Synthesis of a Biodegradable Polymer of Poly (Sodium Alginate/Ethyl Acrylate)
Abstract
:1. Introduction
2. Materials and Methods
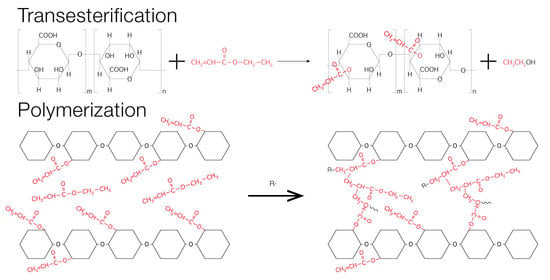
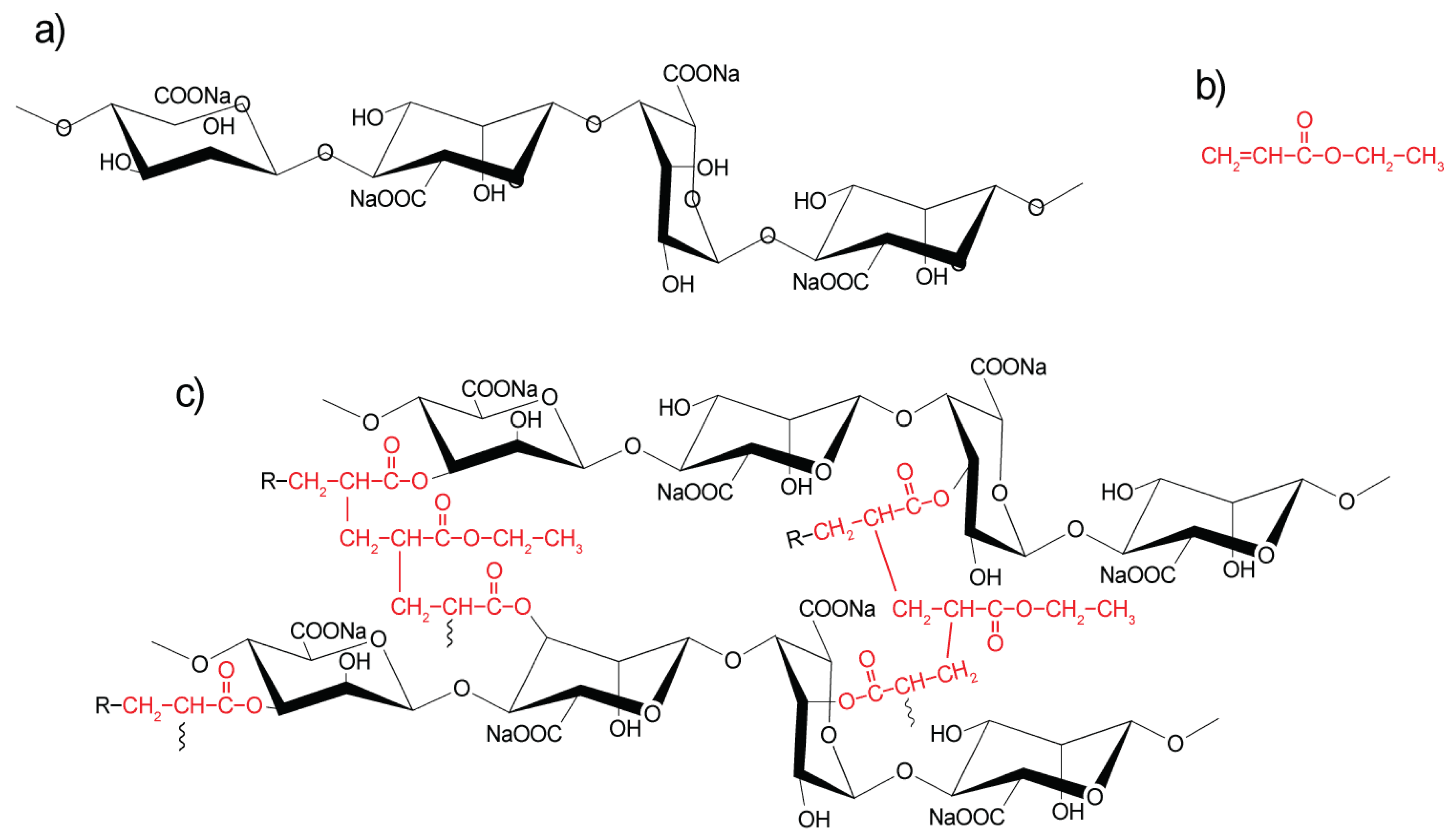
Synthesis
3. Characterization
3.1. FTIR Spectroscopy
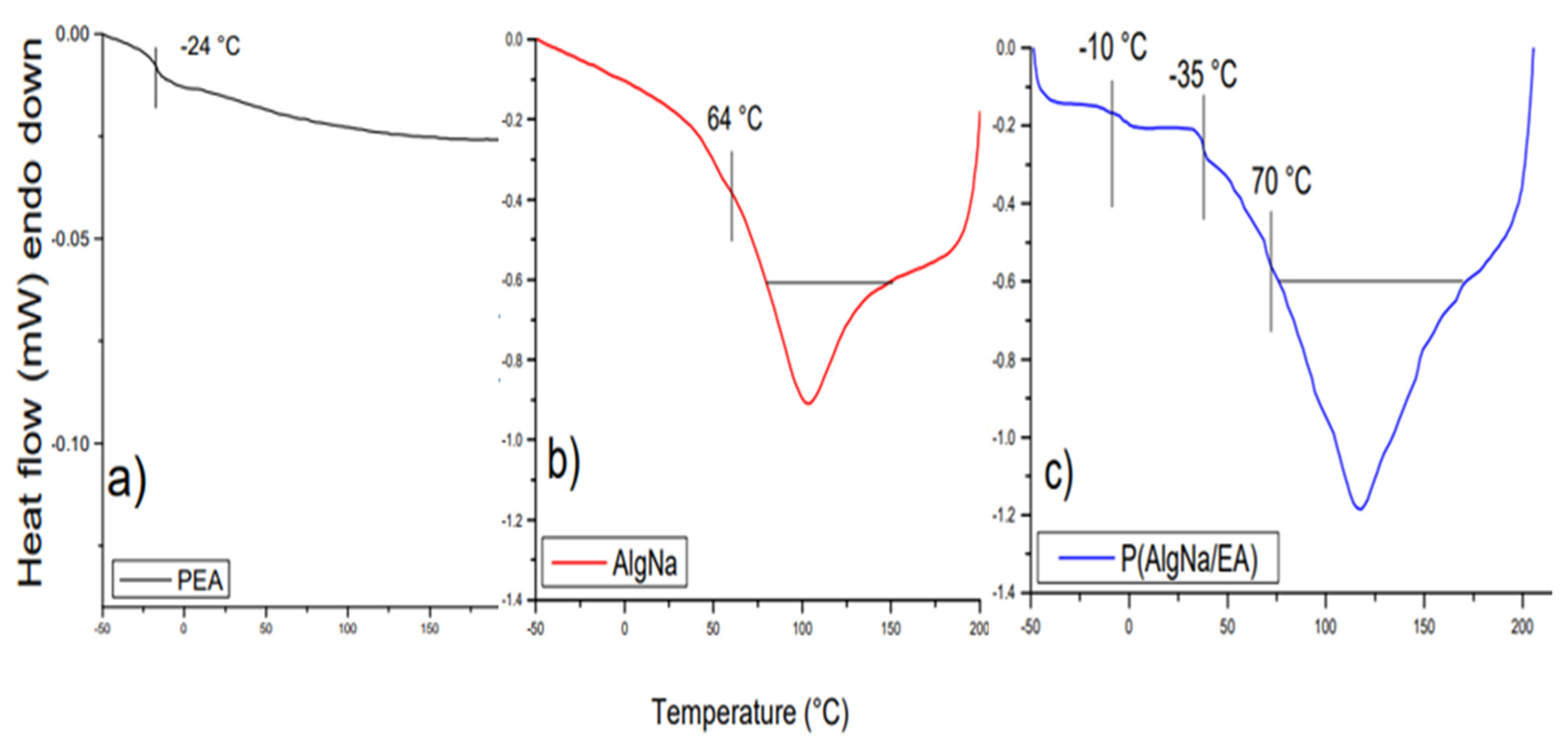
3.2. Differential Scanning Calorimetry (DSC)
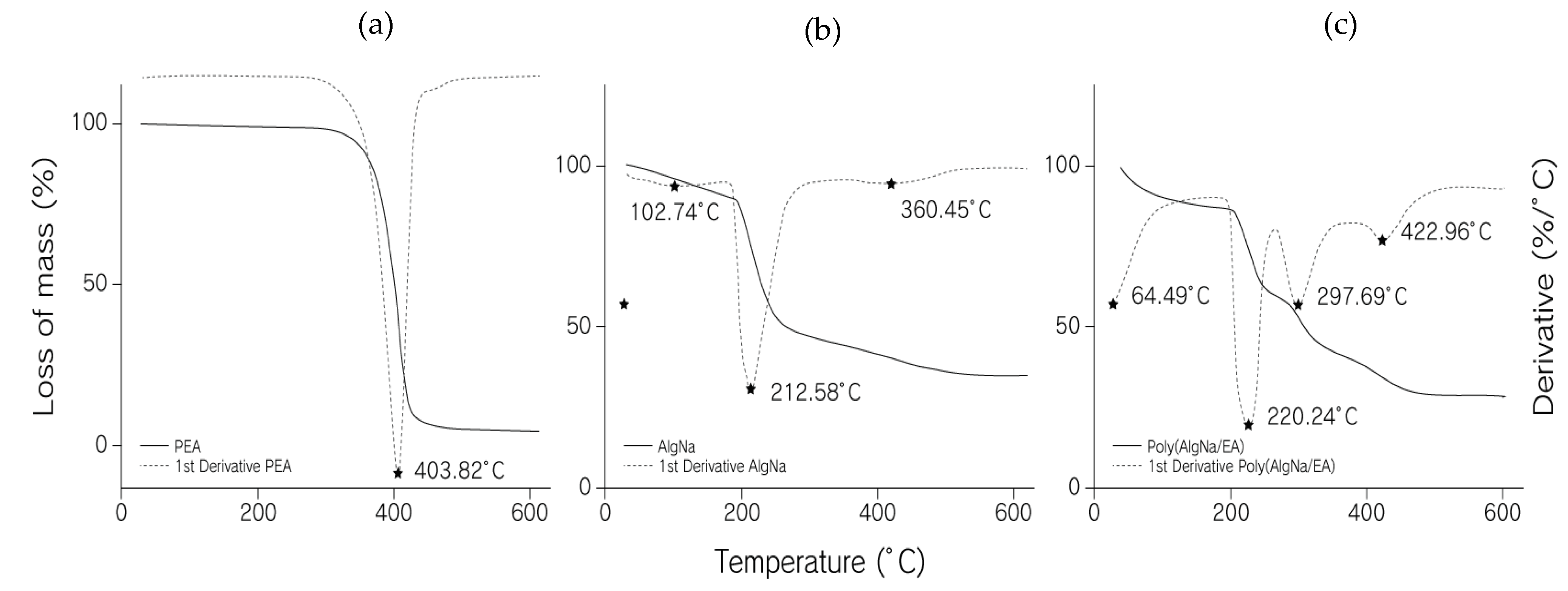
3.3. Thermogravimetric Analyses (TGA)
3.4. Tensile Test
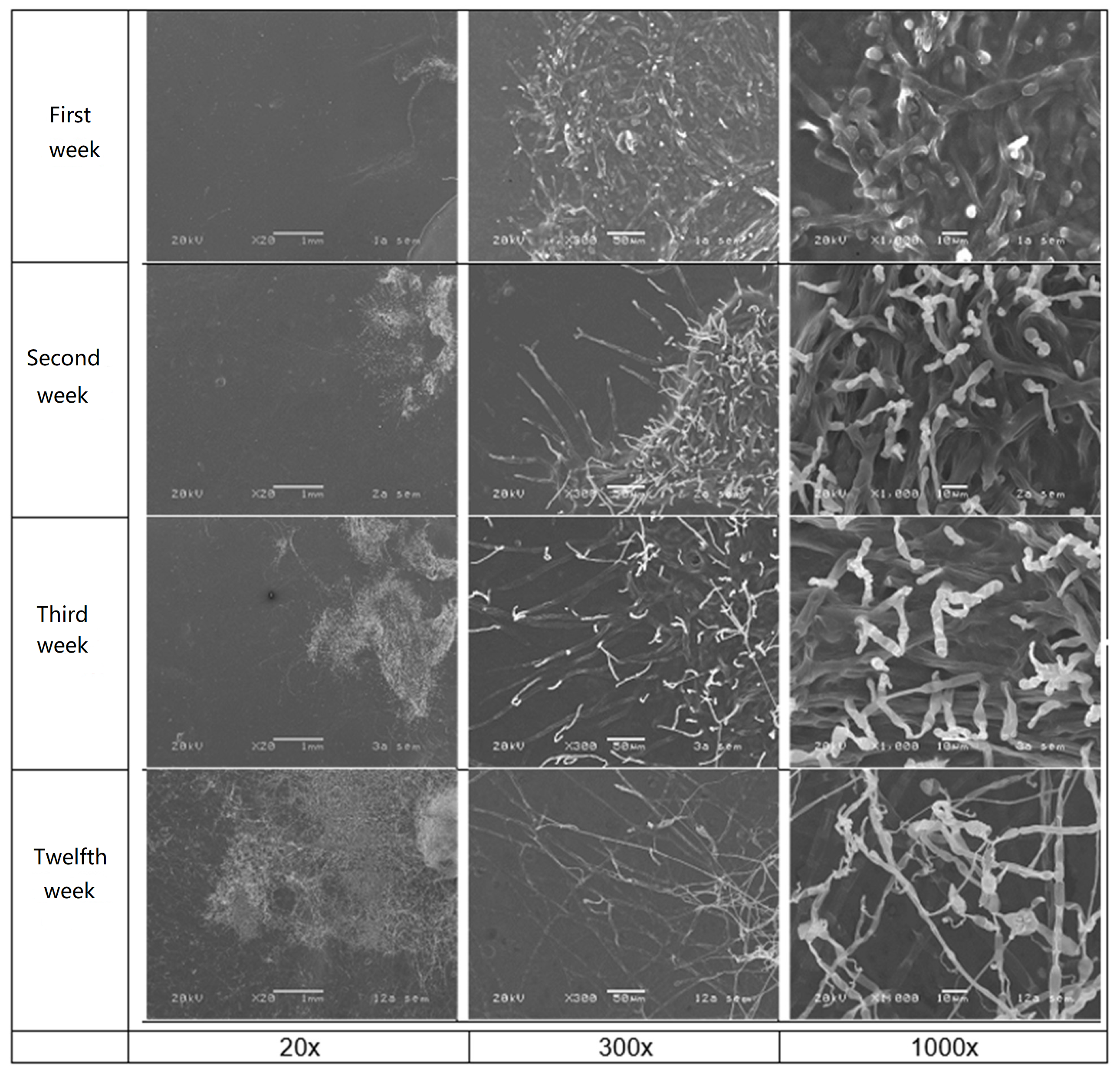
3.5. Evaluation of the Action of a Microorganism of Plastics Biodeterioration
4. Results and discussion
4.1. Fourier-Transform Infrared Spectroscopy (FTIR)
4.2. Thermogravimetric Analysis (TGA)
4.3. Differential Scanning Calorimetry (DSC)
4.4. Tensile Test
4.5. Deterioration of Plastics by Scanning Electron Microscopy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Nessi, V.; Falourd, X.; Maigret, J.-E.; Cahier, K.; D’Orlando, A.; Descamps, N.; Gaucher, V.; Chevigny, C.; Lourdin, D. Cellulose nanocrystals-starch nanocomposites produced by extrusion: Structure and behavior in physiological conditions. Carbohydr. Polym. 2019, 225, 115123. [Google Scholar] [CrossRef]
- Barclay, T.G.; Day, C.M.; Petrovsky, N.; Garg, S. Review of polysaccharide particle-based functional drug delivery. Carbohydr. Polym. 2019, 221, 94–112. [Google Scholar] [CrossRef]
- Reis, A.V.; Guilherme, M.R.; Cavalcanti, O.A.; Rubira, A.F.; Muniz, E.C. Synthesis and characterization of pH-responsive hydrogels based on chemically modified Arabic gum polysaccharide. Polymer 2006, 47, 2023–2029. [Google Scholar] [CrossRef]
- Agüero, L.; Zaldivar-Silva, D.; Peña, L.; Dias, M. Alginate microparticles as oral colon drug delivery device: A review. Carbohydr. Polym. 2017, 168, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, H.; Zhu, Z. Study on the blends of silk fibroin and sodium alginate: Hydrogen bond formation, structure and properties. Polymer 2019, 163, 144–153. [Google Scholar] [CrossRef]
- Yang, J.; He, W. Synthesis of lauryl grafted sodium alginate and optimization of the reaction conditions. Int. J. Biol. Macromol. 2012, 50, 428–431. [Google Scholar] [CrossRef] [PubMed]
- Patel, G.M.; Patel, C.P.; Trivedi, H.C. Ceric-induced grafting of methyl acrylate onto sodium salt of partially carboxymethylated sodium alginate. Eur. Polym. J. 1999, 35, 201–208. [Google Scholar] [CrossRef]
- Trivedi, J.H.; Chourasia, A.V.; Trivedi, H.C. Photo-induced synthesis and characterization of poly(methyl acrylate) grafted sodium salt of partially carboxymethylated sodium alginate. Cellul. Chem. Technol. 2015, 49, 7–19. [Google Scholar]
- Liu, Y.; Li, Y.; Yang, L.; Liu, Y.; Bai, L. Graft copolymerization of methyl acrylate onto sodium alginate initiated by potassium diperiodatocuprate (III). Iran. Polym. J. 2005, 14, 457–463. [Google Scholar] [CrossRef]
- Nizam El-Din, H.M.; Abou Taleb, M.F.; El-Naggar, A.W.M. Metal sorption and swelling characters of acrylic acid and sodium alginate based hydrogels synthesized by gamma irradiation. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms. 2008, 266, 2607–2613. [Google Scholar] [CrossRef]
- Bhattacharyya, R.; Ray, S.K. Adsorption of industrial dyes by semi-IPN hydrogels of Acrylic copolymers and sodium alginate. J. Ind. Eng. Chem. 2015, 22, 92–102. [Google Scholar] [CrossRef]
- Hua, S.; Wang, A. Synthesis, characterization and swelling behaviors of sodium alginate-g-poly(acrylic acid)/sodium humate superabsorbent. Carbohydr. Polym. 2009, 75, 79–84. [Google Scholar] [CrossRef]
- Del Real, A.; Wallander, D.; Maciel, A.; Cedillo, G.; Loza, H. Graft copolymerization of ethyl acrylate onto tamarind kernel powder, and evaluation of its biodegradability. Carbohydr. Polym. 2015, 117, 11–18. [Google Scholar] [CrossRef]
- Zampano, G.; Bertoldo, M.; Bronco, S. Poly(ethyl acrylate) surface-initiated ATRP grafting from wood pulp cellulose fibers. Carbohydr. Polym. 2009, 75, 22–31. [Google Scholar] [CrossRef]
- Laurienzo, P.; Malinconico, M.; Mattia, G.; Russo, R.; La Rotonda, M.I.; Quaglia, F.; Capitani, D.; Mannina, L. Novel alginate–acrylic polymers as a platform for drug delivery. J. Biomed. Mater. Res. Part A 2006, 78A, 523–531. [Google Scholar] [CrossRef]
- Khalid, I.; Ahmad, M.; Usman Minhas, M.; Barkat, K.; Sohail, M. Cross-Linked Sodium Alginate-g-poly(Acrylic Acid) Structure: A Potential Hydrogel Network for Controlled Delivery of Loxoprofen Sodium. Adv. Polym. Technol. 2018, 37, 985–995. [Google Scholar] [CrossRef]
- Mandal, B.; Ray, S.K. Synthesis of interpenetrating network hydrogel from poly(acrylic acid-co-hydroxyethyl methacrylate) and sodium alginate: Modeling and kinetics study for removal of synthetic dyes from water. Carbohydr. Polym. 2013, 98, 257–269. [Google Scholar] [CrossRef]
- Kumar, D.; Kumar verma, D.; Fatma, I. Studies in the Graft Copolymerization of Acrylic Acid onto Plantago Psyllium Mucilage. IOSR J. Appl. Chem. 2014, 7, 1–4. [Google Scholar] [CrossRef]
- Işiklan, N.; Kurşun, F.; Inal, M. Graft copolymerization of itaconic acid onto sodium alginate using benzoyl peroxide. Carbohydr. Polym. 2010, 79, 665–672. [Google Scholar] [CrossRef]
- Işiklan, N.; Kurşun, F. Synthesis and characterization of graft copolymer of sodium alginate and poly(itaconic acid) by the redox system. Polym. Bull. 2013, 70, 1065–1084. [Google Scholar] [CrossRef]
- Mohamed, S.F.; Mahmoud, G.A.; Taleb, M.F.A. Synthesis and characterization of poly(acrylic acid)-g-sodium alginate hydrogel initiated by gamma irradiation for controlled release of chlortetracycline HCl. Monatshefte Chem. 2013, 144, 129–137. [Google Scholar] [CrossRef]
- Mao, X.; Wang, L.; Gu, S.; Duan, Y.; Zhu, Y.; Wang, X.; Lichtfouse, E.; Wang, C. Synthesis of a three-dimensional network sodium alginate-poly(acrylic acid)/attapulgite hydrogel with good mechanic property and reusability for efficient adsorption of Cu2+ and Pb2+. Environ. Chem. Lett. 2018, 16, 653–658. [Google Scholar] [CrossRef]
- Shah, S.B.; Patel, C.P.; Trivedi, H.C. Ceric-induced grafting of ethyl-acrylate onto sodium alginate. Angew. Makromol. Chem. 1994, 214, 75–89. [Google Scholar] [CrossRef]
- ISO. International Standard Plastics—Evaluation of the Action of Microorganisms; ISO 846:2019, 36; International Organization for Standardization: London, UK, 2019. [Google Scholar]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed.; John Wiley & Sons: Chichester, UK, 2002; ISBN 9780471852988. [Google Scholar]
- Subramanian, V.; Ganapathy, K.; Dakshinamoorthy, B. FT-IR, 1 H-NMR and 13 C-NMR Spectroscopy of alginate extracted from Tubinaria decurrens (Phaeophyta). World J. Pharm. Pharm. Sci. 2015, 4, 761–771. [Google Scholar]
- Yang, J.S.; Xie, Y.J.; He, W. Research progress on chemical modification of alginate: A review. Carbohydr. Polym. 2011, 84, 33–39. [Google Scholar] [CrossRef]
- Cardenas-Jiron, G.; Leal, D.; Matsuhiro, B.; Osorio-Roman, I.O. Vibrational spectroscopy and density functional theory calculations of poly- D -mannuronate and heteropolymeric fractions from sodium alginate. J. Raman Spectrosc. 2011. [Google Scholar] [CrossRef]
- Bekin, S.; Sarmad, S.; Gürkan, K.; Keçeli, G.; Gürdaǧ, G. Synthesis, characterization and bending behavior of electroresponsive sodium alginate/poly(acrylic acid) interpenetrating network films under an electric field stimulus. Sens. Actuators B Chem. 2014, 202, 878–892. [Google Scholar] [CrossRef]
- Ford, J. Thermal Analysis—Fundamentals and Applications to Polymer Science, 2nd ed.; Hatakeyama, T., Quinn, F.X., Eds.; Wiley & Sons: Chichester, UK, 1999; ISBN 0-471-98362-4. [Google Scholar]
- Pérez-Garnes, M.; Monleón-Pradas, M. Poly(methacrylated hyaluronan-co-ethyl acrylate) copolymer networks with tunable properties and enzymatic degradation. Polym. Degrad. Stab. 2017, 144, 241–250. [Google Scholar] [CrossRef]
- Park, S.; Venditti, R.A.; Jameel, H.; Pawlak, J.J. Studies of the heat of vaporization of water associated with cellulose fibers characterized by thermal analysis. Cellulose 2007, 14, 195–204. [Google Scholar] [CrossRef]
- Soares, J.P.; Santos, J.E.; Chierice, G.O.; Cavalheiro, E.T.G. Thermal behavior of alginic acid and its sodium salt. Eclet. Quim. 2004, 29, 57–63. [Google Scholar] [CrossRef] [Green Version]
- Gill, P.; Moghadam, T.T.; Ranjbar, B. Differential scanning calorimetry techniques: Applications in biology and nanoscience. J. Biomol. Tech. 2010, 21, 167–193. [Google Scholar]
- Dechun, Z. Chemical and photophysical properties of materials for OLEDs. In Organic Light-Emitting Diodes (OLEDs): Materials, Devices and Applications; Elsevier Ltd.: Amsterdam, The Netherlands, 2013; pp. 114–142. ISBN 9780857094254. [Google Scholar]
- Safranski, D.L.; Griffis, J.C. Applications of Shape-Memory Polymers, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 9780323377973. [Google Scholar]
- Parker, M.J. Test Methods for Physical Properties. In Comprehensive Composite Materials; Elsevier: Amsterdam, The Netherlands, 2000; pp. 183–226. [Google Scholar]
- Liu, Y.; Bhandari, B.; Zhou, W. Glass transition and enthalpy relaxation of amorphous food saccharides: A review. J. Agric. Food Chem. 2006, 54, 5701–5717. [Google Scholar] [CrossRef] [PubMed]
- Ratnayake, W.S.; Otani, C.; Jackson, D.S. DSC enthalpic transitions during starch gelatinisation in excess water, dilute sodium chloride and dilute sucrose solutions. J. Sci. Food Agric. 2009, 89, 2156–2164. [Google Scholar] [CrossRef]
- Bean, S.R.; Zhu, L.; Smith, B.M.; Wilson, J.D.; Ioerger, B.P.; Tilley, M. Starch and Protein Chemistry and Functional Properties. In Sorghum and Millets; Elsevier: Amsterdam, The Netherlands, 2019; pp. 131–170. [Google Scholar]
- Schmidt, S.J. Water and Solids Mobility in Foods. Adv. Food Nutr. Res. 2004, 48, 1–101. [Google Scholar] [CrossRef] [PubMed]
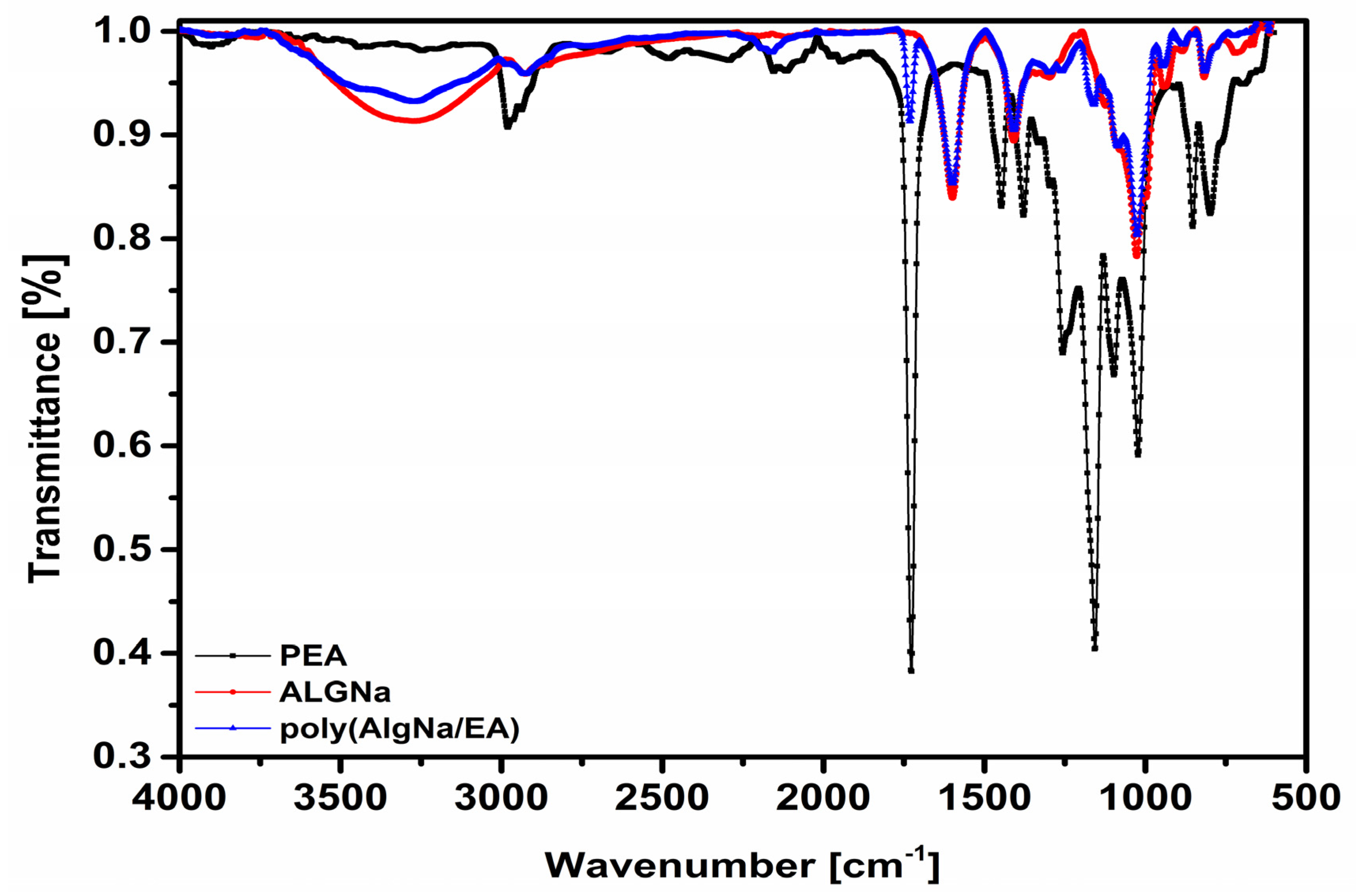
| AlgNa | PEA | poly (AlgNa/EA) | Assignment |
|---|---|---|---|
| 3275 | 3271 | O–H stretching | |
| 2925 | 2925 | C–H stretching of the pyranose ring | |
| 2979 | -------- | Symmetric stretching C–H of CH3 of Ethyl ester | |
| 2964 | -------- | Asymmetric stretching C–H of CH3 of Ethyl ester | |
| 2937 | -------- | Asymmetric stretching C–H of CH2 of Ethyl ester | |
| 2190 | 2190 | Stretching C☰N nitrile of initiator | |
| 1726 | 1732 | stretching O=C of ester | |
| 1598 | 1598 | Asymmetric stretching C–O of COONa | |
| 1469 | ------ | Asymmetric stretching C–H of CH2 of ethyl Deformation vibration of the CH2 ethyl ester | |
| 1446 | ------ | Asymmetric stretching CH3 of ethyl Symmetric stretching CH2 esters acrylic | |
| 1409 | 1386 | Symmetric stretching C–O of the COO− | |
| 1379 | ------ | Symmetric stretching CH3 of Ethyl | |
| 1332 | ------- | Twisting CH2 of the ethyl group | |
| 1257 | 1261 | Asymmetric stretching O=C of R–CO–OR | |
| 1157 | 1161 | Symmetric stretching C=O of R–CO–OR | |
| 1097 | ------ | Stretching of C–O–C of saturated esters | |
| 1027 | 1022 | 1026 | Stretching C–O of C–O–C carbohydrate and ester |
| 945 | 944 | Symmetric stretching C–O and/or symmetric stretching C–C–H pyranose ring | |
| 883 | 881 | Symmetric stretching vibration of C–O–C of 1,4 glycosidic links, characteristic of polysaccharides structure ring | |
| 852 | ------ | Stretching C–C of ethyl | |
| 817 | 815 | δ C–O–C guluronic and mannuronic acid unit | |
| 761 | ------ | CH2 rocking |
| Nomenclature | Elastic Modulus (MPa) | Tensile Strength (MPa) |
|---|---|---|
| AlgNa | 3226 | 93.26 |
| PEA | 0.006 | 0.585 |
| Poly(AlgNa/EA) | 2436 | 97.92 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores-Hernández, C.G.; Cornejo-Villegas, M.d.l.A.; Moreno-Martell, A.; Del Real, A. Synthesis of a Biodegradable Polymer of Poly (Sodium Alginate/Ethyl Acrylate). Polymers 2021, 13, 504. https://doi.org/10.3390/polym13040504
Flores-Hernández CG, Cornejo-Villegas MdlA, Moreno-Martell A, Del Real A. Synthesis of a Biodegradable Polymer of Poly (Sodium Alginate/Ethyl Acrylate). Polymers. 2021; 13(4):504. https://doi.org/10.3390/polym13040504
Chicago/Turabian StyleFlores-Hernández, Cynthia G., Maria de los Angeles Cornejo-Villegas, Abigail Moreno-Martell, and Alicia Del Real. 2021. "Synthesis of a Biodegradable Polymer of Poly (Sodium Alginate/Ethyl Acrylate)" Polymers 13, no. 4: 504. https://doi.org/10.3390/polym13040504
APA StyleFlores-Hernández, C. G., Cornejo-Villegas, M. d. l. A., Moreno-Martell, A., & Del Real, A. (2021). Synthesis of a Biodegradable Polymer of Poly (Sodium Alginate/Ethyl Acrylate). Polymers, 13(4), 504. https://doi.org/10.3390/polym13040504