Green Procedure for Aerobic Oxidation of Benzylic Alcohols with Palladium Supported on Iota-Carrageenan in Ethanol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Polysaccharides and Reagents
2.2. Catalyst Preparation
2.3. Reaction Procedure
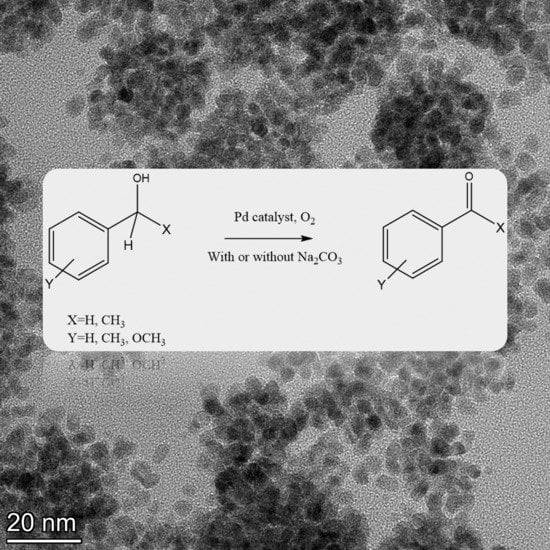
2.4. TEM Analysis
2.5. Surface Analysis by X-ray Photoelectron Spectroscopy (XPS)
2.6. Scanning Electron Microscope (SEM) Energy Dispersive X-ray Spectrometry (EDS)
2.7. Analysis of Total Sugar Concentration
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bäckvall, J.E. Modern Oxidation Methods; John Wiley & Sons: New York, NY, USA, 2011; p. 481. [Google Scholar]
- Kopylovich, M.N.; Ribeiro, A.P.; Alegria, E.C.; Martins, N.M.; Martins, L.M.; Pombeiro, A.J. Catalytic oxidation of alcohols: Recent advances. In Advances in Organometallic Chemistry; Perez, P., Ed.; Academic Press: Cambridge, MA, USA, 2015; pp. 91–174. [Google Scholar]
- Iwabuchi, Y. Green Oxidation in Organic Synthesis; John Wiley & Sons: New York, NY, USA, 2019; pp. 35–78. [Google Scholar]
- Tojo, G.; Fernandez, M.I. Oxidation of Alcohols to Aldehydes and Ketones: A Guide to Current Common Practice; Springer Science & Business Media: New York, NY, USA, 2006; p. 376. [Google Scholar]
- Waters, W.A. Mechanisms of oxidation by compounds of chromium and manganese. Q. Rev. Chem. Soc. 1958, 12, 277–300. [Google Scholar] [CrossRef]
- Sheldon, R.A. Recent advances in green catalytic oxidations of alcohols in aqueous media. Catal. Today 2015, 247, 4–13. [Google Scholar] [CrossRef]
- Yu, Y.; Lu, B.; Wang, X.; Zhao, J.; Wang, X.; Cai, Q. Highly selective oxidation of benzyl alcohol to benzaldehyde with hydrogen peroxide by biphasic catalysis. Chem. Eng. J. 2010, 162, 738–742. [Google Scholar] [CrossRef]
- Ueno, M.; Ohmura, S.D.; Wada, M.; Miyoshi, N. Aerobic oxidation of alcohols using bismuth bromide as a catalyst. Tetrahedron Lett. 2019, 60, 570–573. [Google Scholar] [CrossRef]
- Landaeta, V.R.; Rodríguez-Lugo, R.E. Catalytic Aerobic Oxidations: Aerobic Oxidation Reactions in the Fine Chemicals and Pharmaceutical Industries. In Catalytic Aerobic Oxidations; Mejía, E., Ed.; Royal Society of Chemistry: Cambridge, UK, 2020; pp. 252–290. [Google Scholar]
- Silva, T.F.; Martins, L.M. Recent Advances in Copper Catalyzed Alcohol Oxidation in Homogeneous Medium. Molecules 2020, 25, 748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guðmundsson, A.; Schlipköter, K.E.; Bäckvall, J.E. Iron (II)-Catalyzed Biomimetic Aerobic Oxidation of Alcohols. Angew. Chem. Int. 2020, 132, 5441–5444. [Google Scholar] [CrossRef] [Green Version]
- Parmeggiani, C.; Cardona, F. Transition metal based catalysts in the aerobic oxidation of alcohols. Green Chem. 2012, 14, 47–564. [Google Scholar] [CrossRef]
- Cardona, F.; Parmeggiani, C. Overview: Representative experimental procedures, comparative tables and conclusions. In Transition Metal Catalysis in Aerobic Alcohol Oxidation; Cardona, F., Parmeggiani, C., Eds.; Royal Society of Chemistry: Canbridge, UK, 2014; pp. 256–283. [Google Scholar]
- Stahl, S.S. Palladium oxidase catalysis: Selective oxidation of organic chemicals by direct dioxygen-coupled turnover. Angew. Chem. Int. 2004, 43, 3400–3420. [Google Scholar] [CrossRef]
- Karimi, B.; Zamani, A. Recent advances in the homogeneous palladium-catalyzed aerobic oxidation of alcohols. J. Iran. Chem. Soc. 2008, 5, S1–S20. [Google Scholar] [CrossRef]
- Vino, C.P.; Wilson, K.; Lee, A.F. Recent advances in the heterogeneously catalysed aerobic selective oxidation of alcohols. J. Chem. Technol. Biotechnol. 2011, 86, 161–171. [Google Scholar] [CrossRef]
- Parmeggiani, C.; Matassini, C.; Cardona, F. A step forward towards sustainable aerobic alcohol oxidation: New and revised catalysts based on transition metals on solid supports. Green Chem. 2017, 19, 2030–2050. [Google Scholar] [CrossRef]
- Ali, M.; Rahman, M.; Sarkar, S.M.; Hamid, S.B. Heterogeneous metal catalysts for oxidation reactions. J. Nanomater. 2014, 1, 192038. [Google Scholar] [CrossRef]
- Ji, H.B.; Ebitani, K.; Mizugaki, T.; Kaneda, K. Environmentally friendly alcohol oxidation using heterogeneous catalyst in the presence of air at room temperature. Catal. Commun. 2002, 3, 511–517. [Google Scholar] [CrossRef]
- Sun, H.Y.; Hua, Q.; Guo, F.F.; Wang, Z.Y.; Huang, W.X. Selective aerobic oxidation of alcohols by using manganese oxide nanoparticles as an efficient heterogeneous catalyst. Adv. Synth Catal. 2012, 354, 569–573. [Google Scholar] [CrossRef]
- Zhou, W.; Tao, Q.; Pan, J.; Liu, J.; Qian, J.; He, M.; Chen, Q. Effect of basicity on the catalytic properties of Ni-containing hydrotalcites in the aerobic oxidation of alcohol. J. Mol. Catal. A Chem. 2016, 425, 255–265. [Google Scholar] [CrossRef] [Green Version]
- Kazuya, Y.; Mori, K.; Mizugaki, T.; Ebitani, K.; Kaneda, K. Creation of a monomeric Ru species on the surface of hydroxyapatite as an efficient heterogeneous catalyst for aerobic alcohol oxidation. J. ACS 2000, 122, 7144–7145. [Google Scholar]
- Zhu, J.; Kailasam, K.; Fischer, A.; Thomas, A. Supported cobalt oxide nanoparticles as catalyst for aerobic oxidation of alcohols in liquid phase. ACS Catal. 2011, 1, 342–347. [Google Scholar] [CrossRef]
- Miyamura, H.; Matsubara, R.; Miyazaki, Y.; Kobayashi, S. Aerobic oxidation of alcohols at room temperature and atmospheric conditions catalyzed by reusable gold nanoclusters stabilized by the benzene rings of polystyrene derivatives. Angev. Chem. Int. 2007, 46, 4151–4154. [Google Scholar] [CrossRef]
- Karimi, B.; Zamani, A.; Abedi, S.; Clark, J.H. Aerobic oxidation of alcohols using various types of immobilized palladium catalyst: The synergistic role of functionalized ligands, morphology of support, and solvent in generating and stabilizing nanoparticles. Green Chem. 2009, 11, 109–119. [Google Scholar] [CrossRef]
- Wu, G.; Wang, X.; Guan, N.; Li, L. Palladium on graphene as efficient catalyst for solvent-free aerobic oxidation of aromatic alcohols: Role of graphene support. Appl. Catal. B Environ. 2013, 136, 177–185. [Google Scholar] [CrossRef]
- Kwon, M.S.; Kim, N.; Park, C.M.; Lee, J.S.; Kang, K.Y.; Park, J. Palladium nanoparticles entrapped in aluminum hydroxide: Dual catalyst for alkene hydrogenation and aerobic alcohol oxidation. Org. Lett. 2005, 7, 1077–1079. [Google Scholar] [CrossRef]
- Chen, Y.; Zheng, H.; Guo, Z.; Zhou, C.; Wang, C.; Borgna, A.; Yang, Y. Pd catalysts supported on MnCeOx mixed oxides and their catalytic application in solvent-free aerobic oxidation of benzyl alcohol: Support composition and structure sensitivity. J. Catal. 2011, 283, 34–44. [Google Scholar] [CrossRef]
- Hou, Z.; Theyssen, N.; Brinkmann, A.; Leitner, W. Biphasic Aerobic Oxidation of Alcohols Catalyzed by Poly (ethylene glycol)-Stabilized Palladium Nanoparticles in Supercritical Carbon Dioxide. Angew. Chem. Int. 2005, 44, 1346–1349. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Tripathi, D.; Gupta, P.; Singh, R.; Bahuguna, G.M.; Chauhan, R.K.; Saran, S.; Jain, S.L. Highly dispersed palladium nanoparticles grafted onto nanocrystalline starch for the oxidation of alcohols using molecular oxygen as an oxidant. Dalton Trans. 2013, 42, 11522–11527. [Google Scholar] [CrossRef] [PubMed]
- Johnston, E.V.; Verho, O.; Kärkäs, M.D.; Shakeri, M.; Tai, C.W.; Palmgren, P.; Eriksson, K.; Oscarsson, S.; Bäckvall, J.E. Highly dispersed palladium nanoparticles on mesocellular foam: An efficient and recyclable heterogeneous catalyst for alcohol oxidation. Chem. Euro J. 2012, 18, 12202–12206. [Google Scholar] [CrossRef]
- Tang, L.; Guo, X.; Li, Y.; Zhang, S.; Zha, Z.; Wang, Z. Pt, Pd and Au nanoparticles supported on a DNA–MMT hybrid: Efficient catalysts for highly selective oxidation of primary alcohols to aldehydes, acids and esters. Chem. Commun. 2013, 49, 5213–5215. [Google Scholar] [CrossRef]
- Shakil Hussain, S.M.; Kamal, M.S.; Hossain, M.K. Recent developments in nanostructured palladium and other metal catalysts for organic transformation. J. Nanomater. 2019, 20. [Google Scholar] [CrossRef]
- Wolfson, A.; Levy-Ontman, O. Recent Developments in the Immobilization of Palladium Complexes on Renewable Polysaccharides for Suzuki–Miyaura Cross-Coupling of Halobenzenes and Phenylboronic Acids. Catalysts 2020, 10, 136. [Google Scholar] [CrossRef] [Green Version]
- Wolfson, A.; Levy-Ontman, O. Development and application of palladium nanoparticles on renewable polysaccharides as catalysts for the Suzuki cross-coupling of halobenzenes and phenylboronic acids. Mol. Catal. 2020, 493, 111048. [Google Scholar] [CrossRef]
- Kumbhar, A.; Salunkhe, R. Recent advances in biopolymer supported palladium in organic synthesis. Curr. Org. Chem. 2015, 19, 2075–2121. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M. Green synthesis and catalytic properties of palladium nanoparticles for the direct reductive amination of aldehydes and hydrogenation of unsaturated ketones. New J. Chem. 2014, 38, 5544–5550. [Google Scholar] [CrossRef]
- Levy-Ontman, O.; Biton, S.; Shlomov, B.; Wolfson, A. Renewable polysaccharides as supports for palladium phosphine catalysts. Polymers 2018, 1, 659. [Google Scholar] [CrossRef] [Green Version]
- Wolfson, A.; Biton, S.; Levy-Ontman, O. Study of Pd-based catalysts within red algae-derived polysaccharide supports in a Suzuki cross-coupling reaction. RSC Adv. 2018, 8, 37939–37948. [Google Scholar] [CrossRef] [Green Version]
- Levy-Ontman, O.; Blum, D.; Golden, R.; Pierschel, E.; Leviev, S.; Wolfson, A. Palladium Based-Polysaccharide Hydrogels as Catalysts in the Suzuki Cross-Coupling Reaction. J. Inorg. Organomet. Polym. Mater. 2020, 30, 622–636. [Google Scholar] [CrossRef]
- Leviev, S.; Wolfson, A.; Levy-Ontman, O. RhCl(TPPTS)3 supported on iota-carrageenan as recyclable catalysts for Suzuki cross-coupling. J. Appl. Polym. Sci. 2019, 136, 48200. [Google Scholar] [CrossRef]
- Leviev, S.; Wolfson, A.; Levy-Ontman, O. Novel iota carrageenan-based RhCl3 as an efficient and recyclable catalyst in Suzuki cross coupling. Mol. Catal. 2020, 486, 110841. [Google Scholar] [CrossRef]
- Wolfson, A.; Pierschel, E.; Orzehovsky, T.; Nirenberg, S.; Levy-Ontman, O. Heterogeneous iota carrageenan-based palladium catalysts for organic synthesis. Org. Comm. 2019, 12, 149–159. [Google Scholar] [CrossRef]
- Levy-Ontman, O.; Tabibian, B.; Shaklein, S.; Tzur, E.; Wolfson, A. Acceleration of transfer-hydrogenation of cyclohexene with palladium catalysts in the presence of polysaccharides. Org. Comm. 2020, 13, 138–145. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.T.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Chen, C.C.; Lin, C.L.; Chen, L.C. Functionalized carbon nanomaterial supported palladium nano-catalysts for electrocatalytic glucose oxidation reaction. Electrochim. Acta 2015, 152, 408–416. [Google Scholar] [CrossRef]




| Entry | Catalyst | Base b | Pd(OAc)2 Conversion (%) | PdCl2 Conversion (%) |
|---|---|---|---|---|
| 1 | Homogeneous-No ligand | - | 50 | 0 |
| 2 | Homogeneous-No ligand | + | 37 | 19 |
| 3 | Homogeneous-TPP | - | 23 | 0 |
| 4 | Homogeneous-TPP | + | 28 | 30 |
| 5 | Homogeneous-TPPTS | - | 39 | 0 |
| 6 | Homogeneous-TPPTS | + | 32 | 20 |
| 7 | Heterogeneous | - | 20 | 0 |
| 8 | Heterogeneous | + | 22 | 8 |
| 9 | Heterogeneous c | - | 12 | 0 |
| 10 | Heterogeneous c | + | 20 | 12 |
| 11 | Heterogeneous d | - | 21 | 14 |
| 12 | Heterogeneous e | - | 22 | not tested |
| 13 | Heterogeneous f | - | 40 | not tested |
| Cycle | i-Pd(OAc)2(TPPTS)2 Conversion (%) | i-PdCl2(TPPTS)2 Conversion (%) b |
|---|---|---|
| 1 | 20 | 8 |
| 2 | 29 | 11 |
| 3 | 35 | 15 |
| Element | Atomic % before Reaction | Atomic % before Reaction |
|---|---|---|
| C1s | 56.76 | 55.99 |
| O1s | 31.91 | 31.26 |
| N1s | 0.58 | 0.51 |
| Pd3d | 0.50 | 0.49 |
| Na1s | 2.07 | 2.75 |
| Ca2p | 1.09 | 1.32 |
| S2p | 6.43 | 6.98 |
| K2p | 0.68 | 0.69 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stamker, E.; Levy-Ontman, O.; Wolfson, A. Green Procedure for Aerobic Oxidation of Benzylic Alcohols with Palladium Supported on Iota-Carrageenan in Ethanol. Polymers 2021, 13, 498. https://doi.org/10.3390/polym13040498
Stamker E, Levy-Ontman O, Wolfson A. Green Procedure for Aerobic Oxidation of Benzylic Alcohols with Palladium Supported on Iota-Carrageenan in Ethanol. Polymers. 2021; 13(4):498. https://doi.org/10.3390/polym13040498
Chicago/Turabian StyleStamker, Eliraz, Oshrat Levy-Ontman, and Adi Wolfson. 2021. "Green Procedure for Aerobic Oxidation of Benzylic Alcohols with Palladium Supported on Iota-Carrageenan in Ethanol" Polymers 13, no. 4: 498. https://doi.org/10.3390/polym13040498