Covalent Functionalization of Graphene Oxide with Fructose, Starch, and Micro-Cellulose by Sonochemistry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Organic Molecules
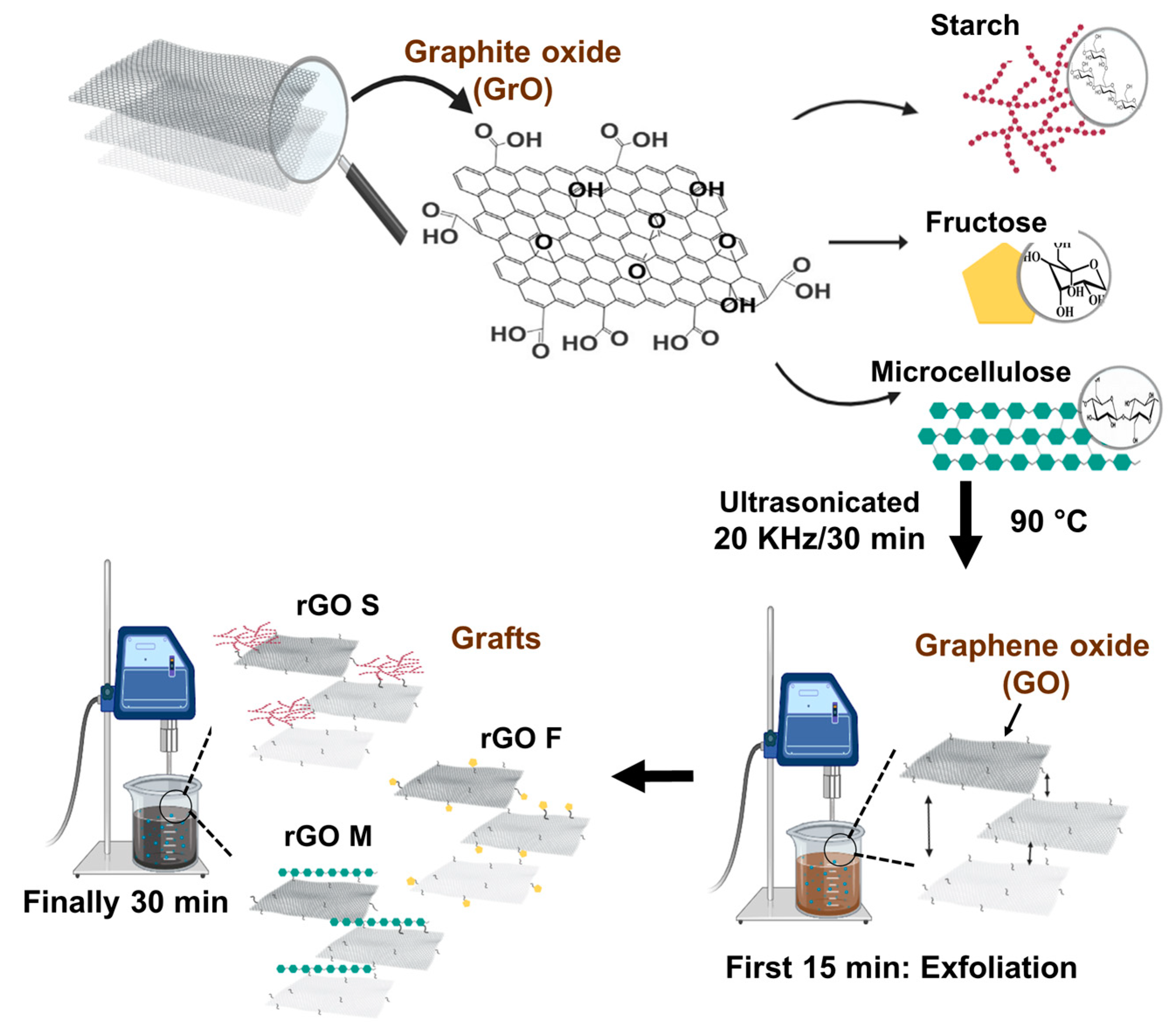
2.3. Graft Synthesis of GO Whit Starch, Fructose, and Micro-Cellulose
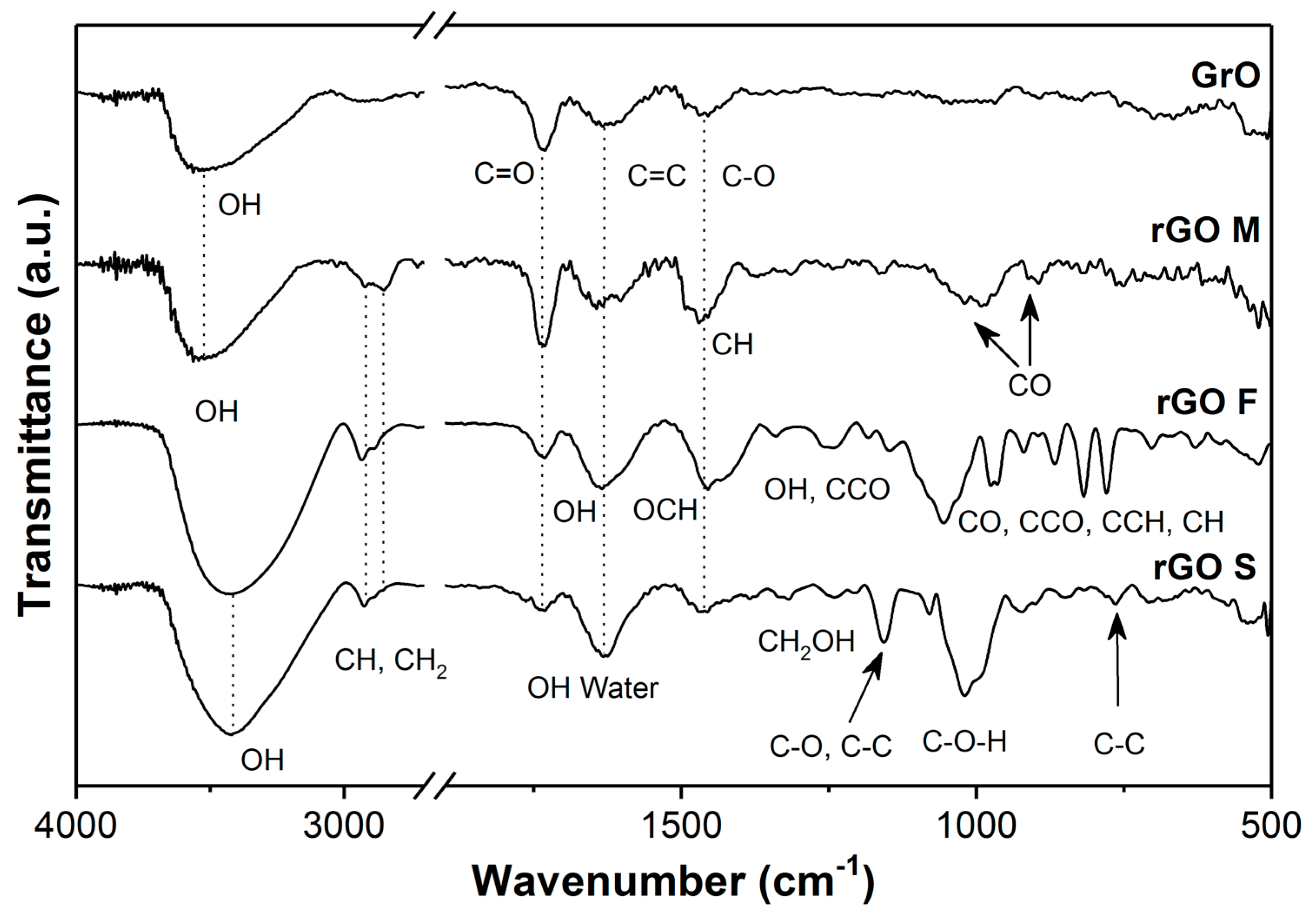
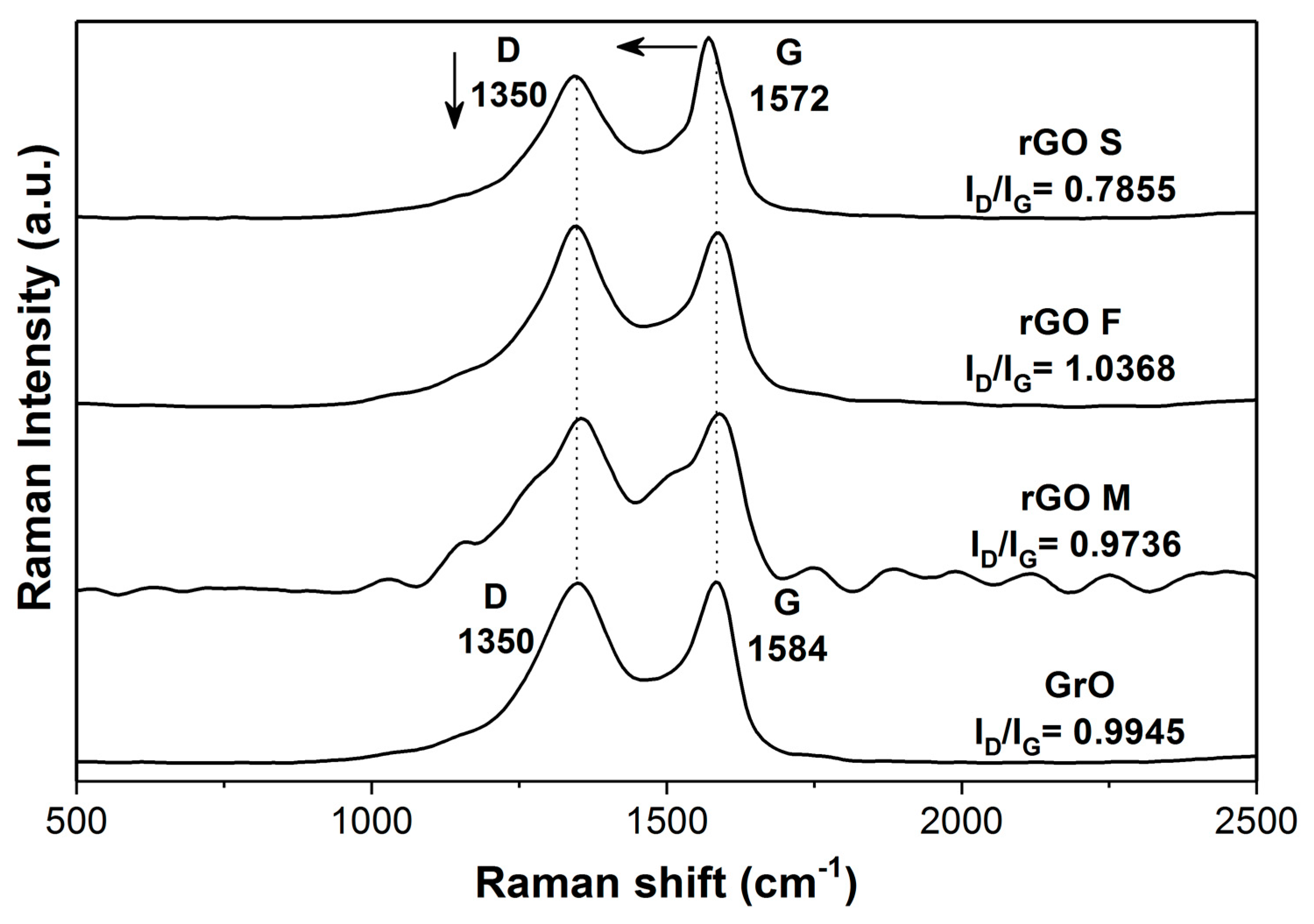
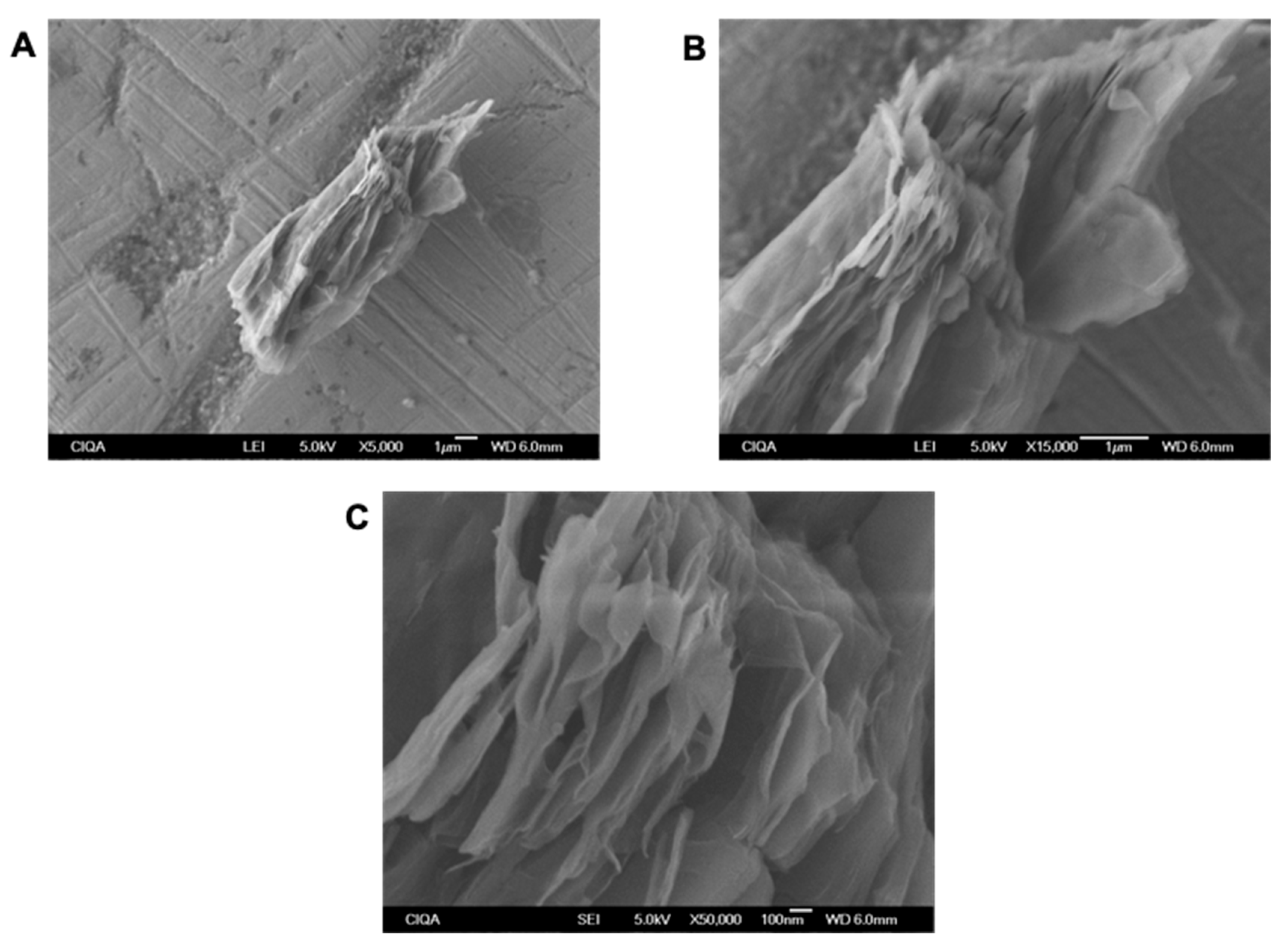
2.4. Characterization
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Castro Neto, A.H.; Guinea, F.; Peres, N.M.R.; Novoselov, K.S.; Geim, A.K. The electronic properties of graphene. Rev. Mod. Phys. 2009, 81, 109–162. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Yin, Z.; Wu, S.; Qi, X.; He, Q.; Zhang, Q.; Yan, Q.; Boey, F.; Zhang, H. Graphene-based materials: Synthesis, characterization, properties, and applications. Small 2011, 7, 1876–1902. [Google Scholar] [CrossRef]
- Sun, X.; Liu, Z.; Welsher, K.; Robinson, J.T.; Goodwin, A.; Zaric, S.; Dai, H. Nano-graphene oxide for cellular imaging and drug delivery. Nano Res. 2008, 1, 203–212. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Polavarapu, L.; Liz-Marzán, L.M. Reduced graphene oxide-supported gold nanostars for improved SERS sensing and drug delivery. ACS Appl. Mater. Interfaces 2014, 6, 21798–21805. [Google Scholar] [CrossRef] [PubMed]
- Valentini, L.; Cardinali, M.; Bittolo Bon, S.; Bagnis, D.; Verdejo, R.; Lopez-Manchado, M.A.; Kenny, J.M. Use of butylamine modified graphene sheets in polymer solar cells. J. Mater. Chem. 2010, 20, 995–1000. [Google Scholar] [CrossRef] [Green Version]
- Ai, W.; Luo, Z.; Jiang, J.; Zhu, J.; Du, Z.; Fan, Z.; Xie, L.; Zhang, H.; Huang, W.; Yu, T. Nitrogen and sulfur codoped graphene: Multifunctional electrode materials for high-performance LI-ion batteries and oxygen reduction reaction. Adv. Mater. 2014, 26, 6186–6192. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.K.; Singh, E.; Singh, P.; Meyyappan, M.; Nalwa, H.S. A review on graphene-based nanocomposites for electrochemical and fluorescent biosensors. RSC Adv. 2019, 9, 8778–8781. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, J.; Song, Q.; Li, N.; Zhang, Z.; Li, K.; Du, Y.; Wu, L.; Tang, M.; Liu, L.; et al. Synthesis of amphiphilic reduced graphene oxide with an enhanced charge injection capacity for electrical stimulation of neural cells. J. Mater. Chem. B 2014, 2, 4331–4337. [Google Scholar] [CrossRef]
- Georgakilas, V.; Otyepka, M.; Bourlinos, A.B.; Chandra, V.; Kim, N.; Kemp, K.C.; Hobza, P.; Zboril, R.; Kim, K.S. Functionalization of graphene: Covalent and non-covalent approach. Chem. Rev. 2012, 112, 6156–6214. [Google Scholar] [CrossRef]
- Kuila, T.; Bose, S.; Mishra, A.K.; Khanra, P.; Kim, N.H.; Lee, J.H. Chemical functionalization of graphene and its applications. Prog. Mater. Sci. 2012, 57, 1061–1105. [Google Scholar] [CrossRef]
- Sedaghat, E.; Rostami, A.A.; Ghaemy, M.; Rostami, A. Characterization, thermal degradation kinetics, and morphological properties of a graphene oxide/poly (vinyl alcohol)/starch nanocomposite. J. Therm. Anal. Calorim. 2019, 136, 759–769. [Google Scholar] [CrossRef]
- Wu, Z.; Huang, Y.; Xiao, L.; Lin, D.; Yang, Y.; Wang, H.; Yang, Y.; Wu, D.; Chen, H.; Zhang, Q.; et al. Physical properties and structural characterization of starch/polyvinyl alcohol/graphene oxide composite films. Int. J. Biol. Macromol. 2019, 123, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Golzari Aqda, T.; Behkami, S.; Raoofi, M.; Bagheri, H. Graphene oxide-starch-based micro-solid phase extraction of antibiotic residues from milk samples. J. Chromatogr. A 2019, 1591, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, F.; Swain, S.K. Nano silver embedded starch hybrid graphene oxide sandwiched poly(ethylmethacrylate) for packaging application. Nano-Struct. Nano-Objects 2019, 18, 100300. [Google Scholar] [CrossRef]
- Sharma, C.; Manepalli, P.H.; Thatte, A.; Thomas, S.; Kalarikkal, N.; Alavi, S. Biodegradable starch/PVOH/laponite RD-based bionanocomposite films coated with graphene oxide: Preparation and performance characterization for food packaging applications. Colloid Polym. Sci. 2017, 295, 1695–1708. [Google Scholar] [CrossRef]
- Amri, A.; Rahmana, H.; Utami, S.P.; Iriyanti, R.S.; Jiang, Z.T.; Rahman, M.M. Conductive composites of tapioca based bioplastic and electrochemical-mechanical liquid exfoliation (emle) graphene. IOP Conf. Ser. Mater. Sci. Eng. 2018, 345. [Google Scholar] [CrossRef]
- Aqlil, M.; Moussemba Nzenguet, A.; Essamlali, Y.; Snik, A.; Larzek, M.; Zahouily, M. Graphene Oxide Filled Lignin/Starch Polymer Bionanocomposite: Structural, Physical, and Mechanical Studies. J. Agric. Food Chem. 2017, 65, 10571–10581. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, W.H.; Dahmouche, K.; Andrade, C.T. Tuning the mechanical and electrical conductivity properties of graphene-based thermoplastic starch/poly(lactic acid) hybrids. Polym. Compos. 2019, 40, E1131–E1142. [Google Scholar] [CrossRef]
- Li, R.; Liu, C.; Ma, J. Studies on the properties of graphene oxide-reinforced starch biocomposites. Carbohydr. Polym. 2011, 84, 631–637. [Google Scholar] [CrossRef]
- Luo, D.; Wang, F.; Vu, B.V.; Chen, J.; Bao, J.; Cai, D.; Willson, R.C.; Ren, Z. Synthesis of graphene-based amphiphilic Janus nanosheets via manipulation of hydrogen bonding. Carbon 2018, 126, 105–110. [Google Scholar] [CrossRef]
- Usman, A.; Hussain, Z.; Riaz, A.; Khan, A.N. Enhanced mechanical, thermal and antimicrobial properties of poly(vinyl alcohol)/graphene oxide/starch/silver nanocomposites films. Carbohydr. Polym. 2016, 153, 592–599. [Google Scholar] [CrossRef]
- Chen, Y.; Dai, G.; Gao, Q. Starch Nanoparticles-Graphene Aerogels with High Supercapacitor Performance and Efficient Adsorption. ACS Sustain. Chem. Eng. 2019, 7, 14064–14073. [Google Scholar] [CrossRef]
- Htut, Z.; Kim, M.; Lee, E.; Lee, G.; Baeck, S.H.; Shim, S.E. Biodegradable polymer-modified graphene/polyaniline electrodes for supercapacitors. Synth. Met. 2017, 227, 61–70. [Google Scholar] [CrossRef]
- Wu, W.; Li, Y.; Yang, L.; Ma, Y.; Pan, D.; Li, Y. A facile one-pot preparation of dialdehyde starch reduced graphene oxide/polyaniline composite for supercapacitors. Electrochim. Acta 2014, 139, 117–126. [Google Scholar] [CrossRef]
- Zhang, X.; Fan, Q.; Qu, N.; Yang, H.; Wang, M.; Liu, A.; Yang, J. Ultrathin 2D nitrogen-doped carbon nanosheets for high performance supercapacitors: Insight into the effects of graphene oxides. Nanoscale 2019, 11, 8588–8596. [Google Scholar] [CrossRef] [PubMed]
- Franco, A.; Cano, M.; Giner-Casares, J.J.; Rodríguez-Castellón, E.; Luque, R.; Puente-Santiago, A.R. Boosting the electrochemical oxygen reduction activity of hemoglobin on fructose@graphene-oxide nanoplatforms. Chem. Commun. 2019, 55, 4671–4674. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, A.; Banerjee, B.; Ghorai, S.; Rana, D.; Roy, I.; Sarkar, G.; Saha, N.R.; De, S.; Ghosh, T.K.; Sadhukhan, S.; et al. Development of an auto-phase separable and reusable graphene oxide-potato starch based cross-linked bio-composite adsorbent for removal of methylene blue dye. Int. J. Biol. Macromol. 2018, 116, 1037–1048. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, H.; Ramin, S. Fast and enhanced removal of mercury from aqueous solutions by magnetic starch-g-poly(acryl amide)/graphene oxide nanocomposite superabsorbents. Polym. Sci. Ser. B 2016, 58, 457–473. [Google Scholar] [CrossRef]
- Lou, Z.; Cao, Z.; Xu, J.; Zhou, X.; Zhu, J.; Liu, X.; Ali Baig, S.; Zhou, J.; Xu, X. Enhanced removal of As(III)/(V) from water by simultaneously supported and stabilized Fe-Mn binary oxide nanohybrids. Chem. Eng. J. 2017, 322, 710–721. [Google Scholar] [CrossRef]
- Jodar, L.V.; Santos, F.A.; Zucolotto, V.; Janegitz, B.C. Electrochemical sensor for estriol hormone detection in biological and environmental samples. J. Solid State Electrochem. 2018, 22, 1431–1438. [Google Scholar] [CrossRef]
- Li, D.; Duan, H.; Ma, Y.; Deng, W. Headspace-Sampling Paper-Based Analytical Device for Colorimetric/Surface-Enhanced Raman Scattering Dual Sensing of Sulfur Dioxide in Wine. Anal. Chem. 2018, 90, 5719–5727. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, F.; Chen, X.; Wang, X.W.; Zhang, W.B.; Peng, J.; Li, J.; Zhai, M. Stretchable, Conductive, and Self-Healing Hydrogel with Super Metal Adhesion. Chem. Mater. 2018, 30, 4289–4297. [Google Scholar] [CrossRef]
- Wu, D.; Samanta, A.; Srivastava, R.K.; Hakkarainen, M. Starch-Derived Nanographene Oxide Paves the Way for Electrospinnable and Bioactive Starch Scaffolds for Bone Tissue Engineering. Biomacromolecules 2017, 18, 1582–1591. [Google Scholar] [CrossRef]
- Liu, K.; Wang, Y.; Li, H.; Duan, Y. A facile one-pot synthesis of starch functionalized graphene as nano-carrier for pH sensitive and starch-mediated drug delivery. Colloids Surf. B Biointerfaces 2015, 128, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Mohan, V.B.; Jayaraman, K.; Bhattacharyya, D. Fabrication of highly conductive graphene particle-coated fiber yarns using polymeric binders through efficient coating techniques. Adv. Polym. Technol. 2018, 37, 3438–3447. [Google Scholar] [CrossRef]
- Yarahmadi, E.; Didehban, K.; Shabanian, M.; Saeb, M.R. High-performance starch-modified graphene oxide/epoxy nanocomposite coatings: A glimpse at cure kinetics and fracture behavior. Prog. Color Color. Coat. 2018, 11, 55–62. [Google Scholar]
- Zheng, P.; Ma, T.; Ma, X. Fabrication and properties of starch-grafted graphene nanosheet/plasticized-starch composites. Ind. Eng. Chem. Res. 2013, 52, 14201–14207. [Google Scholar] [CrossRef]
- Sandhya, P.K.; Sreekala, M.S.; Padmanabhan, M.; Jesitha, K.; Thomas, S. Effect of starch reduced graphene oxide on thermal and mechanical properties of phenol formaldehyde resin nanocomposites. Compos. Part B Eng. 2019, 167, 83–92. [Google Scholar] [CrossRef]
- Soleimani, K.; Dadkhah Tehrani, A.; Adeli, M.; Sattari, S. Convenient method for preparation of a new absorbent based on biofunctionalized graphene oxide hydrogels using nitrene chemistry and click reaction. Iran. Polym. J. 2018, 27, 689–699. [Google Scholar] [CrossRef]
- Srivastava, J.; Kushwaha, A.; Srivastava, M.; Srivastava, A.; Singh, M. Glycoprotein imprinted RGO-starch nanocomposite modified EQCM sensor for sensitive and specific detection of transferrin. J. Electroanal. Chem. 2019, 835, 169–177. [Google Scholar] [CrossRef]
- Srivastava, J.; Kushwaha, A.; Singh, M. Imprinted Graphene-Starch Nanocomposite Matrix-Anchored EQCM Platform for Highly Selective Sensing of Epinephrine. Nano 2018, 13, 1–19. [Google Scholar] [CrossRef]
- Teng, J.; Zeng, X.; Xu, X.; Yu, J.G. Assembly of a novel porous 3D graphene oxide-starch architecture by a facile hydrothermal method and its adsorption properties toward metal ions. Mater. Lett. 2018, 214, 31–33. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X.; Wu, X.; Yu, J.G.; Jiang, X.Y.; Wu, Z.L.; Hao, X. Soluble starch functionalized graphene oxide as an efficient adsorbent for aqueous removal of Cd(II): The adsorption thermodynamic, kinetics and isotherms. J. Sol. Gel. Sci. Technol. 2017, 82, 440–449. [Google Scholar] [CrossRef]
- Wu, D.; Bäckström, E.; Hakkarainen, M. Starch Derived Nanosized Graphene Oxide Functionalized Bioactive Porous Starch Scaffolds. Macromol. Biosci. 2017, 17, 1–11. [Google Scholar] [CrossRef]
- Yarahmadi, E.; Didehban, K.; Sari, M.G.; Saeb, M.R.; Shabanian, M.; Aryanasab, F.; Zarrintaj, P.; Paran, S.M.R.; Mozafari, M.; Rallini, M.; et al. Development and curing potential of epoxy/starch-functionalized graphene oxide nanocomposite coatings. Prog. Org. Coat. 2018, 119, 194–202. [Google Scholar] [CrossRef]
- Hernández-Hernández, E.; Hernández-Belmares, P.J.; Ceniceros-Reyes, M.A.; Rodríguez-Fernández, O.S.; González-Morones, P. Graphite Oxide: A Simple and Reproducible Synthesis Route. In Graphene Production and Application; IntechOpen: London, UK, 2016; p. 13. [Google Scholar] [CrossRef] [Green Version]
- Krishnamoorthy, K.; Veerapandian, M.; Yun, K.; Kim, S.J. The chemical and structural analysis of graphene oxide with different degrees of oxidation. Carbon 2013, 53, 38–49. [Google Scholar] [CrossRef]
- Ma, T.; Chang, P.R.; Zheng, P.; Ma, X. The composites based on plasticized starch and graphene oxide/reduced graphene oxide. Carbohydr. Polym. 2013, 94, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.M.; Tong, D.S.; Zhao, L.Z.; Yu, W.H.; Zhou, C.H.; Wang, H. Fourier transform infrared spectroscopy analysis for hydrothermal transformation of microcrystalline cellulose on montmorillonite. Appl. Clay Sci. 2014, 95, 74–82. [Google Scholar] [CrossRef]
- Ibrahim, M.; Alaam, M.; El-Haes, H.; Jalbout, A.F.; De Leon, A. Analysis of the structure and vibrational spectra of glucose and fructose. Eclet. Quim. 2006, 31, 15–21. [Google Scholar] [CrossRef]
- Kizil, R.; Irudayaraj, J.; Seetharaman, K. Characterization of irradiated starches by using FT-Raman and FTIR spectroscopy. J. Agric. Food Chem. 2002, 50, 3912–3918. [Google Scholar] [CrossRef]
- Brenner, T.; Kiessler, B.; Radosta, S.; Arndt, T. Processing surface sizing starch using oxidation, enzymatic hydrolysis and ultrasonic treatment methods—Preparation and application. Carbohydr. Polym. 2016, 138, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Qi, H.; Wang, Z.; Bi, Y.; Zou, J.; Xu, B.; Ren, X.; Ma, H. The ex-situ and in-situ ultrasonic assisted oxidation of corn starch: A comparative study. Ultrason. Sonochem. 2020, 61, 104854. [Google Scholar] [CrossRef] [PubMed]
- Ambre, J.P.; Dhopte, K.B.; Nemade, P.R.; Dalvi, V.H. High flux hyperbranched starch-graphene oxide piperazinamide composite nanofiltration membrane. J. Environ. Chem. Eng. 2019, 7. [Google Scholar] [CrossRef]
- Acik, M.; Lee, G.; Mattevi, C.; Pirkle, A.; Wallace, R.M.; Chhowalla, M.; Cho, K.; Chabal, Y. The role of oxygen during thermal reduction of graphene oxide studied by infrared absorption spectroscopy. J. Phys. Chem. C 2011, 115, 19761–19781. [Google Scholar] [CrossRef]
- O’Connell, C. The effects of methylparaben on the gelatinization and thermal decomposition of corn starch. Thermochim. Acta 1999, 340–341, 183–194. [Google Scholar] [CrossRef]
- Abdolmaleki, A.; Mallakpour, S.; Rostami, M. Performance evaluation of fructose-functionalized multiwalled carbon nanotubes/biodegradable poly(amide-imide) based on N, N′ -(pyromellitoyl)-bis- S -valine bionanocomposite. High Perform. Polym. 2015, 27, 903–910. [Google Scholar] [CrossRef]
- Mallakpour, S.; Abdolmaleki, A.; Rostami, M. Morphology and thermal properties of environmental friendly nanocomposites using biodegradable poly(amide–imide) based on N-trimellitylimido-S-valine matrix reinforced by fructose-functionalized multi-walled carbon nanotubes. Colloid Polym. Sci. 2014, 293, 545–553. [Google Scholar] [CrossRef]
- Mallakpour, S.; Behranvand, V. Sonochemical production and characterization of d-fructose functionalized MWCNTs/alanine-based poly(amide-imide) nanocomposites. Colloid Polym. Sci. 2015, 293, 1817–1826. [Google Scholar] [CrossRef]
- Mallakpour, S.; Madani, M. Synthesis, structural characterization, and tensile properties of fructose functionalized multi-walled carbon nanotubes/chitosan nanocomposite films. J. Plast. Film Sheeting 2016, 32, 56–73. [Google Scholar] [CrossRef]
- Mallakpour, S.; Behranvand, V. Improved solubilization of multiwalled carbon nanotubes (MWCNTs) in water by surface functionalization with d -glucose and d fructose: Properties comparison of functionalized MWCNTs/alanine-based poly(amide-imide) nanocomposites. High Perform. Polym. 2016, 28, 936–944. [Google Scholar] [CrossRef]
- Khenblouche, A.; Bechki, D.; Gouamid, M.; Charradi, K.; Segni, L.; Hadjadj, M.; Boughali, S. Extraction and characterization of cellulose microfibers from Retama raetam stems. Polimeros 2019, 29, 1–8. [Google Scholar] [CrossRef]
- Guinesi, L.S.; da Róz, A.L.; Corradini, E.; Mattoso, L.H.C.; de Teixeira, E.M.; Curvelo, A.A.D.S. Kinetics of thermal degradation applied to starches from different botanical origins by non-isothermal procedures. Thermochim. Acta 2006, 447, 190–196. [Google Scholar] [CrossRef]
- Manek, R.V.; Builders, P.F.; Kolling, W.M.; Emeje, M.; Kunle, O.O. Physicochemical and binder properties of starch obtained from Cyperus esculentus. AAPS PharmSciTech 2012, 13, 379–388. [Google Scholar] [CrossRef]
- Rotaru, P.; Scorei, R.; Hrbor, A.; Dumitru, M.D. Thermal analysis of a calcium fructoborate sample. Thermochim. Acta 2010, 506, 8–13. [Google Scholar] [CrossRef]
- Gong, J.; Li, J.; Xu, J.; Xiang, Z.; Mo, L. Research on cellulose nanocrystals produced from cellulose sources with various polymorphs. RSC Adv. 2017, 7, 33486–33493. [Google Scholar] [CrossRef] [Green Version]
- Abulizi, A.; Okitsu, K.; Zhu, J.J. Ultrasound assisted reduction of graphene oxide to graphene in l-ascorbic acid aqueous solutions: Kinetics and effects of various factors on the rate of graphene formation. Ultrason. Sonochem. 2014, 21, 1174–1181. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Kaur, P.; Juglan, K.C. Synthesis and Ultrasonic Investigation of Reduced Graphene Oxide Nanosuspension with Water. J. Phys. Conf. Ser. 2020, 1531. [Google Scholar] [CrossRef]
- Narayanan, K.B.; Park, G.T.; Han, S.S. Antibacterial properties of starch-reduced graphene oxide–polyiodide nanocomposite. Food Chem. 2020, 128385. [Google Scholar] [CrossRef]




| Functional Group | Position (eV)/Concentration (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| GrO | rGO S | rGO F | rGO M | |||||
| eV | % | eV | % | eV | % | eV | % | |
| C–C and C=C | 284.7 | 46.8 | 284.5 | 14.3 | 284.8 | 16.0 | 284.7 | 4.5 |
| C–O | 286.3 | 10.2 | - | - | - | - | ||
| C–O–C | 286.9 | 33.5 | 286.3 | 24.7 | 286.5 | 26.4 | 286.1 | 20.7 |
| C=O | 288.2 | 7.4 | 287.9 | 38.7 | 287.9 | 38.6 | 287.4 | 44.5 |
| O=C–OH | 289.4 | 2.1 | 288.8 | 16.9 | 289.3 | 10.4 | 288.6 | 22.4 |
| O=C–O | - | 290.1 | 5.3 | 290.7 | 8.5 | 289.8 | 7.8 | |
| Relation % (C–C/C=O) | 6.3 | 0.4 | 0.4 | 0.1 | ||||
| Weight Loss | rGO S | |||
| Temperature (°C) | Assignation | Weight% | Maximum Peak (°C) | |
| 1 | 40 a 150 | Water | 9.72 | ---- |
| 2 | 150–242 | RGrO (OH, C–O–C) | 9.23 | 218.1 |
| 3 | 242–450 | SD | 47.54 | 301.8 |
| Hybridization% | 47.54 | |||
| % rGO | 33.51 | |||
| Weight Loss | rGO F | |||
| Temperature (°C) | Assignation | Weight% | Maximum Peak (°C) | |
| 1 | 40–145 | Water | 11.34 | 129.1 |
| 2 | 145–283 | Fructose degradation | 38.2 | 204.2 |
| 3 | 283–422 | 2.88 | 339.1 | |
| Hybridization% | 41.08 | |||
| % rGO | 47.58 | |||
| Weight Loss | rGO M | |||
| Temperature (°C) | Assignation | Weight% | Maximum Peak (°C) | |
| 1 | 120–267 | TDH | 13.36 | 230.1, 255.5 |
| 2 | 267–600 | DC + DL | 50.63 | 324.4, 446.8 |
| Hybridization% | 63.99 | |||
| % rGO | 36.01 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz-Benítez, M.M.; Gónzalez-Morones, P.; Hernández-Hernández, E.; Villagómez-Ibarra, J.R.; Castro-Rosas, J.; Rangel-Vargas, E.; Fonseca-Florido, H.A.; Gómez-Aldapa, C.A. Covalent Functionalization of Graphene Oxide with Fructose, Starch, and Micro-Cellulose by Sonochemistry. Polymers 2021, 13, 490. https://doi.org/10.3390/polym13040490
Cruz-Benítez MM, Gónzalez-Morones P, Hernández-Hernández E, Villagómez-Ibarra JR, Castro-Rosas J, Rangel-Vargas E, Fonseca-Florido HA, Gómez-Aldapa CA. Covalent Functionalization of Graphene Oxide with Fructose, Starch, and Micro-Cellulose by Sonochemistry. Polymers. 2021; 13(4):490. https://doi.org/10.3390/polym13040490
Chicago/Turabian StyleCruz-Benítez, María Montserrat, Pablo Gónzalez-Morones, Ernesto Hernández-Hernández, José Roberto Villagómez-Ibarra, Javier Castro-Rosas, Esmeralda Rangel-Vargas, Heidi Andrea Fonseca-Florido, and Carlos Alberto Gómez-Aldapa. 2021. "Covalent Functionalization of Graphene Oxide with Fructose, Starch, and Micro-Cellulose by Sonochemistry" Polymers 13, no. 4: 490. https://doi.org/10.3390/polym13040490






