Solvent-Free Synthesis of Iron-Based Metal-Organic Frameworks (MOFs) as Slow-Release Fertilizers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of MOFs
2.3. Element Composition
2.4. Spectral Characterization
2.5. Principal Component Analysis
2.6. Nutrient Release Behavior
3. Results
3.1. Elemental Analysis
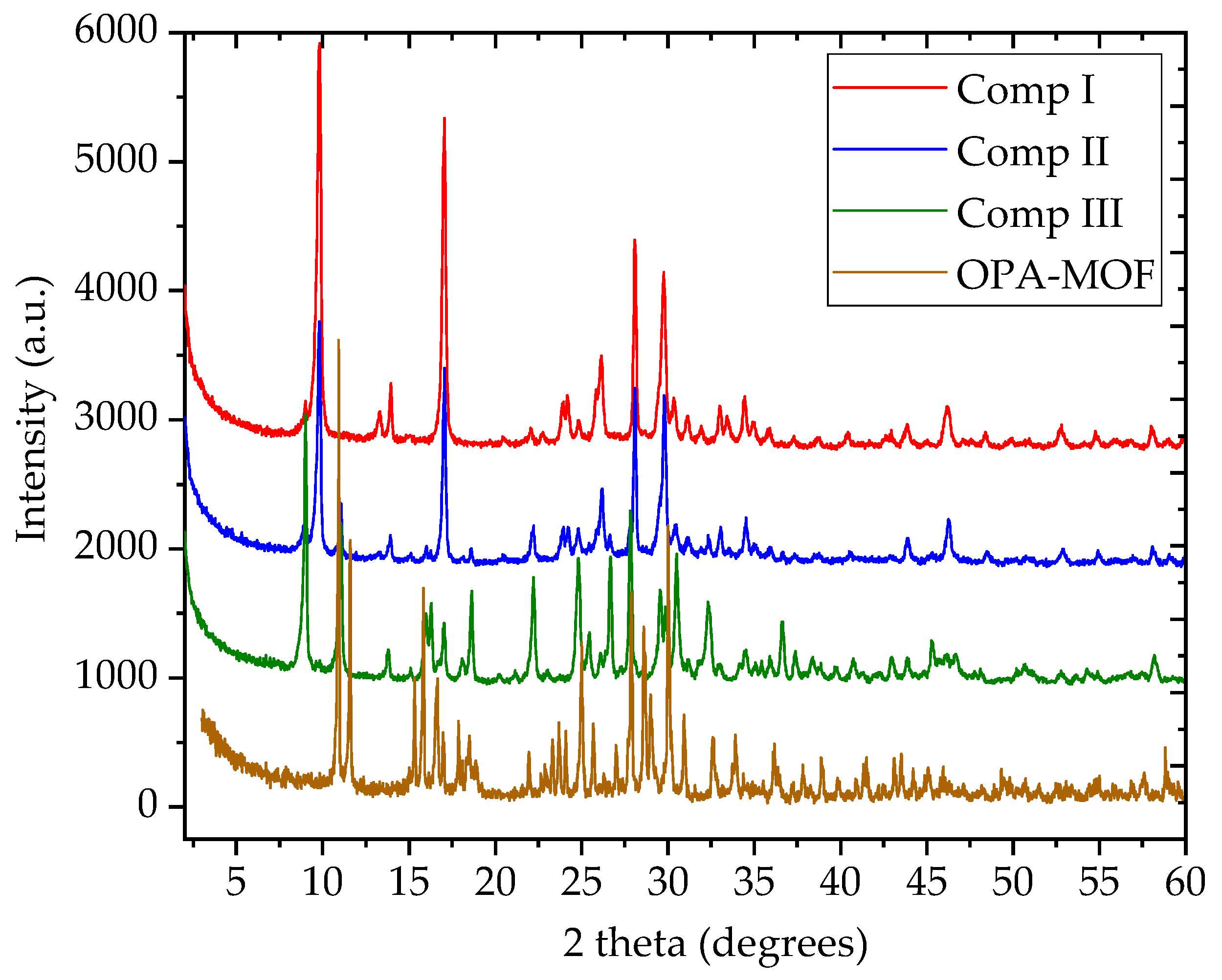
3.2. X-ray Powder Diffraction
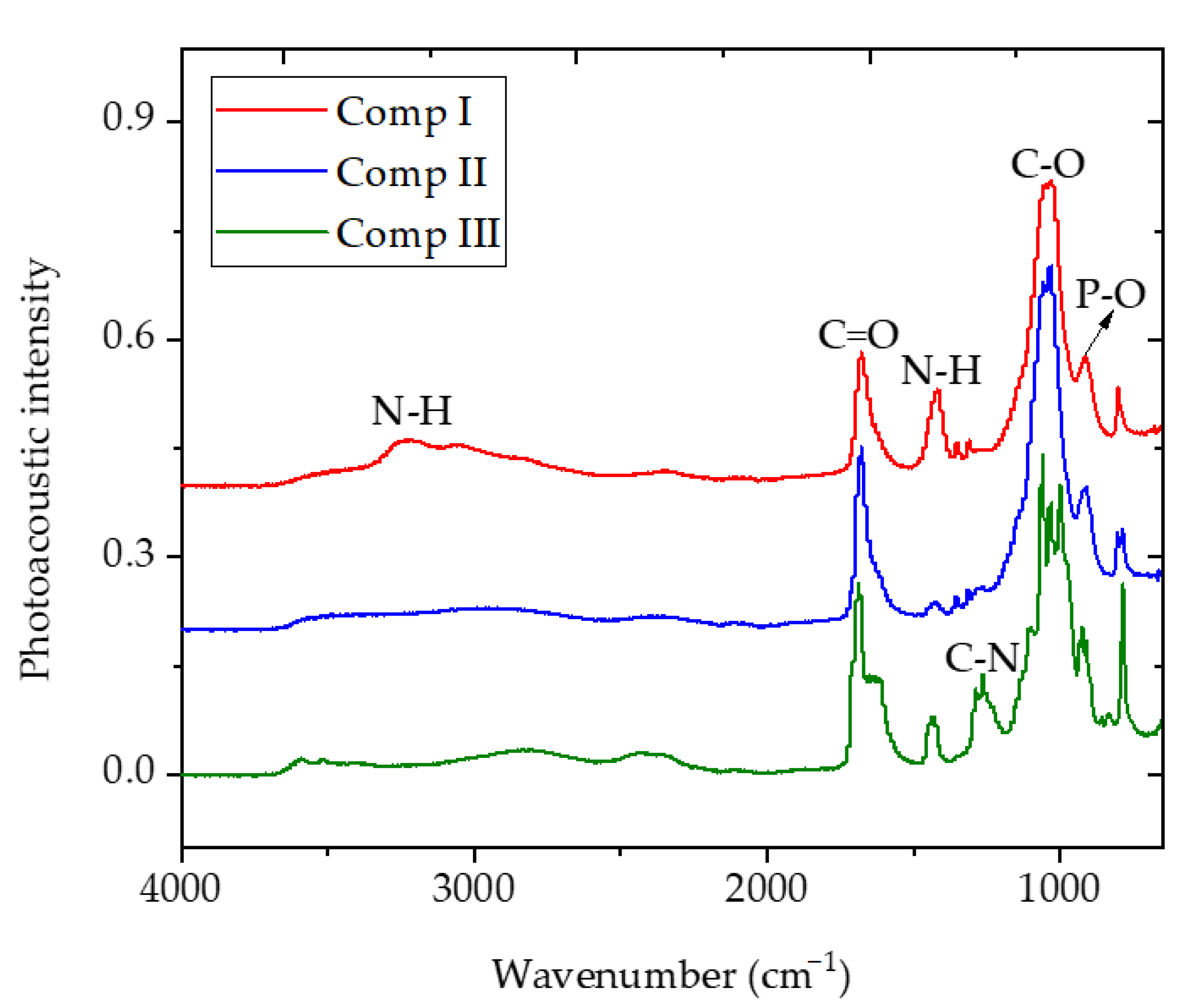
3.3. FTIR-ATR Characterization
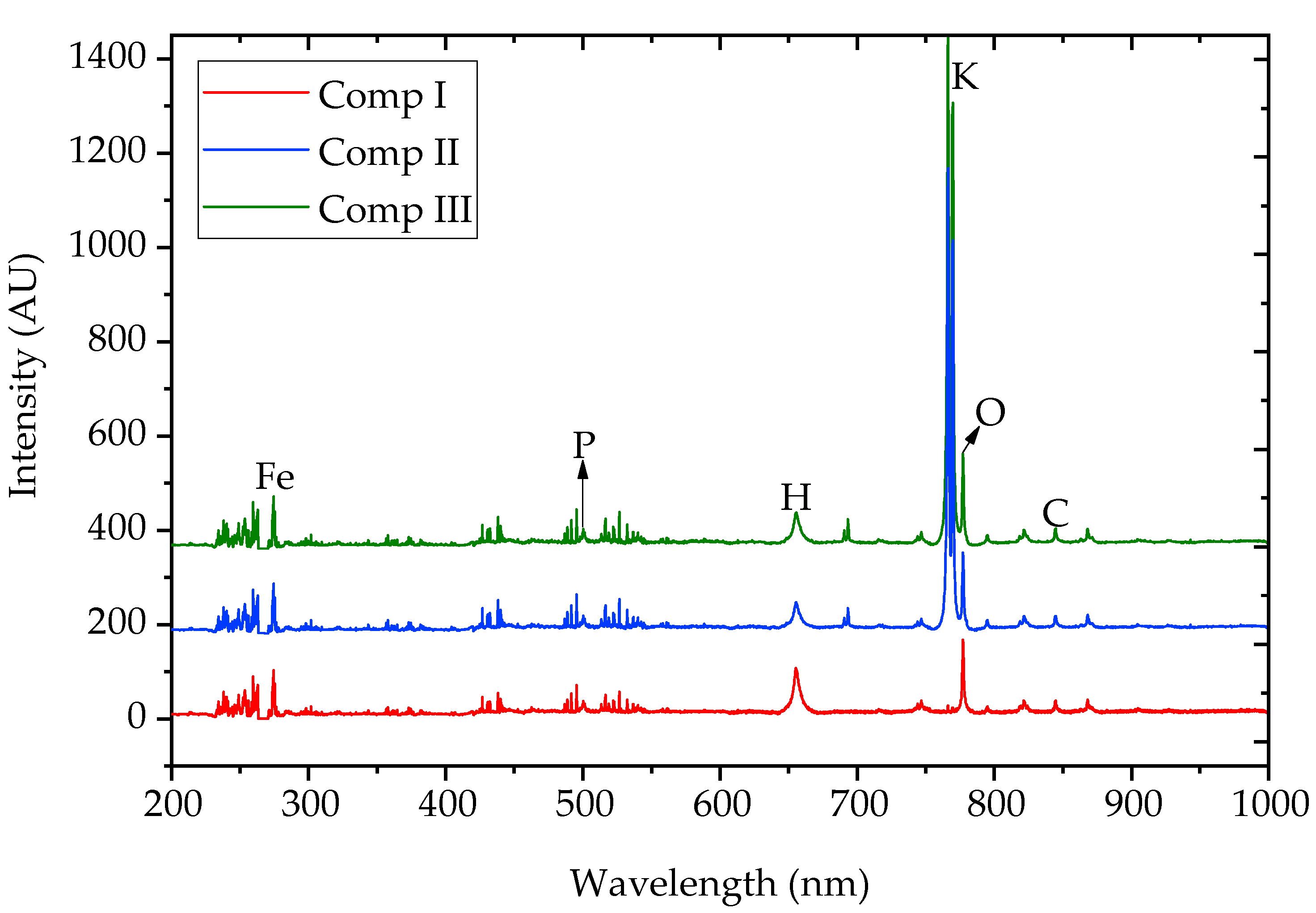
3.4. LIBS Characterization
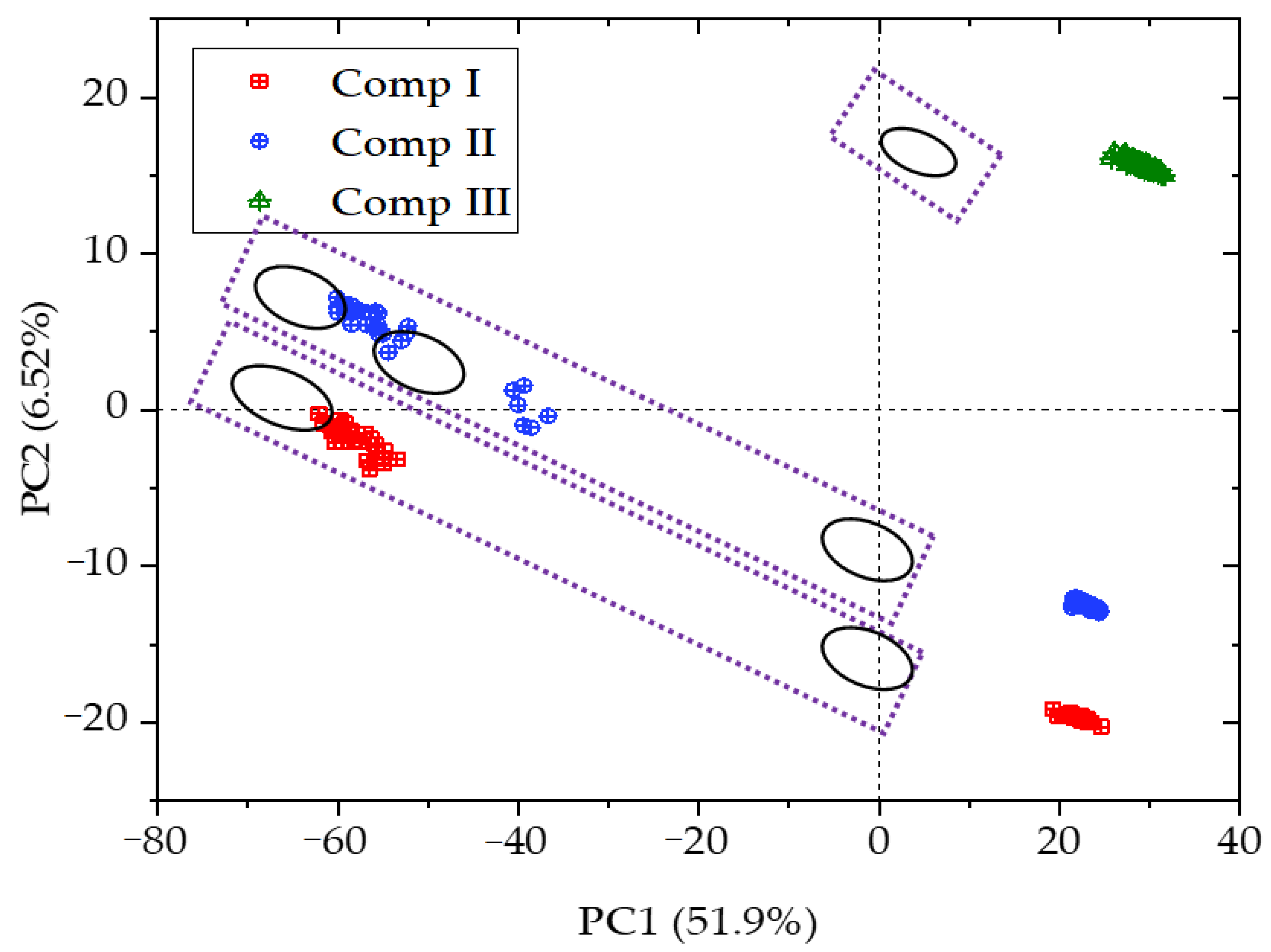
3.5. Principal Component Analysis of Fused Spectra
3.6. Nutrient Release Profile
4. Discussion
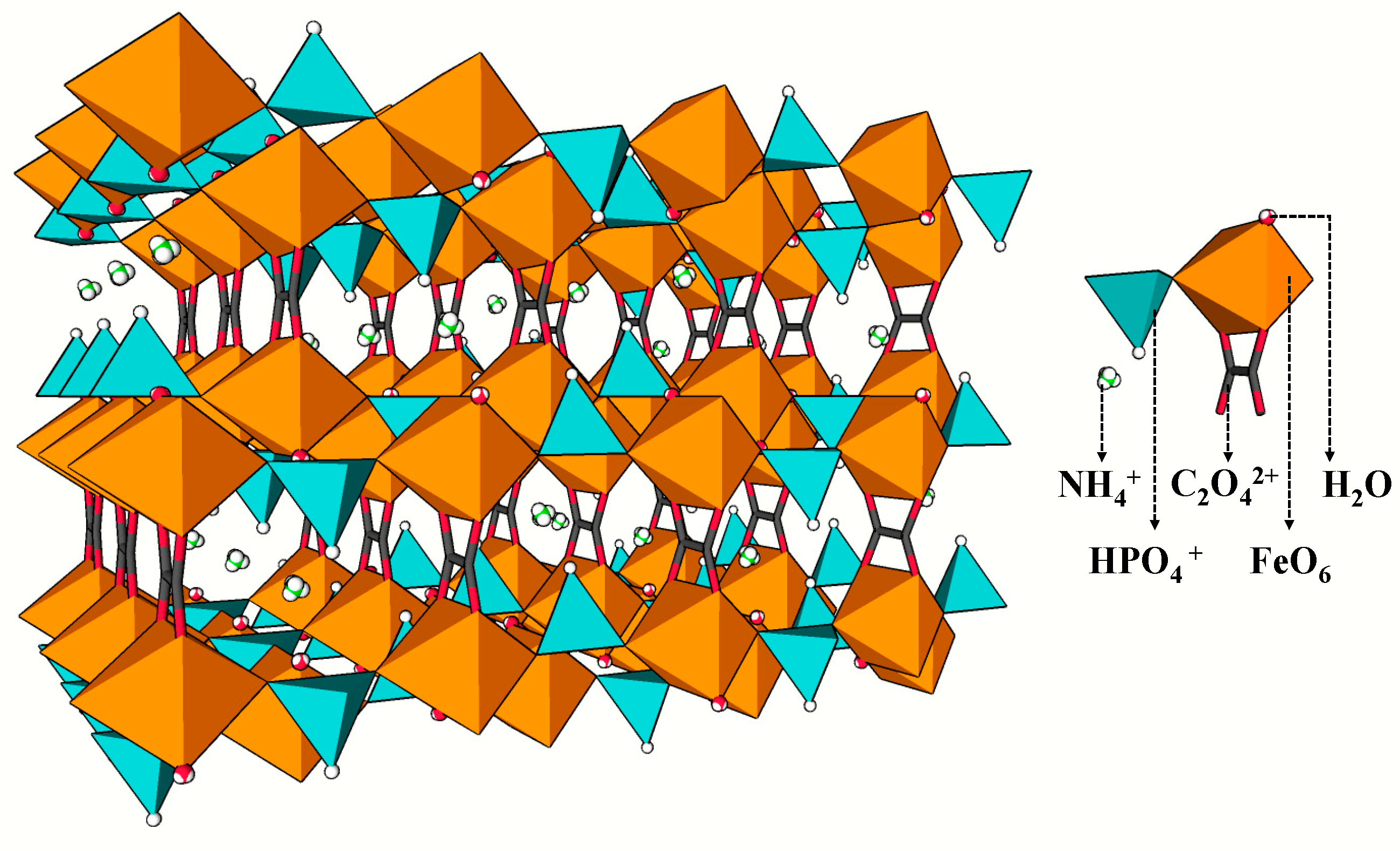
4.1. Structure of MOFs
4.2. MOFs as Fertilizers
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mikula, K.; Izydorczyk, G.; Skrzypczak, D.; Mironiuk, M.; Moustakas, K.; Witek-Krowiak, A.; Chojnacka, K. Controlled release micronutrient fertilizers for precision agriculture—A review. Sci. Total Environ. 2020, 712, 136365. [Google Scholar] [CrossRef]
- Hao, T.; Zhu, Q.; Zeng, M.; Shen, J.; Shi, X.; Liu, X.; Zhang, F.; de Vries, W. Impacts of nitrogen fertilizer type and application rate on soil acidification rate under a wheat-maize double cropping system. J. Environ. Manag. 2020, 270, 110888. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lü, S.; Zhang, Z.; Zhao, X.; Li, X.; Ning, P.; Liu, M. Environmentally friendly fertilizers: A review of materials used and their effects on the environment. Sci. Total Environ. 2018, 613–614, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Trenkel, M.E. Improving Fertilizer Use Efficiency Controlled-Release and Stabilized Fertilizers in Agriculture. Int. Fertil. Ind. Assoc. Paris 1997, 53–102. [Google Scholar]
- Jiao, L.; Seow, J.Y.R.; Skinner, W.S.; Wang, Z.U.; Jiang, H.L. Metal-organic frameworks: Structures and functional applications. Mater. Today 2019, 27, 43–68. [Google Scholar] [CrossRef]
- Anstoetz, M.; Rose, T.; Clark, M.; Yee, L.; Raymond, C.; Vancov, T. Novel Applications for Oxalate-Phosphate-Amine Metal-Organic-Frameworks (OPA-MOFs): Can an Iron-Based OPA-MOF Be Used as Slow-Release Fertilizer? PLoS ONE 2015, 10, e0144169. [Google Scholar] [CrossRef]
- Anstoetz, M. Synthesis Optimisation, Characterisation and Evaluation of an Iron-Based Oxalate-Phosphate-Amine MOF (OPA-MOF) for Innovative Application in Agriculture. Ph.D. Thesis, Southern Cross University, Lismore, NSW, Australia, 2016. [Google Scholar]
- Anstoetz, M.; Clark, M.; Yee, L. Response Surface Optimisation of an Oxalate–Phosphate–Amine Metal-organic Framework (OPA-MOF) of Iron and Urea. J. Inorg. Organomet. Polym. Mater. 2017, 27, 996–1013. [Google Scholar] [CrossRef]
- Wu, K.; Du, C.; Shen, Y.; Ma, F. Exploration of metal-organic framework (MOF) material as a new type of fertilizer applied in rice. J. Plant Nutr. Fertil. 2019, 25, 2170–2177. [Google Scholar]
- Wu, K.; Du, C.; Ma, F.; Shen, Y.; Dong, L.; Zhou, J. Degradation of Metal-Organic Framework Materials as Controlled-Release Fertilizers in Crop Fields. Polymers 2019, 11, 947. [Google Scholar] [CrossRef]
- Do, J.L.; Friscic, T. Mechanochemistry: A Force of Synthesis. ACS Cent. Sci. 2016, 3. [Google Scholar] [CrossRef]
- Tan, D.; Garcia, F. Main group mechanochemistry: From curiosity to established protocols. Chem. Soc. Rev. 2019, 48. [Google Scholar] [CrossRef]
- Tanaka, S. Chapter 10—Mechanochemical synthesis of MOFs. In Metal-Organic Frameworks for Biomedical Applications; Mozafari, M., Ed.; Woodhead Publishing: Cambridge, UK, 2020; pp. 197–222. [Google Scholar] [CrossRef]
- Al Amery, N.; Abid, H.R.; Al-Saadi, S.; Wang, S.; Liu, S. Facile directions for synthesis, modification and activation of MOFs. Mater. Today Chem. 2020, 17, 100343. [Google Scholar] [CrossRef]
- Pichon, A.; Lazuen, A.; James, S. Solvent-free synthesis of a microporous metal-organic framework. CrystEngComm 2006, 8, 211–214. [Google Scholar] [CrossRef]
- Chaturvedi, G.; Kaur, A.; Umar, A.; Khan, M.A.; Algarni, H.; Kansal, S.K. Removal of fluoroquinolone drug, levofloxacin, from aqueous phase over iron based MOFs, MIL-100(Fe). J. Solid State Chem. 2020, 281, 121029. [Google Scholar] [CrossRef]
- Tella, A.C.; Oladipo, A.C.; Adeyemi, O.G.; Oluwafemi, O.S.; Oguntoye, S.O.; Alimi, L.O.; Ajayi, J.T.; Degni, S.K. Solid state synthesis, spectroscopic and X-ray studies of metal complexes of 2-picolinic acid and vapochromic behavior of [Co(Pic)2(H2O)2]·2H2O. Solid State Sci. 2017, 68, 1–9. [Google Scholar] [CrossRef]
- Nadizadeh, Z.; Naimi-Jamal, M.R.; Panahi, L. Mechanochemical solvent-free in situ synthesis of drug-loaded {Cu2(1,4-bdc)2(dabco)}n MOFs for controlled drug delivery. J. Solid State Chem. 2018, 259, 35–42. [Google Scholar] [CrossRef]
- Srinivasan, B.R.; Shetgaonkar, S.Y.; Näther, C.; Bensch, W. Solid state synthesis and characterization of a triple chain calcium(II) coordination polymer showing two different bridging 4-nitrobenzoate coordination modes. Polyhedron 2009, 28, 534–540. [Google Scholar] [CrossRef]
- Singh, N.K.; Gupta, S.; Pecharsky, V.K.; Balema, V.P. Solvent-free mechanochemical synthesis and magnetic properties of rare-earth based metal-organic frameworks. J. Alloys Compd. 2017, 696, 118–122. [Google Scholar] [CrossRef]
- Wu, K.; Du, C.; Ma, F.; Shen, Y.; Zhou, J. Optimization of metal-organic (citric acid) frameworks for controlled release of nutrients. RSC Adv. 2019, 9, 32270–32277. [Google Scholar] [CrossRef]
- Usman, K.; Buenviaje, S.; Edañol, Y.; Conato, M.; Payawan, L. Facile Fabrication of a Potential Slow-Release Fertilizer Based on Oxalate-Phosphate-Amine Metal-Organic Frameworks (OPA-MOFs). Mater. Sci. Forum 2018, 936, 14–19. [Google Scholar] [CrossRef]
- Wu, K. Synthesis and Application of Controlled Release Fertilizers Based on Metal Organic Frameworks (MOFs). Ph.D. Thesis, University of Chinese Academy of Sciences, Peking, China, 2020. [Google Scholar]
- Yang, X.; Li, J.; Hou, Y.; Shi, S.; Shan, Y. K2Fe(C2O4)(HPO4)(OH2)·H2O: A layered oxalatophosphate hybrid material. Inorg. Chim. Acta 2008, 361, 1510–1514. [Google Scholar] [CrossRef]
- Lin, H.M.; Li, K.H.; Jiang, Y.C.; Wang, S.L. Organically templated inorganic/organic hybrid materials: Synthesis and structures of (C4H12N2)[FeII4(C2O4)3(HPO4)2] and (C5H14N2)[FeIII2(C2O4)(HPO4)3]. Chem. Mater. 1999, 11. [Google Scholar] [CrossRef]
- Meng, H.; Li, G.H.; Xing, Y.; Yang, Y.L.; Cui, Y.J.; Liu, L.; Ding, H.; Pang, W.Q. Hydrothermal synthesis and characterization of a new 3D iron phosphatooxalate templated by organo-amine. Polyhedron 2004, 23, 2357–2362. [Google Scholar] [CrossRef]
- Lethbridge, Z.; Clarkson, G.; Turner, S.; Walton, R. Polymorphism and variable structural dimensionality in the iron(III) phosphate oxalate system: A new polymorph of 3D [Fe2(HPO4)2(C2O4)(H2O)2]·2H2O and the layered material [Fe 2(HPO4)2(C2O4)(H2O)2]. Dalton Trans. 2009, 9176–9182. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Qi, Y.; Zhang, Y.; Liu, Z.Y.; Zhao, Y.F.; Liu, Z.M. Synthesis, structure and magnetic properties of a new iron phosphonate-oxalate with 3D framework: [Fe(O3PCH3)(C2O4)0.5(H2O)]. Mater. Res. Bull. 2007, 42, 1531–1538. [Google Scholar] [CrossRef]
- Rajic, N.; Stojakovic, D.; Hanzel, D.; Zabukovec Logar, N.; Kaucic, V. Preparation and characterization of iron(III) phosphate–oxalate using 1,2-diaminopropane as the structure-directing agent. Microporous Mesoporous Mater. 2002, 55, 313–319. [Google Scholar] [CrossRef]
- Kizewski, F.; Boyle, P.; Hesterberg, D.; Martin, J. Mixed Anion (Phosphate/Oxalate) Bonding to Iron(III) Materials. J. Am. Chem. Soc. 2010, 132, 2301–2308. [Google Scholar] [CrossRef]
- Chang, W.J.; Lin, H.M.; Lii, K.H. Synthesis and characterization of new iron phosphatooxalates: [(S)-C5H14N2] [Fe4(C2O4)3(HPO4)2(H2O)2] and [(S)-C5H14N2] [Fe4(C2O4)3(HPO4)2]. J. Solid State Chem. 2001, 157, 233–239. [Google Scholar] [CrossRef]
- Huang, Z.W.; Hu, K.Q.; Mei, L.; Wang, C.Z.; Chen, Y.M.; Wu, W.S.; Chai, Z.F.; Shi, W.Q. Potassium ions induced framework interpenetration for enhancing the stability of uranium-based porphyrin mof with visible-light- driven photocatalytic activity. Inorg. Chem. 2021, 60, 651–659. [Google Scholar] [CrossRef]

| N | K | C | H | P | Fe | Yield | Nutrients | |
|---|---|---|---|---|---|---|---|---|
| Comp I | 4.91 | 0 | 4.53 | 3.60 | 15.71 | 18.60 | 33.21 | 40.89 |
| Comp II | 0 | 11.48 | 4.75 | 1.66 | 14.16 | 16.22 | 33.93 | 45.97 |
| Comp III | 0.11 | 12.59 | 6.52 | 2.05 | 11.76 | 15.45 | 33.79 | 41.78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, Y.; Xu, X.; Ma, F.; Du, C. Solvent-Free Synthesis of Iron-Based Metal-Organic Frameworks (MOFs) as Slow-Release Fertilizers. Polymers 2021, 13, 561. https://doi.org/10.3390/polym13040561
Du Y, Xu X, Ma F, Du C. Solvent-Free Synthesis of Iron-Based Metal-Organic Frameworks (MOFs) as Slow-Release Fertilizers. Polymers. 2021; 13(4):561. https://doi.org/10.3390/polym13040561
Chicago/Turabian StyleDu, Yaxiao, Xuebin Xu, Fei Ma, and Changwen Du. 2021. "Solvent-Free Synthesis of Iron-Based Metal-Organic Frameworks (MOFs) as Slow-Release Fertilizers" Polymers 13, no. 4: 561. https://doi.org/10.3390/polym13040561
APA StyleDu, Y., Xu, X., Ma, F., & Du, C. (2021). Solvent-Free Synthesis of Iron-Based Metal-Organic Frameworks (MOFs) as Slow-Release Fertilizers. Polymers, 13(4), 561. https://doi.org/10.3390/polym13040561







