [Google Scholar] [CrossRef]
- Lu, F.; Yang, G.; Xu, Q.; Zhang, J.; Zhang, B.; Feng, Y. Tailoring the benzotriazole (BTZ) auxiliary acceptor in a DA′-π-A type sensitizer for high performance dye-sensitized solar cells (DSSCs). Dyes Pigments 2018, 158, 195–203. [Google Scholar] [CrossRef]
- Lee, S.; Ha, J.-W.; Park, H.J.; Hwang, D.-H. Synthesis and Characterization of Benzotriazole-Based Polymer Donors with Good Planarity for Organic Photovoltaics. Macromol. Res. 2020, 28, 903–909. [Google Scholar] [CrossRef]
- Qiu, Z.; Xu, X.; Zhang, S.; Wang, P.; Wang, Y.; Pei, Y.; Peng, Q.; Liu, Y. Efficient strategies to improve photovoltaic performance of ADA type small molecules by introducing rigidly fluorinated central cores. Dyes Pigments 2017, 147, 505–513. [Google Scholar] [CrossRef]
- Feng, K.; Yuan, J.; Bi, Z.; Ma, W.; Xu, X.; Zhang, G.; Peng, Q. Low-energy-loss polymer solar cells with 14.52% efficiency enabled by wide-band-gap copolymers. IScience 2019, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Song, Y.; Wang, Z.; He, B.; Yang, X.; Li, L.; Xu, B.; Zhang, J.; Huang, F.; Cao, Y. Synthesis of medium bandgap copolymers based on benzotriazole for non-fullerene organic solar cells. Polymer 2019, 179, 121580. [Google Scholar] [CrossRef]
- Lee, K.-S.; Kim, T.-D.; Kajzar, F.; Rau, I. Molecular Crystals and Liquid Crystals. In Proceedings of the 14th International Conference on Frontiers of Polymers and Advanced Materials (14th ICFPAM), Aejeon, Korea, 31 October–4 November 2016. [Google Scholar]
- Kim, J.J.; Choi, H.; Lee, J.W.; Kang, M.S.; Song, K.; Kang, S.O.; Ko, J. A polymer gel electrolyte to achieve ≥ 6% power conversion efficiency with a novel organic dye incorporating a low-band-gap chromophore. J. Mater. Chem. 2008, 18, 5223–5229. [Google Scholar] [CrossRef]
- Hızalan, G.; Balan, A.; Baran, D.; Toppare, L. Spray processable ambipolar benzotriazole bearing electrochromic polymers with multi-colored and transmissive states. J. Mater. Chem. 2011, 21, 1804–1809. [Google Scholar] [CrossRef]
- Kim, B.; Yeom, H.R.; Yun, M.H.; Kim, J.Y.; Yang, C. A selenophene analogue of PCDTBT: Selective fine-tuning of LUMO to lower of the bandgap for efficient polymer solar cells. Macromolecules 2012, 45, 8658–8664. [Google Scholar] [CrossRef]
- Kyaw, A.K.K.; Wang, D.H.; Gupta, V.; Zhang, J.; Chand, S.; Bazan, G.C.; Heeger, A.J. Efficient solution-processed small-molecule solar cells with inverted structure. Adv. Mater. 2013, 25, 2397–2402. [Google Scholar] [CrossRef]
- Yu, W.; Huang, L.; Yang, D.; Fu, P.; Zhou, L.; Zhang, J.; Li, C. Efficiency exceeding 10% for inverted polymer solar cells with a ZnO/ionic liquid combined cathode interfacial layer. J. Mater. Chem. A 2015, 3, 10660–10665. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).