Formation and Evaluation of a Two-Phase Polymer System in Human Plasma as a Method for Extracellular Nanovesicle Isolation
Abstract
1. Introduction
2. Materials and Methods
2.1. Biological Samples
2.2. Components of PTPS
2.3. Plasma Preparation
2.4. ENV Isolation by Differential Centrifugation (UC)
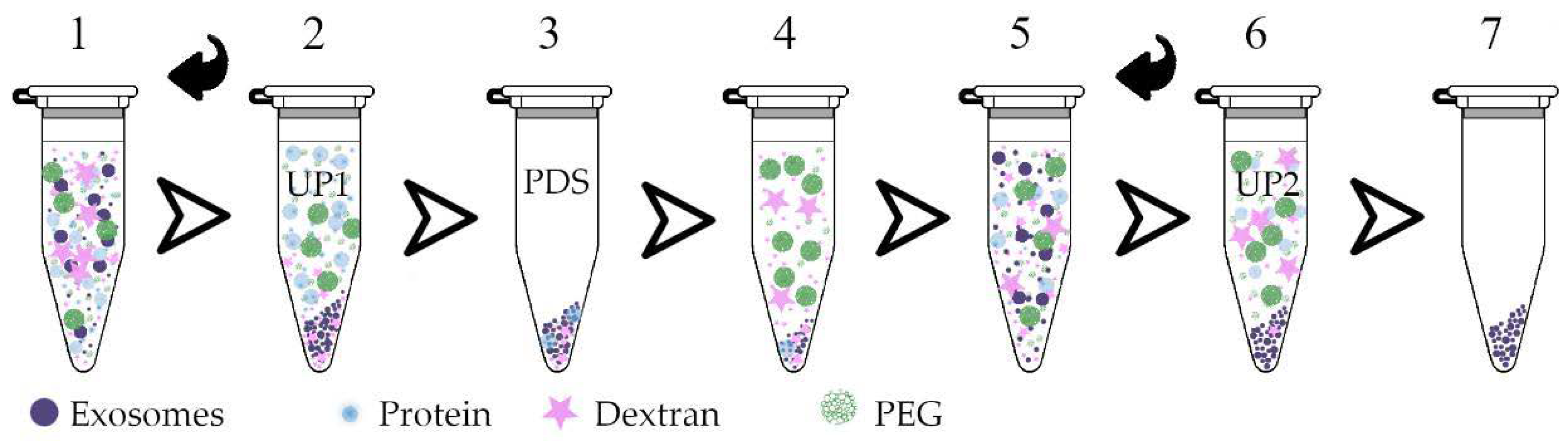
2.5. ENV Isolation by Plasma Two-Phase Polymer System (PTPS)
2.6. ENV Isolation by Commercial Kits
2.7. Viscosimetry and Dynamic Light Scattering (DLS)
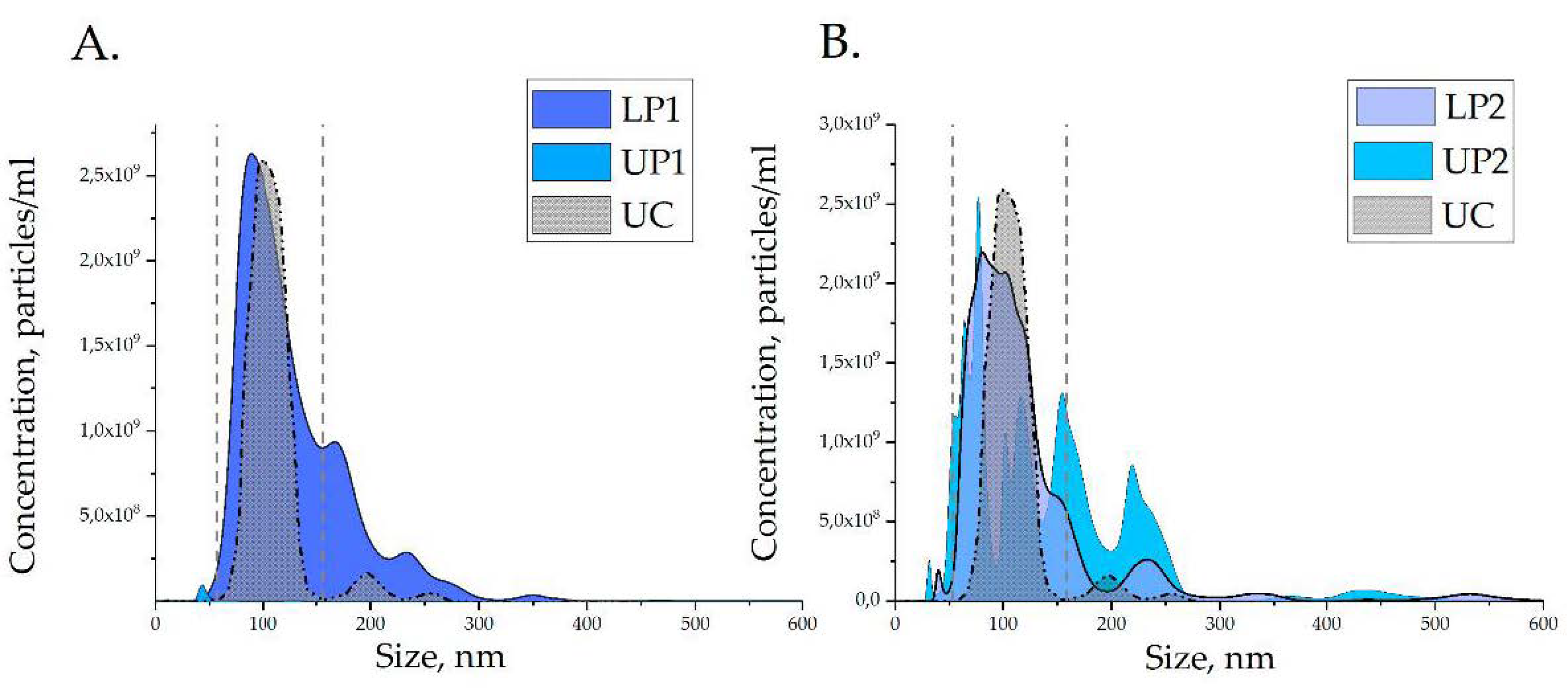
2.8. Nanoparticle Tracking Analysis (NTA)
2.9. Atomic Force Microscopy (AFM)
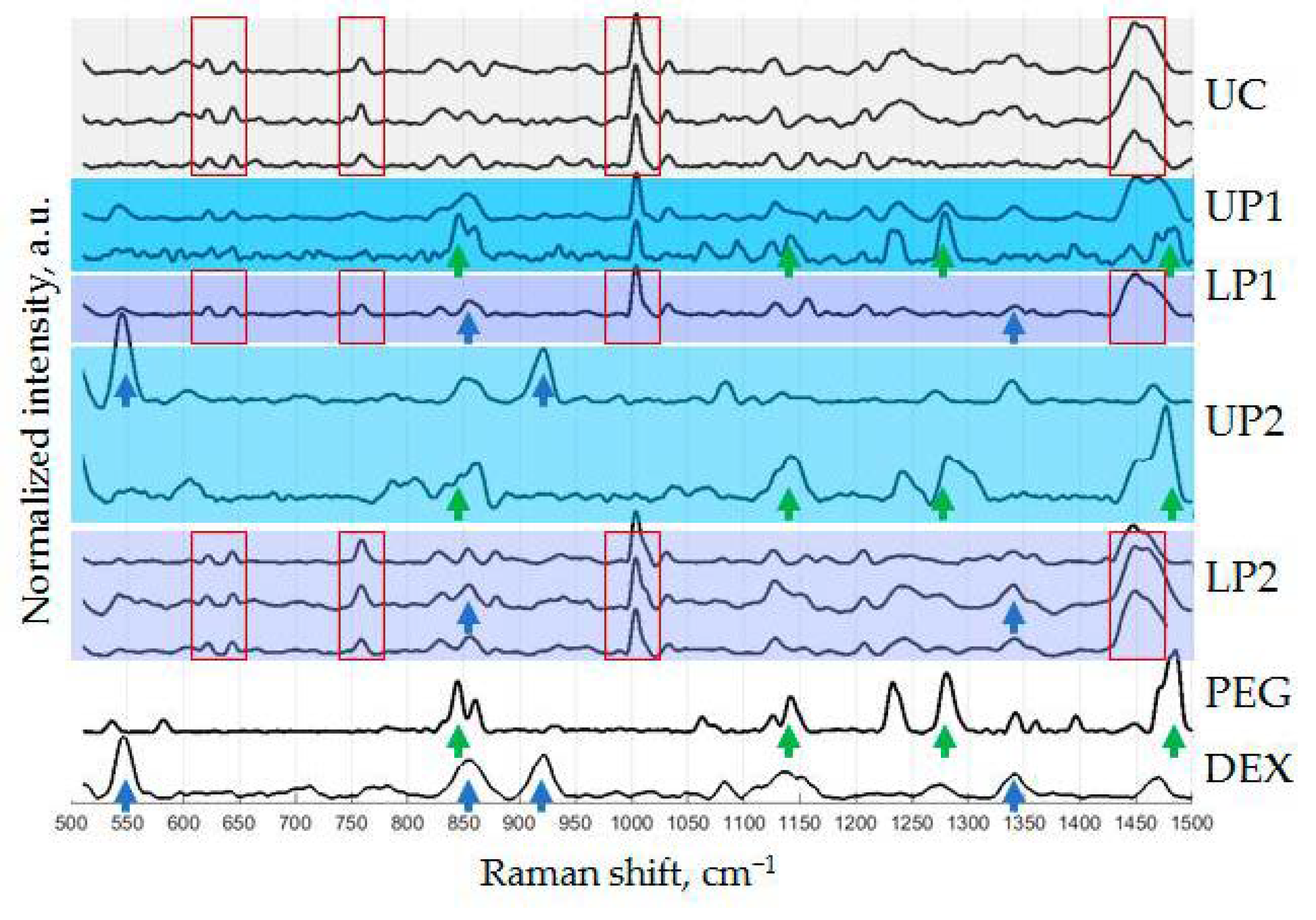
2.10. Raman Spectroscopy (RS)
2.11. Dot Blotting
2.12. Isolation of RNA and RT-PCR Analysis of MicroRNA
2.13. Flow Cytometry (FC)
3. Results
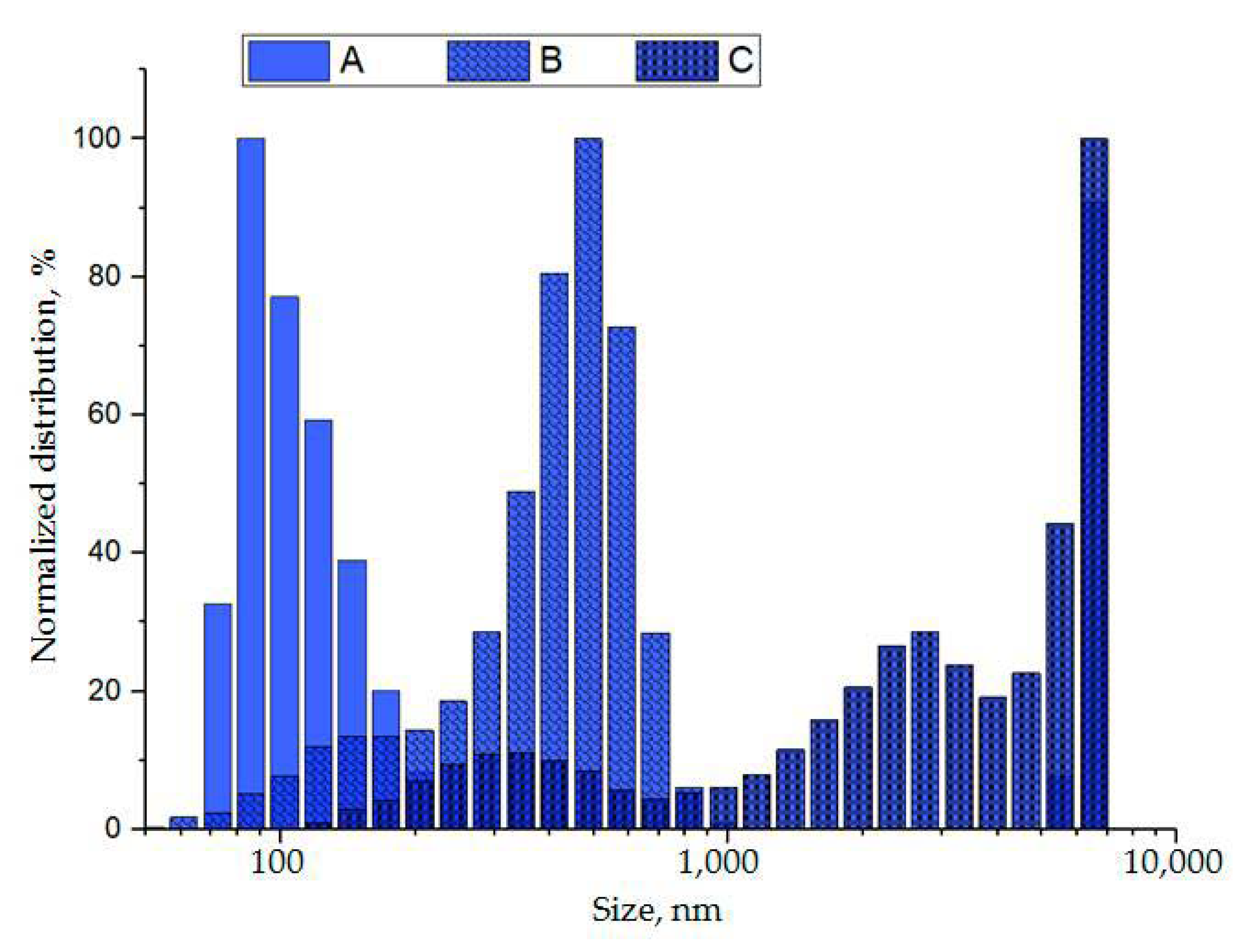
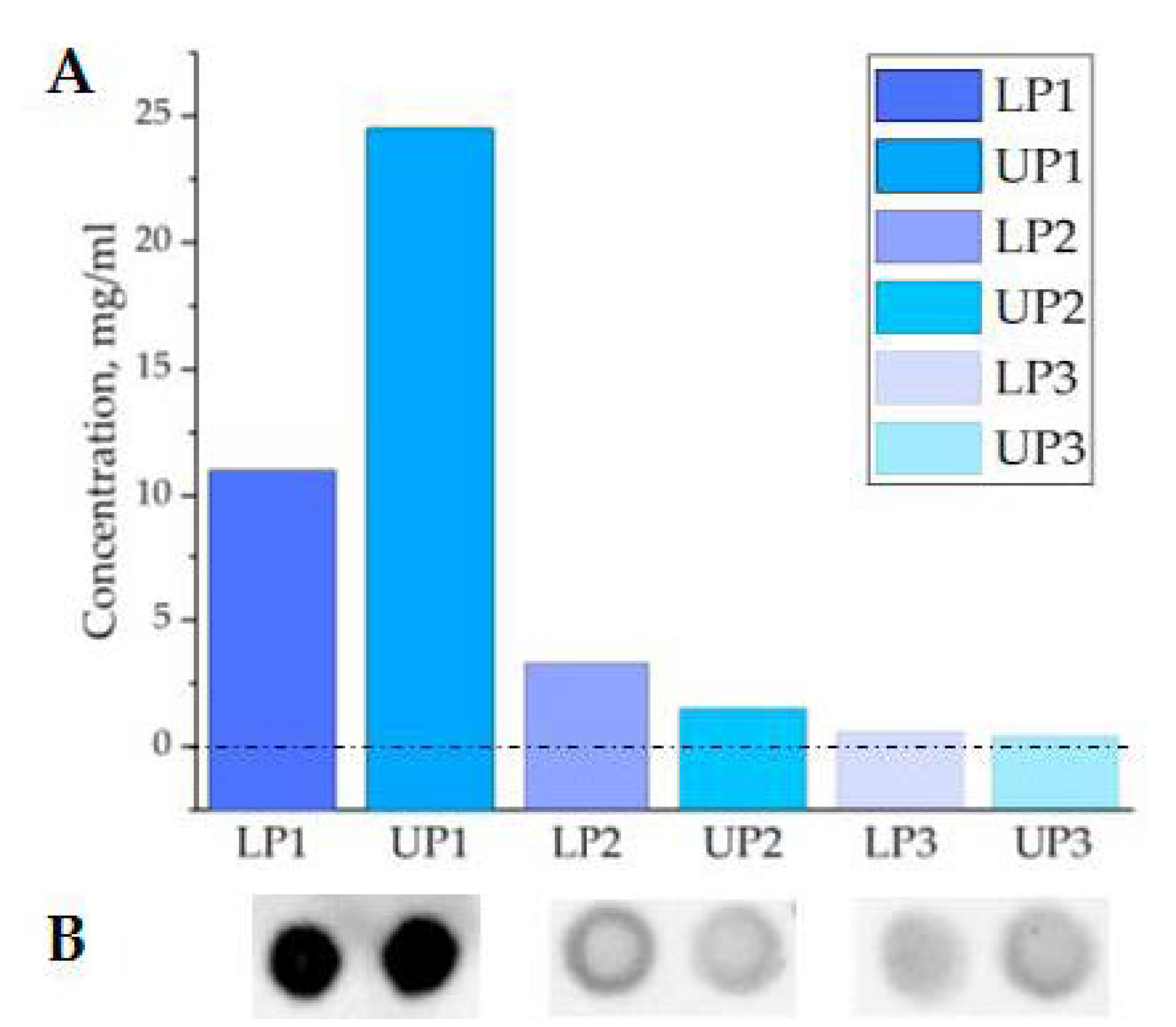
3.1. Optimization of the PTPS Parameters: Polymer Molecular Mass and Concentration
3.2. Plasma Two-Phase Polymer System Characteristics
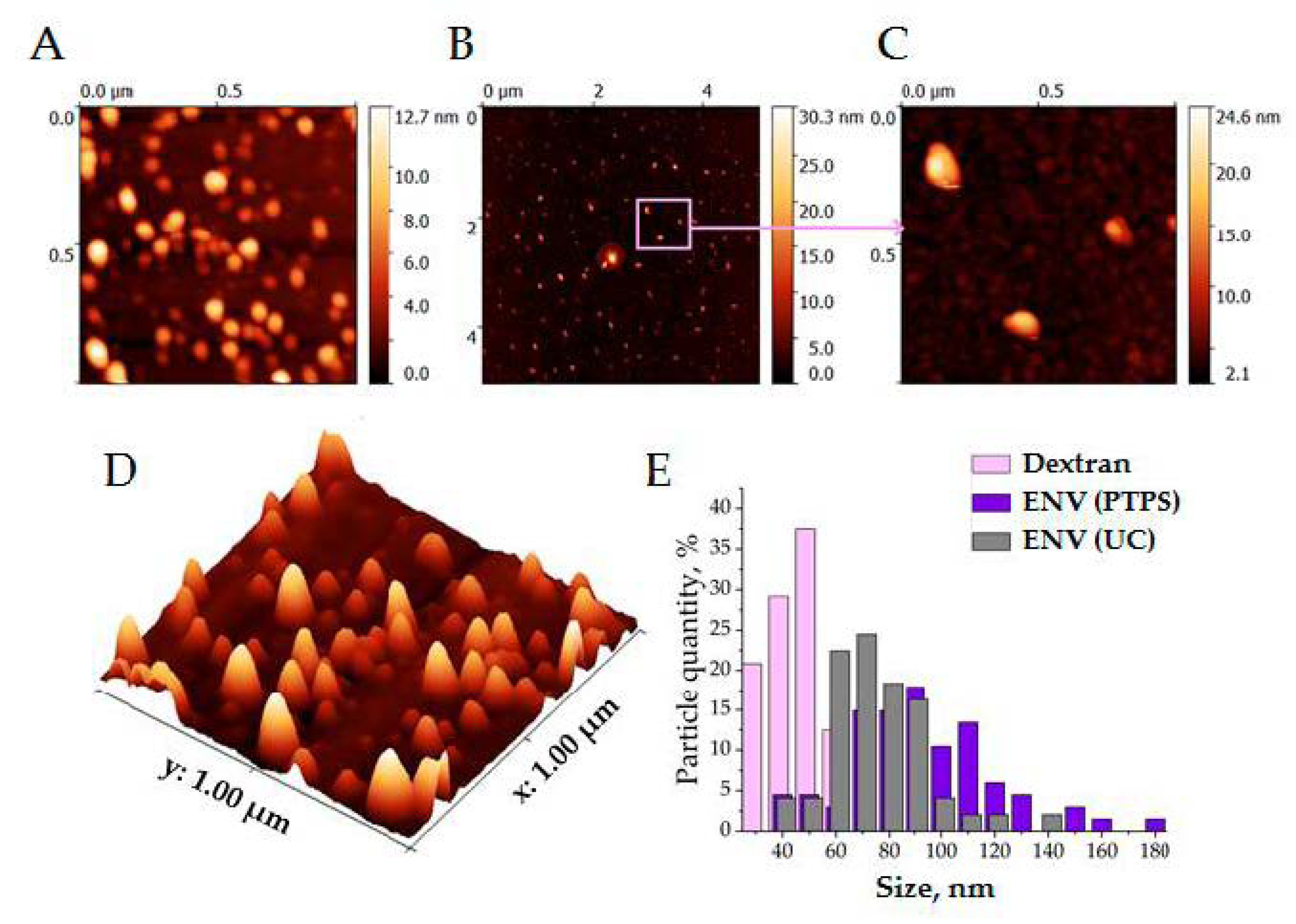
3.3. Characteristics of Isolated Vesicles
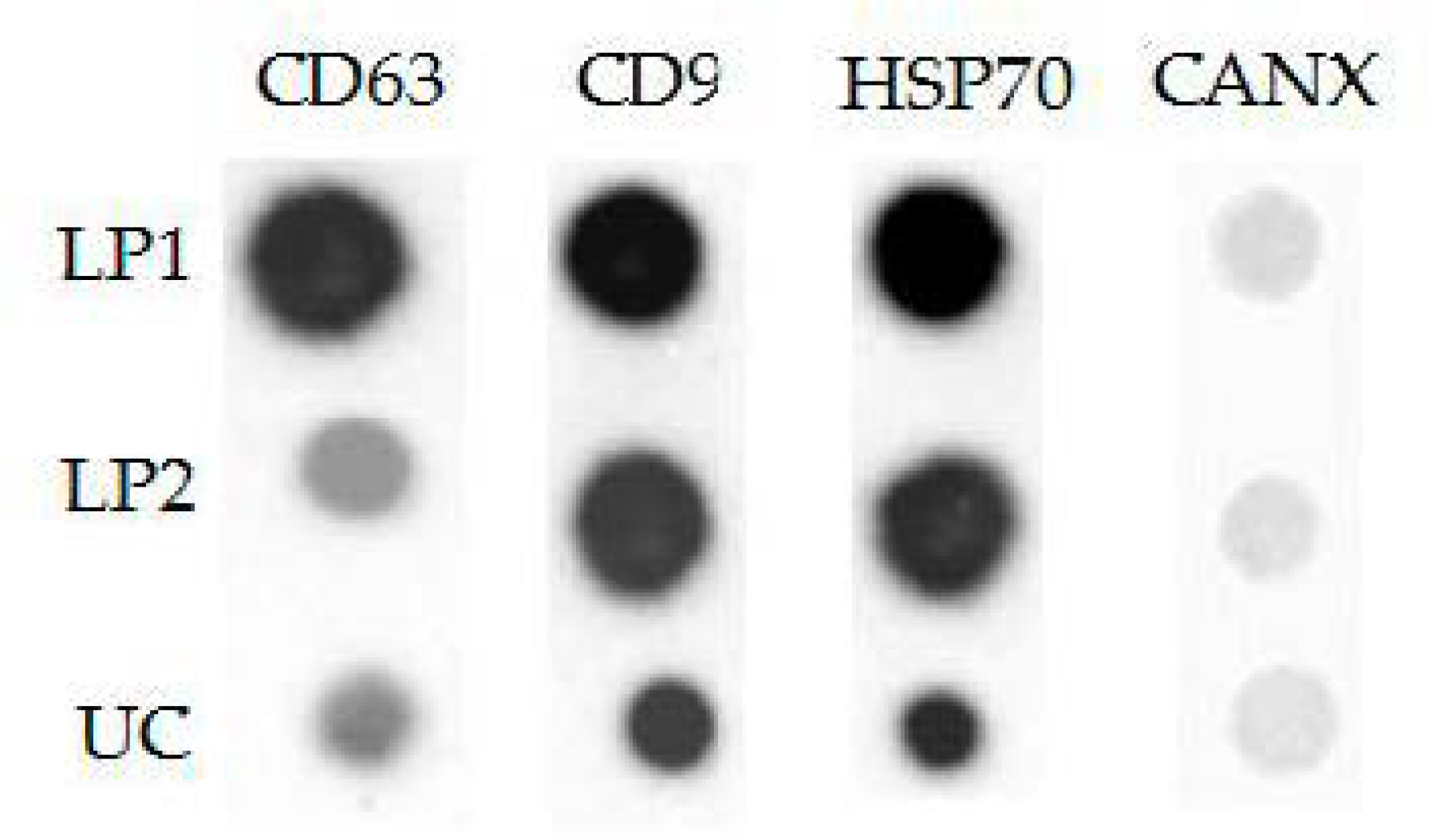
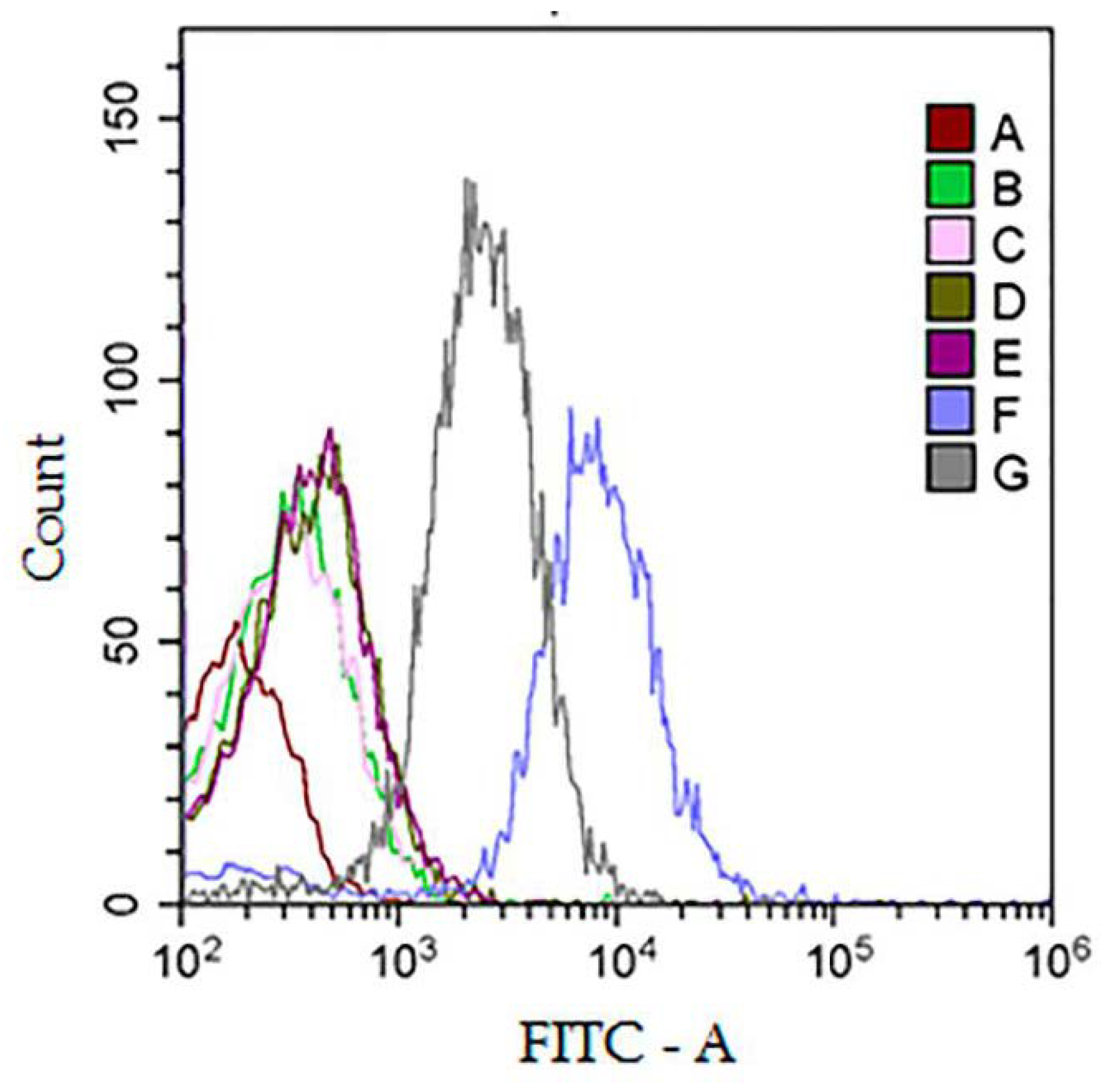
3.4. Analysis of Protein Surface Markers and miRNAs in Isolated Vesicles
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tkach, M.; Théry, C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell 2016, 164, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Tkach, M.; Kowal, J.; Théry, C. Why the need and how to approach the functional diversity of extracellular vesicles. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20160479. [Google Scholar] [CrossRef] [PubMed]
- Arraud, N.; Linares, R.; Tan, S.; Gounou, C.; Pasquet, J.-M.; Mornet, S.; Brisson, A.R. Extracellular vesicles from blood plasma: Determination of their morphology, size, phenotype and concentration. J. Thromb. Haemost. 2014, 12, 614–627. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Pertierra, E.; Oliveira-Rodríguez, M.; Rivas, M.; Oliva, P.; Villafani, J.; Navarro, A.; Blanco-López, M.C.; Cernuda-Morollón, E. Characterization of Plasma-Derived Extracellular Vesicles Isolated by Different Methods: A Comparison Study. Bioengineering 2019, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Gong, M.; Hu, Y.; Liu, H.; Zhang, W.; Zhang, M.; Hu, X.; Aubert, D.; Zhu, S.; Wu, L.; et al. Quality and efficiency assessment of six extracellular vesicle isolation methods by nano-flow cytometry. J. Extracell. Vesicles 2020, 9, 1697028. [Google Scholar] [CrossRef]
- Kang, H.; Kim, J.; Park, J. Methods to isolate extracellular vesicles for diagnosis. Micro Nano Syst. Lett. 2017, 5, 15. [Google Scholar] [CrossRef]
- Konoshenko, M.Y.; Lekchnov, E.A.; Vlassov, A.V.; Laktionov, P.P. Isolation of Extracellular Vesicles: General Methodologies and Latest Trends. BioMed Res. Int. 2018, 2018, 1–27. [Google Scholar] [CrossRef]
- Théry, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and Characterization of Exosomes from Cell Culture Supernatants and Biological Fluids. Curr. Protoc. Cell Biol. 2006, 30, 3.22.1–3.22.29. [Google Scholar] [CrossRef]
- Busatto, S.; Vilanilam, G.K.; Ticer, T.; Lin, W.-L.; Dickson, D.; Shapiro, S.A.; Bergese, P.; Wolfram, J. Tangential Flow Filtration for Highly Efficient Concentration of Extracellular Vesicles from Large Volumes of Fluid. Cells 2018, 7, 273. [Google Scholar] [CrossRef]
- Deregibus, M.C.; Figliolini, F.; D’Antico, S.; Manzini, P.M.; Pasquino, C.; De Lena, M.; Tetta, C.; Brizzi, M.F.; Camussi, G. Charge-based precipitation of extracellular vesicles. Int. J. Mol. Med. 2016, 38, 1359–1366. [Google Scholar] [CrossRef]
- Zhang, K.; Yue, Y.; Wu, S.; Liu, W.; Shi, J.; Zhang, Z. Rapid Capture and Nondestructive Release of Extracellular Vesicles Using Aptamer-Based Magnetic Isolation. ACS Sensors 2019, 4, 1245–1251. [Google Scholar] [CrossRef] [PubMed]
- Zabegina, L.; Nazarova, I.; Knyazeva, M.; Nikiforova, N.; Slyusarenko, M.; Titov, S.E.; Vasilyev, D.; Sleptsov, I.V.; Malek, A.V. MiRNA let-7 from TPO(+) Extracellular Vesicles is a Potential Marker for a Differential Diagnosis of Follicular Thyroid Nodules. Cells 2020, 9, 1917. [Google Scholar] [CrossRef] [PubMed]
- Albertsson, P. Fractionation of particles and macromolecules in aqueous two-phase systems. Biochem. Pharmacol. 1961, 5, 351–358. [Google Scholar] [CrossRef]
- Zaslavsky, B.Y. Bioanalytical Applications of Partitioning in Aqueous Polymer Two-Phase Systems. Anal. Chem. 1992, 64, 765A–773A. [Google Scholar] [CrossRef]
- Hatti-Kaul, R. Aqueous Two-Phase Systems: A General Overview. Mol. Biotechnol. 2001, 19, 269–278. [Google Scholar] [CrossRef]
- Shin, H.; Han, C.; Labuz, J.M.; Kim, J.; Kim, J.; Cho, S.; Gho, Y.S.; Takayama, S.; Park, J. High-yield isolation of extracellular vesicles using aqueous two-phase system. Sci. Rep. 2015, 5, srep13103. [Google Scholar] [CrossRef]
- Shin, H.; Park, Y.H.; Kim, Y.-G.; Lee, J.Y.; Park, J. Aqueous two-phase system to isolate extracellular vesicles from urine for prostate cancer diagnosis. PLoS ONE 2018, 13, e0194818. [Google Scholar] [CrossRef]
- Supplement, C. The Fifth International Meeting of ISEV, ISEV2016, Rotterdam, The Netherlands, 4–7 May, 2016. J. Extracell. Vesicles 2016, 5, 31552. [Google Scholar] [CrossRef]
- Kırbaş, O.K.; Bozkurt, B.T.; Asutay, A.B.; Mat, B.; Ozdemir, B.; Öztürkoğlu, D.; Ölmez, H.; Işlek, Z.; Şahin, F.; Taşli, P.N. Optimized Isolation of Extracellular Vesicles From Various Organic Sources Using Aqueous Two-Phase System. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Shin, H.; Jeong, H.; Park, J.; Hong, S.; Choi, Y. Correlation between Cancerous Exosomes and Protein Markers Based on Surface-Enhanced Raman Spectroscopy (SERS) and Principal Component Analysis (PCA). ACS Sens. 2018, 3, 2637–2643. [Google Scholar] [CrossRef]
- Kennedy, H.L. Improving the frequency response of Savitzky-Golay filters via colored-noise models. Digit. Signal Process. 2020, 102, 102743. [Google Scholar] [CrossRef]
- Albertsson, P.-Å. Particle fractionation in liquid two-phase systems The composition of some phase systems and the behaviour of some model particles in them application to the isolation of cell walls from microorganisms. Biochim. Biophys. Acta (BBA) Bioenerg. 1958, 27, 378–395. [Google Scholar] [CrossRef]
- Diamond, A.D.; Hsu, J.T. Phase diagrams for dextran-PEG aqueous two-phase systems at 22 °C. Biotechnol. Tech. 1989, 3, 119–124. [Google Scholar] [CrossRef]
- Hernández, B.; Pflüger, F.; Kruglik, S.G.; Ghomi, M. Characteristic Raman Lines of Phenylalanine Analyzed by a Multiconformational Approach. J. Raman Spectrosc. 2013, 44, 827–833. [Google Scholar] [CrossRef]
- Lee, C.; Carney, R.P.; Hazari, S.; Smith, Z.J.; Knudson, A.; Robertson, C.S.; Lam, K.S.; Wachsmann-Hogiu, S. 3D Plasmonic Nanobowl Platform for the Study of Exosomes in Solution. Nanoscale 2015, 7, 9290–9297. [Google Scholar] [CrossRef]
- Stone, N.; Kendall, C.; Smith, J.; Crow, P.; Barr, H. Raman Spectroscopy for Identification of Epithelial Cancers. Faraday Discuss. 2004, 126, 141. [Google Scholar] [CrossRef] [PubMed]
- Notingher, I.; Verrier, S.; Haque, S.; Polak, J.M.; Hench, L.L. Spectroscopic Study of Human Lung Epithelial Cells (A549) in Culture: Living Cells versus Dead Cells. Biopolymers 2003, 72, 230–240. [Google Scholar] [CrossRef]
- Takeuchi, H. Raman Structural Markers of Tryptophan and Histidine Side Chains in Proteins. Biopolymers 2003, 72, 305–317. [Google Scholar] [CrossRef]
- Harada, I.; Miura, T.; Takeuchi, H. Origin of the Doublet at 1360 and 1340 Cm-1 in the Raman Spectra of Tryptophan and Related Compounds. Spectrochim. Acta Part A Mol. Spectrosc. 1986, 42, 307–312. [Google Scholar] [CrossRef]
- Enciso-Martinez, A.; Van Der Pol, E.; Hau, C.M.; Nieuwland, R.; Van Leeuwen, T.G.; Terstappen, L.W.M.M.; Otto, C. Label-free Identification and Chemical Characterisation of Single Extracellular Vesicles and Lipoproteins by Synchronous Rayleigh and Raman Scattering. J. Extracell. Vesicles 2020, 9, 1730134. [Google Scholar] [CrossRef]
- Stremersch, S.; Marro, M.; Pinchasik, B.-E.; Baatsen, P.; Hendrix, A.; De Smedt, S.C.; Loza-Alvarez, P.; Skirtach, A.G.; Raemdonck, K.; Braeckmans, K. Identification of Individual Exosome-Like Vesicles by Surface Enhanced Raman Spectroscopy. Small 2016, 12, 3292–3301. [Google Scholar] [CrossRef]
- Mohammadi, M.R.; Malkovskiy, A.V.; Jothimuthu, P.; Kim, K.-M.; Parekh, M.; Inayathullah, M.; Zhuge, Y.; Rajadas, J. PEG/Dextran Double Layer Influences Fe Ion Release and Colloidal Stability of Iron Oxide Nanoparticles. Sci. Rep. 2018, 8, 4286. [Google Scholar] [CrossRef] [PubMed]
- Koenig, L.; Angood, A.C. Raman spectra of poly(ethylene glycols) in solution. J. Polym. Sci. Part B 2010, 8, 1787–1796. [Google Scholar] [CrossRef]
- Zhbankov, R.; Andrianov, V.; Marchewka, M. Fourier Transform IR and Raman Spectroscopy and Structure of Carbohydrates. J. Mol. Struct. 1997, 436, 637–654. [Google Scholar] [CrossRef]
- Siew, D.C.W.; Cooney, R.P.; Taylor, M.J.; Wiggins, P.M. Vibrational Spectroscopic Studies of Aqueous Dextran Sulphate. J. Raman Spectrosc. 1994, 25, 727–733. [Google Scholar] [CrossRef]
- Onozato, M.; Tanaka, Y.; Arita, M.; Sakamoto, T.; Ichiba, H.; Sadamoto, K.; Kondo, M.; Fukushima, T. Amino acid analyses of the exosome-eluted fractions from human serum by HPLC with fluorescence detection. Pr. Lab. Med. 2018, 12, e00099. [Google Scholar] [CrossRef] [PubMed]
- Van Berlo, M.; Luyben, K.C.; Van Der Wielen, L.A. Poly(ethylene glycol)–salt aqueous two-phase systems with easily recyclable volatile salts. J. Chromatogr. B Biomed. Sci. Appl. 1998, 711, 61–68. [Google Scholar] [CrossRef]
- Johansson, G. Studies on aqueous dextran-poly(ethylene glycol) two-phase systems containing charged poly(ethylene glycol) I. Partition of albumins. Biochim. Biophys. Acta (BBA) Gen. Subj. 1970, 222, 381–389. [Google Scholar] [CrossRef]
- Settanni, G.; Zhou, J.; Schmid, F. Interactions between proteins and poly(ethylene-glycol) investigated using molecular dynamics simulations. J. Phys. Conf. Ser. 2017, 921, 12002. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slyusarenko, M.; Nikiforova, N.; Sidina, E.; Nazarova, I.; Egorov, V.; Garmay, Y.; Merdalimova, A.; Yevlampieva, N.; Gorin, D.; Malek, A. Formation and Evaluation of a Two-Phase Polymer System in Human Plasma as a Method for Extracellular Nanovesicle Isolation. Polymers 2021, 13, 458. https://doi.org/10.3390/polym13030458
Slyusarenko M, Nikiforova N, Sidina E, Nazarova I, Egorov V, Garmay Y, Merdalimova A, Yevlampieva N, Gorin D, Malek A. Formation and Evaluation of a Two-Phase Polymer System in Human Plasma as a Method for Extracellular Nanovesicle Isolation. Polymers. 2021; 13(3):458. https://doi.org/10.3390/polym13030458
Chicago/Turabian StyleSlyusarenko, Maria, Nadezhda Nikiforova, Elena Sidina, Inga Nazarova, Vladimir Egorov, Yuri Garmay, Anastasiia Merdalimova, Natalia Yevlampieva, Dmitry Gorin, and Anastasia Malek. 2021. "Formation and Evaluation of a Two-Phase Polymer System in Human Plasma as a Method for Extracellular Nanovesicle Isolation" Polymers 13, no. 3: 458. https://doi.org/10.3390/polym13030458
APA StyleSlyusarenko, M., Nikiforova, N., Sidina, E., Nazarova, I., Egorov, V., Garmay, Y., Merdalimova, A., Yevlampieva, N., Gorin, D., & Malek, A. (2021). Formation and Evaluation of a Two-Phase Polymer System in Human Plasma as a Method for Extracellular Nanovesicle Isolation. Polymers, 13(3), 458. https://doi.org/10.3390/polym13030458










