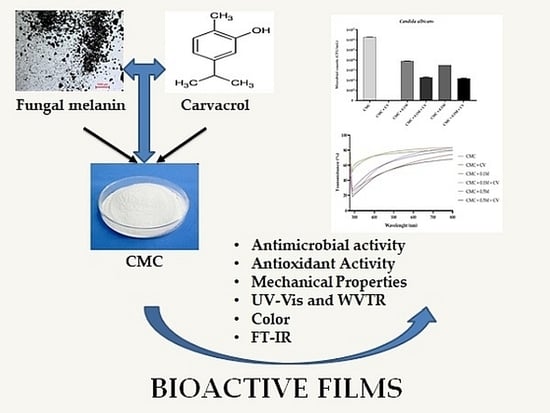
Preparation and Characterization of Carboxymethyl Cellulose-Based Bioactive Composite Films Modified with Fungal Melanin and Carvacrol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Isolation, Purification and Preparation of Melanin from Agaricus Bisporus Waste
2.3. Determination of Carvacrol Minimum Inhibitory Concentration (MIC)
2.4. Preparation of Films
2.5. Thickness, Moisture Content (MC) and Mechanical Properties of The Films
2.6. DSC Measurements
2.7. The Water Vapour Transmission Rate (WVTR) of the Films
2.8. Spectral Analysis
2.9. Colour Analysis
2.10. Antioxidant Activity and Reducing Power
2.11. Antimicrobial Activity
2.12. Statistical Analysis
3. Results and Discussion
3.1. Antimicrobial Activity of The Films
3.2. Radicals Scavenging Activities and Reducing Power
3.3. The Thickness, Moisture Content, Mechanical, Thermal and Water Vapour Barrier Properties of The Films
3.4. Appearance, Colour, Opacity, and Transparency Changes
3.5. The Changes of UV-Vis Blocking Properties
3.6. FT-IR Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roy, S.; Rhim, J.W. Fabrication of copper sulfide nanoparticles and limonene incorporated pullulan/carrageenan based film with improved mechanical and antibacterial properties. Polymers 2020, 12, 2665. [Google Scholar] [CrossRef]
- Łopusiewicz, Ł.; Drozłowska, E.; Trocer, P.; Kostek, M.; Śliwiński, M.; Henriques, M.H.F.; Bartkowiak, A.; Sobolewski, P. Whey protein concentrate/isolate biofunctional films modified with melanin from watermelon (Citrullus lanatus) seeds. Materials 2020, 13, 3876. [Google Scholar] [CrossRef]
- Łopusiewicz, Ł.; Jędra, F.; Mizielińska, M. New poly(lactic acid) active packaging composite films incorporated with fungal melanin. Polymers 2018, 10, 386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Łopusiewicz, Ł.; Jędra, F.; Bartkowiak, A. New Active Packaging Films Made from Gelatin Modified with Fungal Melanin. World Sci. News 2018, 101, 1–30. [Google Scholar]
- Flores, Z.; San-Martin, D.; Beldarraín-Iznaga, T.; Leiva-Vega, J.; Villalobos-Carvajal, R. Effect of Homogenization Method and Carvacrol Content on Microstructural and Physical Properties of Chitosan-Based Films. Foods 2021, 10, 141. [Google Scholar] [CrossRef] [PubMed]
- Ezati, P.; Rhim, J.W.; Moradi, M.; Tajik, H.; Molaei, R. CMC and CNF-based alizarin incorporated reversible pH-responsive color indicator films. Carbohydr. Polym. 2020, 246, 116614. [Google Scholar] [CrossRef]
- Lei, K.; Wang, X.; Li, X.; Wang, L. The innovative fabrication and applications of carvacrol nanoemulsions, carboxymethyl chitosan microgels and their composite films. Colloids Surf. B Biointerfaces 2019, 175, 688–696. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, H.M.S.; Riaz, A.; Hamed, Y.S.; Abdin, M.; Chen, G.; Wan, P.; Zeng, X. Production and characterization of CMC-based antioxidant and antimicrobial films enriched with chickpea hull polysaccharides. Int. J. Biol. Macromol. 2018, 118, 469–477. [Google Scholar] [CrossRef]
- Jannatyha, N.; Shojaee-Aliabadi, S.; Moslehishad, M.; Moradi, E. Comparing mechanical, barrier and antimicrobial properties of nanocellulose/CMC and nanochitosan/CMC composite films. Int. J. Biol. Macromol. 2020, 164, 2323–2328. [Google Scholar] [CrossRef]
- Tabari, M. Investigation of Carboxymethyl Cellulose (CMC) on Mechanical Properties of Cold Water Fish Gelatin Biodegradable Edible Films. Foods 2017, 6, 41. [Google Scholar] [CrossRef] [Green Version]
- Ghanbarzadeh, B.; Almasi, H.; Entezami, A.A. Physical properties of edible modified starch/carboxymethyl cellulose films. Innov. Food Sci. Emerg. Technol. 2010, 11, 697–702. [Google Scholar] [CrossRef]
- Ancuţa, P.; Sonia, A. Oil press-cakes and meals valorization through circular economy approaches: A review. Appl. Sci. 2020, 10, 7432. [Google Scholar] [CrossRef]
- Łopusiewicz, Ł. Waste from the harvesting of button mushroom (Agaricus bisporus) as a source of natural melanin. Folia Pomeranae Univ. Technol. Stetin. Agric. Aliment. Piscaria Zootech. 2018, 343, 23–42. [Google Scholar] [CrossRef]
- Dueñas, M.; García-Estévez, I. Agricultural and food waste: Analysis, characterization and extraction of bioactive compounds and their possible utilization. Foods 2020, 9, 817. [Google Scholar] [CrossRef] [PubMed]
- Solano, F. Melanins: Skin Pigments and Much More—Types, Structural Models, Biological Functions, and Formation Routes. New J. Sci. 2014, 2014, 498276. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Chen, T.; Li, J.; Jin, M.; Ye, M. The structural analysis and its hepatoprotective activity of melanin isolated from Lachnum sp. Process. Biochem. 2020, 90, 249–256. [Google Scholar] [CrossRef]
- Łopusiewicz, Ł. Antioxidant, antibacterial properties and the light barrier assessment of raw and purified melanins isolated from Citrullus lanatus (watermelon) seeds. Herba Pol. 2018, 64, 25–36. [Google Scholar] [CrossRef] [Green Version]
- Al-Tayib, O.A.; Elbadwi, S.M.; Bakhiet, A.O. Cytotoxicity assay for herbal melanin derived from Nigella sativa seeds using in vitro cell lines. IOSR J. Humanit. Soc. Sci. 2017, 22, 43. [Google Scholar]
- Ye, M.; Wang, Y.; Guo, G.Y.; He, Y.L.; Lu, Y.; Ye, Y.W.; Yang, Q.H.; Yang, P.Z. Physicochemical characteristics and antioxidant activity of arginine-modified melanin from Lachnum YM-346. Food Chem. 2012, 135, 2490–2497. [Google Scholar] [CrossRef]
- Yang, M.; Li, L.; Yu, S.; Liu, J.; Shi, J. High performance of alginate/polyvinyl alcohol composite film based on natural original melanin nanoparticles used as food thermal insulating and UV–vis block. Carbohydr. Polym. 2020, 233, 115884. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.W. Agar-based antioxidant composite films incorporated with melanin nanoparticles. Food Hydrocoll. 2019, 94, 391–398. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.W. Carrageenan-based antimicrobial bionanocomposite films incorporated with ZnO nanoparticles stabilized by melanin. Food Hydrocoll. 2019, 90, 500–507. [Google Scholar] [CrossRef]
- Roy, S.; Kim, H.C.; Kim, J.W.; Zhai, L.; Zhu, Q.Y.; Kim, J. Incorporation of melanin nanoparticles improves UV-shielding, mechanical and antioxidant properties of cellulose nanofiber based nanocomposite films. Mater. Today Commun. 2020, 24, 100984. [Google Scholar] [CrossRef]
- Roy, S.; Van Hai, L.; Kim, H.C.; Zhai, L.; Kim, J. Preparation and characterization of synthetic melanin-like nanoparticles reinforced chitosan nanocomposite films. Carbohydr. Polym. 2020, 231, 115729. [Google Scholar] [CrossRef]
- Dong, W.; Wang, Y.; Huang, C.; Xiang, S.; Ma, P.; Ni, Z.; Chen, M. Enhanced thermal stability of poly (vinyl alcohol) in presence of melanin. J. Therm. Anal. Calorim. 2014, 115, 1661–1668. [Google Scholar] [CrossRef]
- Bang, Y.J.; Shankar, S.; Rhim, J.W. Preparation of polypropylene/poly (butylene adipate-co-terephthalate) composite films incorporated with melanin for prevention of greening of potatoes. Packag. Technol. Sci. 2020, 33, 1–9. [Google Scholar] [CrossRef]
- Kiran, G.S.; Jackson, S.A.; Priyadharsini, S.; Dobson, A.D.W.; Selvin, J. Synthesis of Nm-PHB (nanomelanin-polyhydroxy butyrate) nanocomposite film and its protective effect against biofilm-forming multi drug resistant Staphylococcus aureus. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Di Mauro, E.; Camaggi, M.; Vandooren, N.; Bayard, C.; De Angelis, J.; Pezzella, A.; Baloukas, B.; Silverwood, R.; Ajji, A.; Pellerin, C.; et al. Eumelanin for nature-inspired UV-absorption enhancement of plastics. Polym. Int. 2019, 68, 984–991. [Google Scholar] [CrossRef]
- Kwiatkowski, P.; Pruss, A.; Wojciuk, B.; Dołęgowska, B.; Wajs-Bonikowska, A.; Sienkiewicz, M.; Mężyńska, M.; Łopusiewicz, Ł. The influence of essential oil compounds on antibacterial activity of mupirocin-susceptible and induced low-level mupirocin-resistant MRSA strains. Molecules 2019, 24, 3105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhumal, C.V.; Ahmed, J.; Bandara, N.; Sarkar, P. Improvement of antimicrobial activity of sago starch/guar gum bi-phasic edible films by incorporating carvacrol and citral. Food Packag. Shelf Life 2019, 21, 100380. [Google Scholar] [CrossRef]
- Ramos, M.; Jiménez, A.; Peltzer, M.; Garrigós, M.C. Characterization and antimicrobial activity studies of polypropylene films with carvacrol and thymol for active packaging. J. Food Eng. 2012, 109, 513–519. [Google Scholar] [CrossRef]
- Magi, G.; Marini, E.; Facinelli, B. Antimicrobial activity of essential oils and carvacrol, and synergy of carvacrol and erythromycin, against clinical, erythromycin-resistant Group A Streptococci. Front. Microbiol. 2015, 6, 165. [Google Scholar] [CrossRef] [Green Version]
- Lima, I.O.; De Oliveira Pereira, F.; De Oliveira, W.A.; De Oliveira Lima, E.; Menezes, E.A.; Cunha, F.A.; De Fátima Formiga Melo Diniz, M. Antifungal activity and mode of action of carvacrol against Candida albicans strains. J. Essent. Oil Res. 2013, 25, 138–142. [Google Scholar] [CrossRef]
- Lambert, R.J.W.; Skandamis, P.N.; Coote, P.J.; Nychas, G.J.E. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J. Appl. Microbiol. 2001, 91, 453–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marchese, A.; Arciola, C.R.; Coppo, E.; Barbieri, R.; Barreca, D.; Chebaibi, S.; Sobarzo-Sánchez, E.; Nabavi, S.F.; Nabavi, S.M.; Daglia, M. The natural plant compound carvacrol as an antimicrobial and anti-biofilm agent: Mechanisms, synergies and bio-inspired anti-infective materials. Biofouling 2018, 34, 630–656. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Qiu, W.; Mei, J.; Xie, J. Effect of Sonication on the Properties of Flaxseed Gum Films Incorporated with Carvacrol. Int. J. Mol. Sci. 2020, 21, 1637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharifi-Rad, M.; Varoni, E.M.; Iriti, M.; Martorell, M.; Setzer, W.N.; del Mar Contreras, M.; Salehi, B.; Soltani-Nejad, A.; Rajabi, S.; Tajbakhsh, M.; et al. Carvacrol and human health: A comprehensive review. Phyther. Res. 2018, 32, 1675–1687. [Google Scholar] [CrossRef]
- Suntres, Z.E.; Coccimiglio, J.; Alipour, M. The Bioactivity and Toxicological Actions of Carvacrol. Crit. Rev. Food Sci. Nutr. 2015, 55, 304–318. [Google Scholar] [CrossRef] [PubMed]
- Du, W.X.; Olsen, C.W.; Avena-Bustillos, R.J.; McHugh, T.H.; Levin, C.E.; Friedman, M. Storage stability and antibacterial activity against Escherichia coli O157:H7 of carvacrol in edible apple films made by two different casting methods. J. Agric. Food Chem. 2008, 56, 3082–3088. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Lv, H.; Yang, B.; Chen, X.; Sun, H. Physical properties, antioxidant and antimicrobial activity of chitosan films containing carvacrol and pomegranate peel extract. Molecules 2015, 20, 11034–11045. [Google Scholar] [CrossRef] [Green Version]
- Medina-Jaramillo, C.; Quintero-Pimiento, C.; Díaz-Díaz, D.; Goyanes, S.; López-Córdoba, A. Improvement of andean blueberries postharvest preservation using carvacrol/alginate-edible coatings. Polymers 2020, 12, 2352. [Google Scholar] [CrossRef]
- Sapper, M.; Martin-Esparza, M.E.; Chiralt, A.; Martinez, C.G. Antifungal polyvinyl alcohol coatings incorporating carvacrol for the postharvest preservation of golden delicious apple. Coatings 2020, 10, 1027. [Google Scholar] [CrossRef]
- Ochoa-Velasco, C.E.; Pérez-Pérez, J.C.; Varillas-Torres, J.M.; Navarro-Cruz, A.R.; Hernández-Carranza, P.; Munguía-Pérez, R.; Cid-Pérez, T.S.; Avila-Sosa, R. Starch Edible Films/Coatings Added with Carvacrol and Thymol: In Vitro and In Vivo Evaluation against Colletotrichum gloeosporioides. Foods 2021, 10, 175. [Google Scholar] [CrossRef]
- Fang, S.; Zhou, Q.; Hu, Y.; Liu, F.; Mei, J.; Xie, J. Antimicrobial Carvacrol Incorporated in Flaxseed Gum-Sodium Alginate Active Films to Improve the Quality Attributes of Chinese Sea bass (Lateolabrax maculatus) during Cold Storage. Molecules 2019, 24, 3292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krepker, M.; Prinz-Setter, O.; Shemesh, R.; Vaxman, A.; Alperstein, D.; Segal, E. Antimicrobial carvacrol-containing polypropylene films: Composition, structure and function. Polymers 2018, 10, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canales, D.; Montoille, L.; Rivas, L.M.; Ortiz, J.A.; Yañez-S, M.; Rabagliati, F.M.; Ulloa, M.T.; Alvarez, E.; Zapata, P.A. Fungicides Films of Low-Density Polyethylene (LDPE)/Inclusion Complexes (Carvacrol and Cinnamaldehyde) Against Botrytis cinerea. Coatings 2019, 9, 795. [Google Scholar] [CrossRef] [Green Version]
- Kwiatkowski, P.; Pruss, A.; Grygorcewicz, B.; Wojciuk, B.; Dołęgowska, B.; Giedrys-Kalemba, S.; Kochan, E.; Sienkiewicz, M. Preliminary study on the antibacterial activity of essential oils alone and in combination with gentamicin against extended-spectrum β-lactamase-producing and New Delhi metallo-β-lactamase-1-producing Klebsiella pneumoniae isolates. Microb. Drug Resist. 2018, 24, 1368–1375. [Google Scholar] [CrossRef]
- Kwiatkowski, P.; Łopusiewicz, Ł.; Kostek, M.; Drozłowska, E.; Pruss, A.; Wojciuk, B.; Sienkiewicz, M.; Zielińska-Bliźniewska, H.; Dołęgowska, B. The Antibacterial Activity of Lavender Essential Oil Alone and In Combination with Octenidine Dihydrochloride against MRSA Strains. Molecules 2019, 25, 95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizielińska, M.; Kowalska, U.; Salachna, P.; Łopusiewicz, Ł.; Jarosz, M. The influence of accelerated UV-A and Q-SUN irradiation on the antibacterial properties of hydrophobic coatings containing Eucomis comosa extract. Polymers 2018, 10, 421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizielińska, M.; Łopusiewicz, Ł.; Mężyńska, M.; Bartkowiak, A. The influence of accelerated UV-A and Q-sun irradiation on the antimicrobial properties of coatings containing ZnO nanoparticles. Molecules 2017, 22, 1556. [Google Scholar] [CrossRef] [Green Version]
- López-Mata, M.A.; Ruiz-Cruz, S.; Silva-Beltrán, N.P.; Ornelas-Paz, J.D.J.; Zamudio-Flores, P.B.; Burruel-Ibarra, S.E. Physicochemical, antimicrobial and antioxidant properties of chitosan films incorporated with carvacrol. Molecules 2013, 18, 13735–13753. [Google Scholar] [CrossRef] [Green Version]
- Lukic, I.; Vulic, J.; Ivanovic, J. Antioxidant activity of PLA/PCL films loaded with thymol and/or carvacrol using scCO2 for active food packaging. Food Packag. Shelf Life 2020, 26, 100578. [Google Scholar] [CrossRef]
- Scaffaro, R.; Lopresti, F.; D’Arrigo, M.; Marino, A.; Nostro, A. Efficacy of poly (lactic acid)/carvacrol electrospun membranes against Staphylococcus aureus and Candida albicans in single and mixed cultures. Appl. Microbiol. Biotechnol. 2018, 102, 4171–4181. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yu, H.; Tian, B.; Jiang, B.; Xu, J.; Li, D.; Feng, Z.; Liu, C. Novel Edible Coating with Antioxidant and Antimicrobial Activities Based on Whey Protein Isolate Nanofibrils and Carvacrol and Its Application on Fresh-Cut Cheese. Coatings 2019, 9, 583. [Google Scholar] [CrossRef] [Green Version]
- Łopusiewicz, Ł.; Jędra, F.; Bartkowiak, A. The application of melanin modified gelatin coatings for packaging and the oxidative stability of pork lard. World Sci. News 2018, 101, 108–119. [Google Scholar]
- Tongdeesoontorn, W.; Mauer, L.J.; Wongruong, S.; Sriburi, P.; Rachtanapun, P. Effect of carboxymethyl cellulose concentration on physical properties of biodegradable cassava starch-based films. Chem. Cent. J. 2011, 5, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oun, A.A.; Rhim, J.W. Preparation and characterization of sodium carboxymethyl cellulose/cotton linter cellulose nanofibril composite films. Carbohydr. Polym. 2015, 127, 101–109. [Google Scholar] [CrossRef]






| Sample | RP (700 nm) | DPPH (%) | ABTS (%) | O2− (%) |
|---|---|---|---|---|
| CMC | 0.030 ± 0.001 f | 15.23 ± 0.01 f | 7.98 ± 0.14 f | 9.68 ± 0.05 f |
| CMC + 0.1 M | 0.206 ± 0.005 d | 64.20 ± 0.06 d | 55.72 ± 0.08 e | 63.92 ± 0.07 d |
| CMC + 0.5 M | 0.244 ± 0.010 c | 65.73 ± 0.01 c | 57.65 ± 0.08 d | 68.08 ± 0.09 c |
| CMC + CV | 0.192 ± 0.002 e | 45.44 ± 0.02 e | 69.18 ± 0.05 c | 61.63 ± 0.09 e |
| CMC + 0.1 M + CV | 0.259 ± 0.001 b | 69.70 ± 0.06 b | 77.61 ± 0.01 b | 81.83 ± 0.18 b |
| CMC + 0.5 M + CV | 0.389 ± 0.006 a | 72.70 ± 0.05 a | 93.54 ± 0.03 a | 89.84 ± 0.07 a |
| Sample | Thickness (mm) | TSC (%) | WVTR (g/(m2 × Day)) | TS (N) | EB (%) | Tm (°C) | ΔHm (J/g) |
|---|---|---|---|---|---|---|---|
| CMC | 0.05 ± 0.00 c | 89.00 ± 0.31 b | 1098.68 ± 6.74 a | 18.11 ± 1.25 d | 9.91 ± 0.25 a | 130.54 | −252.30 |
| CMC + 0.1 M | 0.06 ± 0.01 c | 89.33 ± 0.28 ab | 962.42 ± 3.51 c | 19.61 ± 1.03 d | 8.74 ± 0.76 b | 105.51 | −250.33 |
| CMC + 0.5 M | 0.04 ± 0.01 c | 89.35 ± 0.12 ab | 933.07 ± 4.32 d | 25.89 ± 3.06 c | 7.31 ± 0.66 cd | 113.04 | −283.25 |
| CMC + CV | 0.12 ± 0.02 a | 89.02 ± 0.17 b | 1092.38 ± 11.73 ab | 25.30 ± 1.52 bc | 7.88 ± 0.31 bc | 144.93 | −153.91 |
| CMC + 0.1 M + CV | 0.08 ± 0.00 b | 89.43 ± 0.19 ab | 992.03 ± 3.84 b | 29.20 ± 2.44 ab | 6.33 ± 0.49 d | 102.69 | −201.18 |
| CMC + 0.5 M + CV | 0.05 ± 0.01 c | 89.57 ± 0.03 a | 961.74 ± 9.13 bc | 34.29 ± 1.83 a | 4.11 ± 0.72 e | 107.72 | −288.62 |
| Sample | L* | a* | b* | ΔE | C | H° | YI | WI | Opacity | T660 (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| CMC | 89.77 ± 0.92 a | −0.66 ± 0.02 c | 4.38 ± 0.38 d | used as standard | 4.43 ± 0.38 c | −8.68 ± 0.83 d | 6.97 ± 0.68 e | 94.54 ± 0.39 a | 7.62 ± 0.20 a | 82.07 a |
| CMC + 0.1 M | 86.08 ± 0.61 ab | −0.31 ± 0.09 c | 6.75 ± 0.93 c | 2.78 ± 0.82 bc | 6.96 ± 0.94 b | −2.71 ± 1.07 c | 10.98 ± 1.54 c | 92.84 ± 1.25 c | 7.50 ± 0.17 a | 78.72 c |
| CMC + 0.5 M | 83.81 ± 1.44 bc | 0.88 ± 0.03 b | 16.61 ± 0.38 b | 13.76 ± 0.64 a | 17.47 ± 1.40 a | 3.17 ± 0.64 a | 28.38 ± 4.47 a | 82.88 ± 2.37 d | 7.02 ± 0.13 b | 67.97 d |
| CMC + CV | 89.28 ± 0.50 a | −0.08 ± 0.13 c | 4.94 ± 0.50 d | 1.13 ± 0.68 c | 5.04 ± 0.43 bc | −0.56 ± 1.13 b | 7.91 ± 0.84 de | 94.07 ± 0.46 a | 7.22 ± 0.13 b | 79.10 b |
| CMC + 0.1 M + CV | 88.62 ± 0.22 ab | −0.19 ± 0.09 c | 6.32 ± 0.93 cd | 3.62 ± 0.78 b | 6.06 ± 0.75 bc | −1.91 ± 0.83 bc | 10.20 ± 1.51 c | 92.82 ± 0.83 b | 7.14 ± 0.15 b | 75.29 e |
| CMC + 0.5 M + CV | 82.50 ± 0.28 c | 1.35 ± 0.84 a | 19.85 ± 0.28 a | 14.24 ± 0.72 a | 17.82 ± 3.02 a | 4.26 ± 1.49 a | 20.13 ± 0.13 b | 86.64 ± 0.96 d | 6.54 ± 0.17 c | 64.37 f |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łopusiewicz, Ł.; Kwiatkowski, P.; Drozłowska, E.; Trocer, P.; Kostek, M.; Śliwiński, M.; Polak-Śliwińska, M.; Kowalczyk, E.; Sienkiewicz, M. Preparation and Characterization of Carboxymethyl Cellulose-Based Bioactive Composite Films Modified with Fungal Melanin and Carvacrol. Polymers 2021, 13, 499. https://doi.org/10.3390/polym13040499
Łopusiewicz Ł, Kwiatkowski P, Drozłowska E, Trocer P, Kostek M, Śliwiński M, Polak-Śliwińska M, Kowalczyk E, Sienkiewicz M. Preparation and Characterization of Carboxymethyl Cellulose-Based Bioactive Composite Films Modified with Fungal Melanin and Carvacrol. Polymers. 2021; 13(4):499. https://doi.org/10.3390/polym13040499
Chicago/Turabian StyleŁopusiewicz, Łukasz, Paweł Kwiatkowski, Emilia Drozłowska, Paulina Trocer, Mateusz Kostek, Mariusz Śliwiński, Magdalena Polak-Śliwińska, Edward Kowalczyk, and Monika Sienkiewicz. 2021. "Preparation and Characterization of Carboxymethyl Cellulose-Based Bioactive Composite Films Modified with Fungal Melanin and Carvacrol" Polymers 13, no. 4: 499. https://doi.org/10.3390/polym13040499