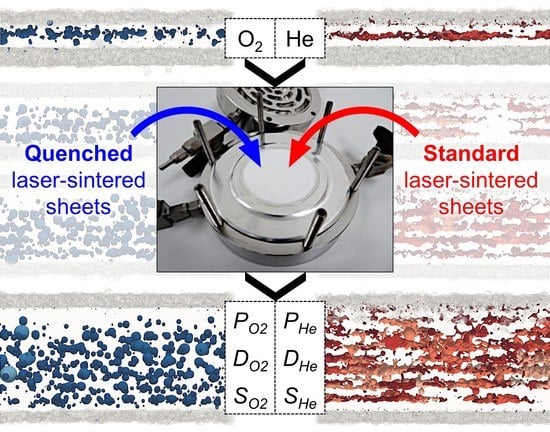
Thickness-Dependent Permeation Properties of Quenched and Standard Laser-Sintered Polyamide 12 Sheets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Production of Quenched and Standard Laser-Sintered Sheets
2.2. Analysis of Structural Properties
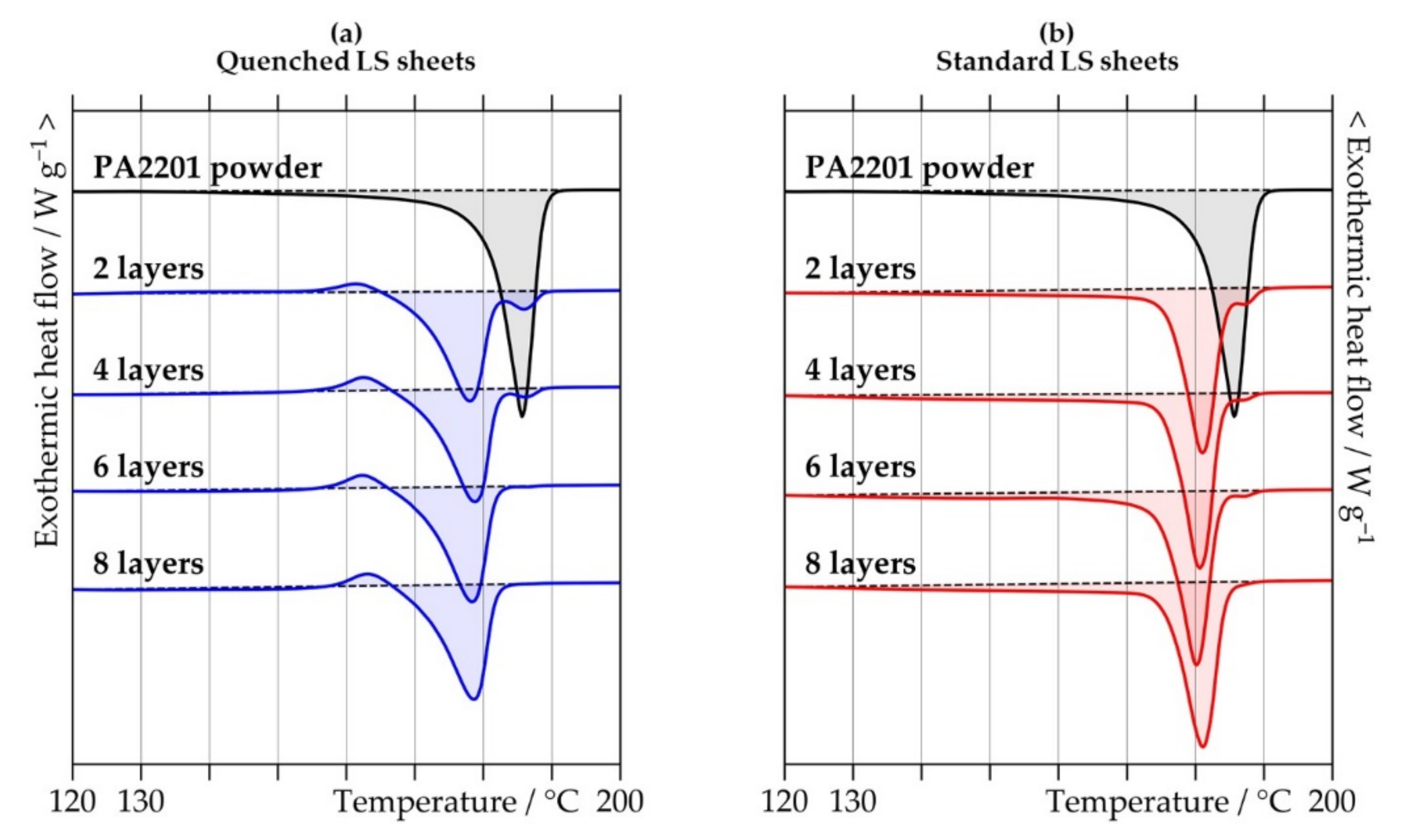
2.2.1. Differential Scanning Calorimetry
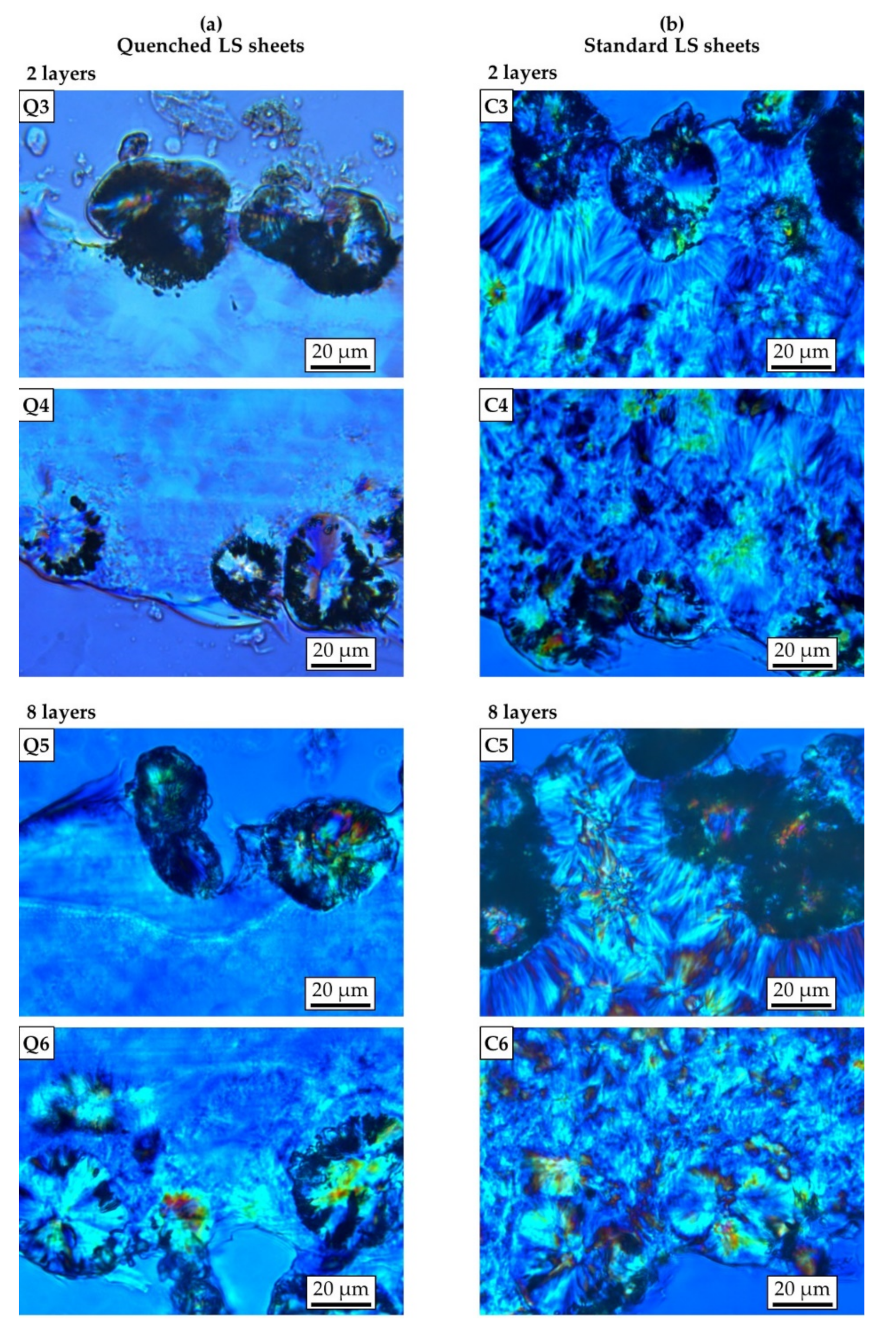
2.2.2. Light Microscopy
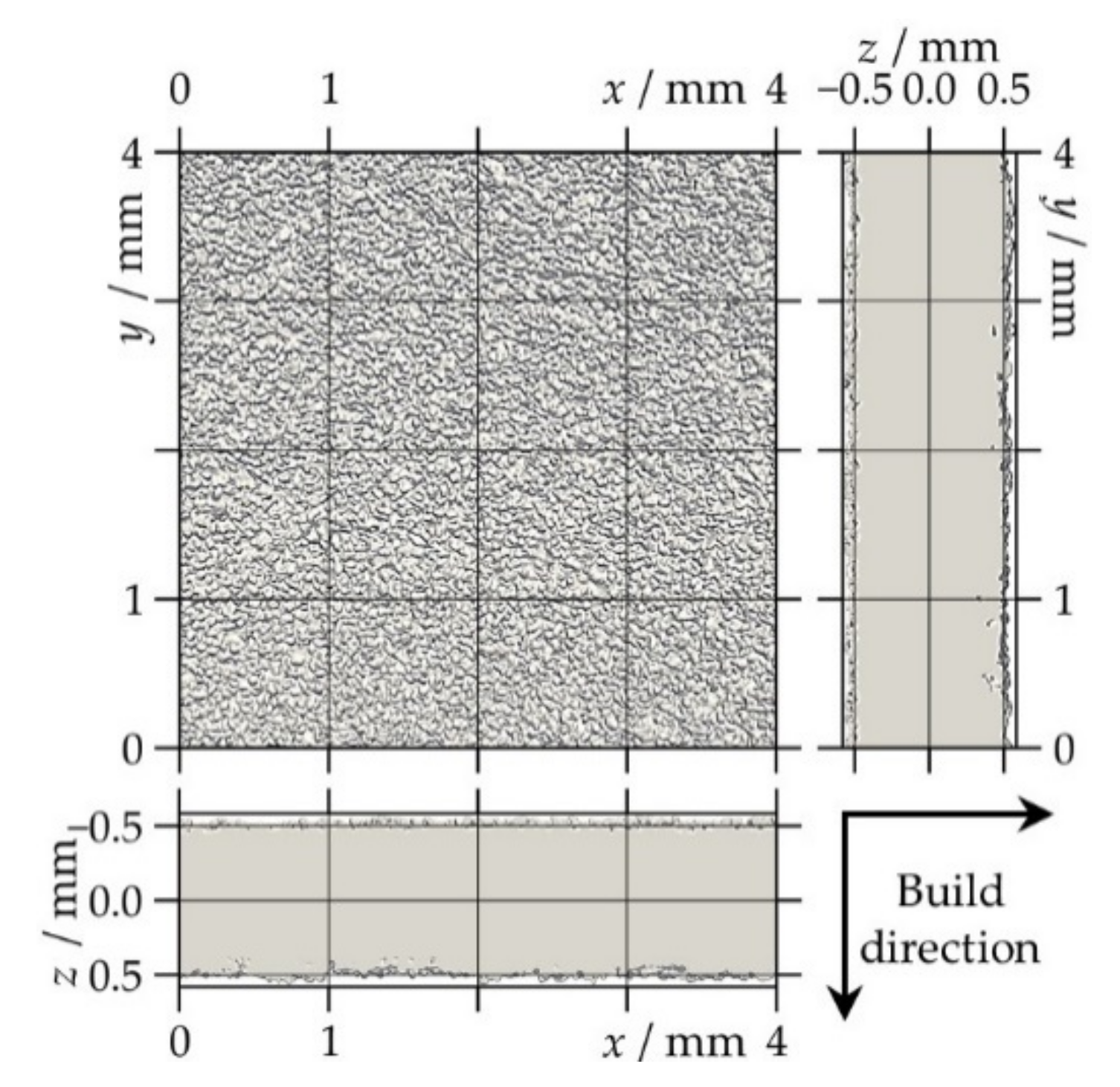
2.2.3. X-ray Micro-Computed Tomography
- Porosity: Porosity was measured as the fraction of pore volume over the total volume (=pore and solid volume).
- Pore number density: The pore number density was determined by dividing the number of disjoint pores by the total volume.
- Two-dimensional (2D) porosity: 2D porosity was defined as the fraction of pore volume over the total volume in one particular x–y slice of the VOI.
- Effective thickness: The effective thickness leff as used in the permeation analysis corresponds to the average value of the thickness distribution collected from the total volume in the z-direction.
2.3. Permeation Measurements
2.3.1. Sample Preparation
2.3.2. Oxygen Permeation Measurement
2.3.3. Helium Permeation Measurement
2.3.4. Evaluation of Permeation Measurements
3. Results and Discussion
3.1. Crystallinity and Crystalline Structure
3.2. Porosity and Pore Structure
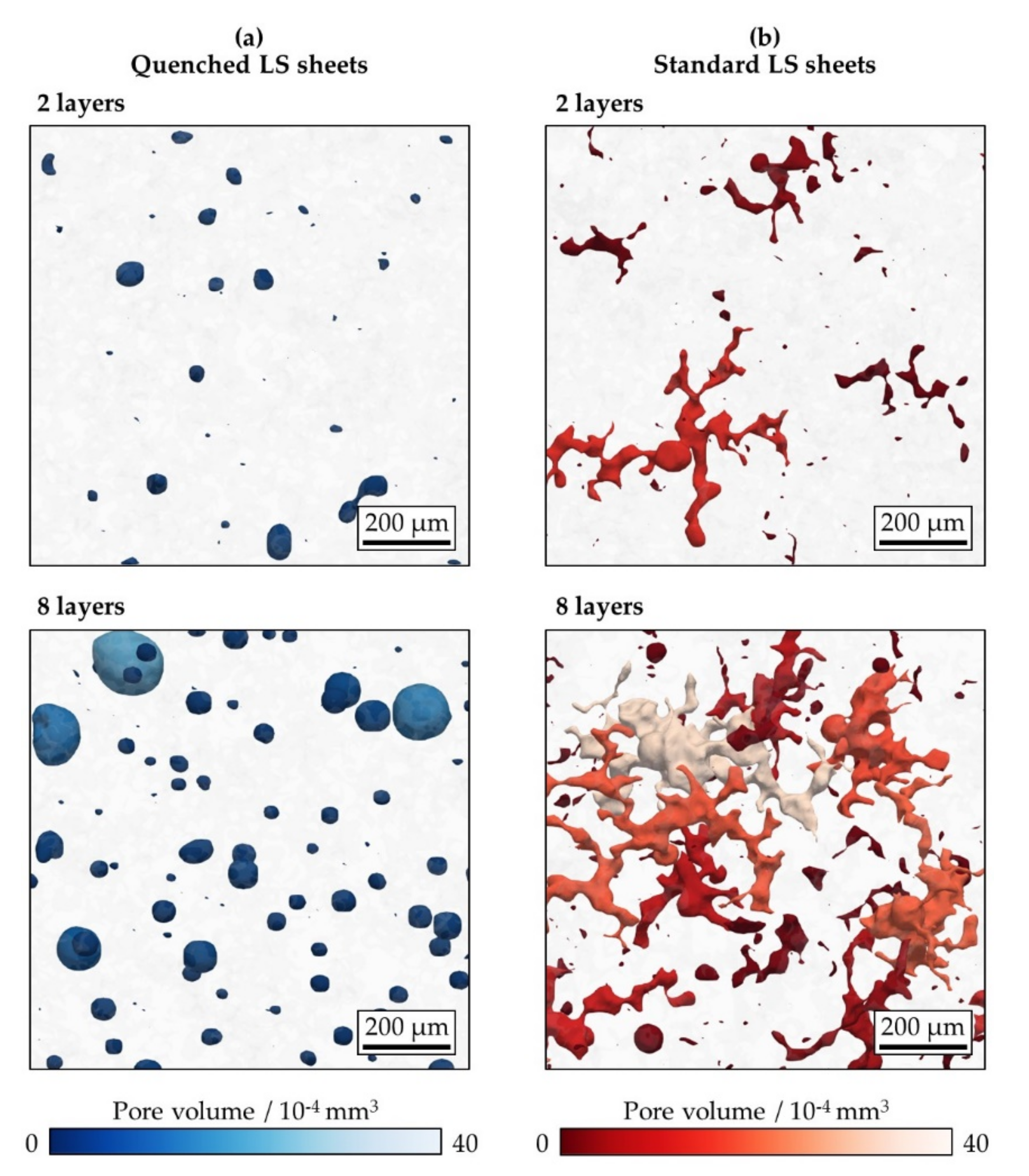
3.2.1. Overall Porosity and Pore Number Density
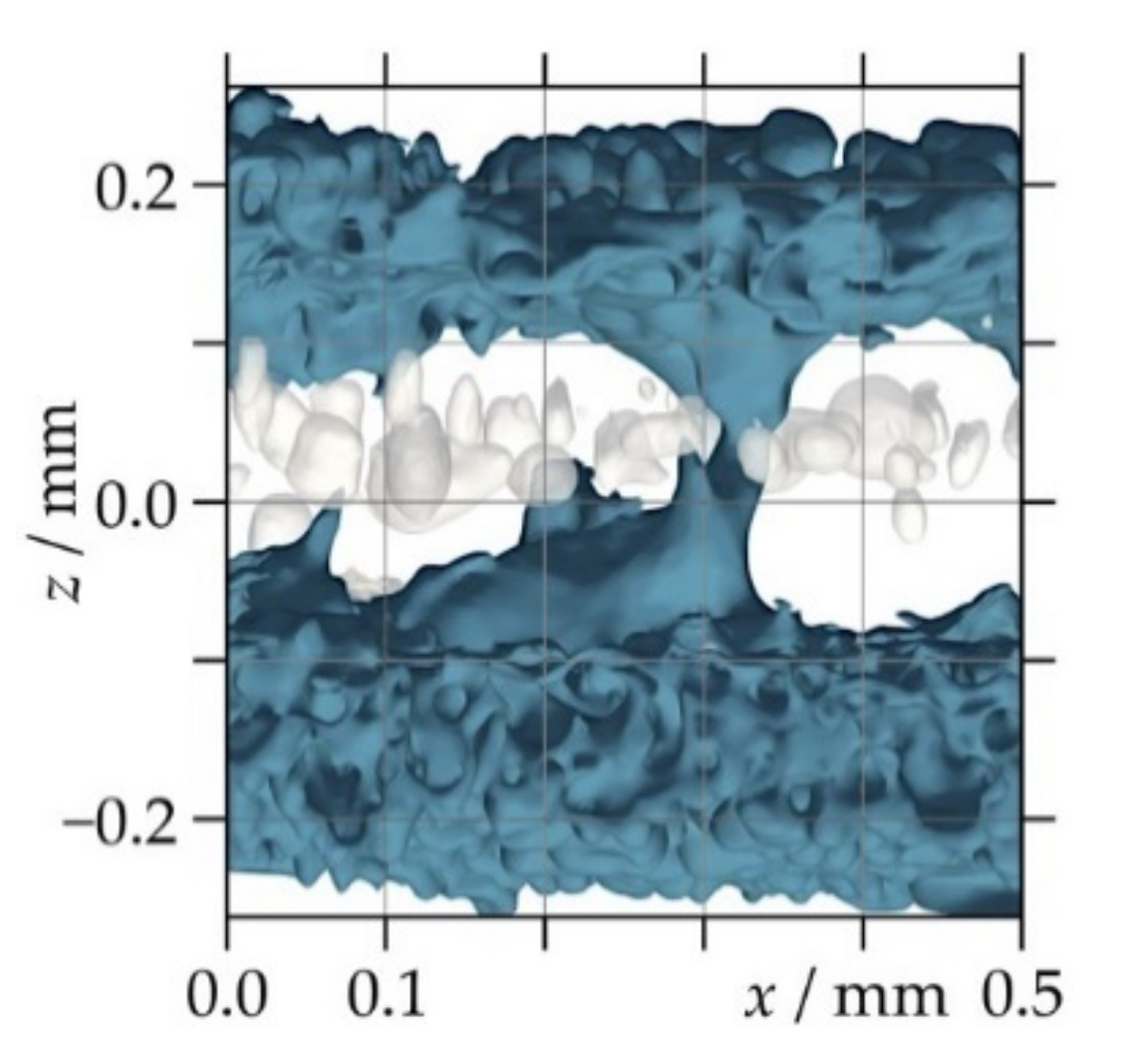
3.2.2. Pore Size and Morphology
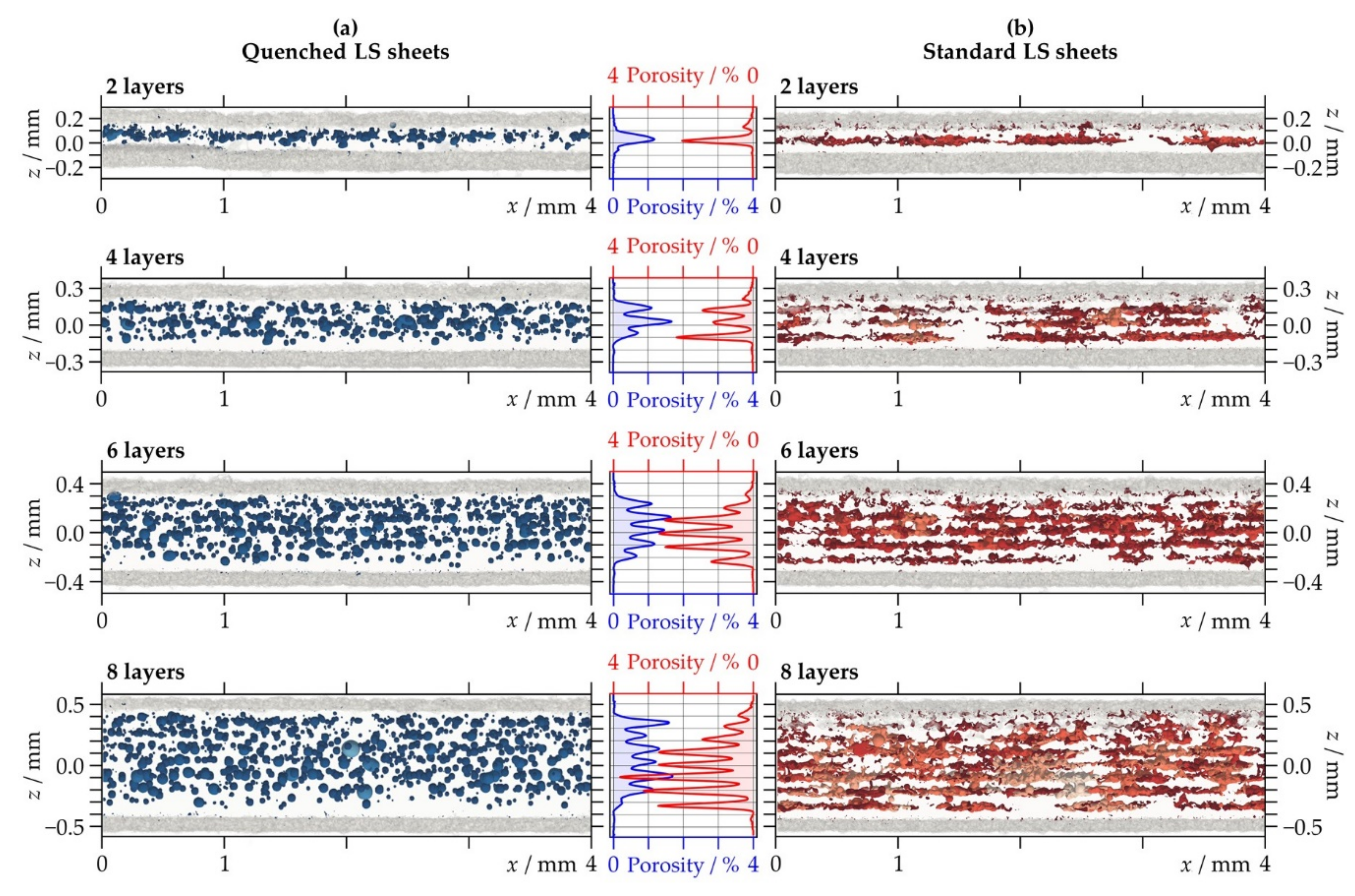
3.2.3. Porosity Distribution
3.3. Thickness Distribution and Effective Thickness
3.4. Permeation Properties
4. Conclusions
- Quenched and standard LS sheets showed a similar increase in diffusion coefficients with decreasing thickness. The main source for this behavior thus might be the high volume fraction of the border zones constituted by poorly sintered powder particles as well as incomplete melting and poor consolidation of the polymer and therefore low material integrity when producing very thin structures.
- The standard LS sheets showed a significant increase in solubility coefficients with increasing thickness. This suggested that the amount of absorbed gases could depend on the pore volume including additional nanometer-sized pores that are not detectable by light microscopy or CT.
- Opposite behavior of the diffusion and solubility coefficients, especially for the standard LS sheets, led to virtually unchanged values of the permeation coefficients for all thicknesses.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Berman, B. 3-D printing: The new industrial revolution. Bus. Horiz. 2012, 55, 155–162. [Google Scholar] [CrossRef]
- Attaran, M. The rise of 3-D printing: The advantages of additive manufacturing over traditional manufacturing. Bus. Horiz. 2017, 60, 677–688. [Google Scholar] [CrossRef]
- Tofail, S.A.; Koumoulos, E.P.; Bandyopadhyay, A.; Bose, S.; O’Donoghue, L.; Charitidis, C. Additive manufacturing: Scientific and technological challenges, market uptake and opportunities. Mater. Today 2018, 21, 22–37. [Google Scholar] [CrossRef]
- Gebhardt, A.; Kessler, J.; Thurn, L. 3D Printing: Understanding Additive Manufacturing; Hanser: Munich, Germany, 2018. [Google Scholar]
- Bai, Y.; Wagner, G.; Williams, C.B. Effect of Particle Size Distribution on Powder Packing and Sintering in Binder Jetting Additive Manufacturing of Metals. J. Manuf. Sci. Eng. 2017, 139, 081019. [Google Scholar] [CrossRef] [Green Version]
- Hwang, H.H.; Zhu, W.; Victorine, G.; Lawrence, N.; Chen, S. 3D-Printing of Functional Biomedical Microdevices via Light-and Extrusion-Based Approaches. Small Methods 2018, 2, 1700277. [Google Scholar] [CrossRef]
- Zeng, M.; Zhang, Y. Colloidal nanoparticle inks for printing functional devices: Emerging trends and future prospects. J. Mater. Chem. A 2019, 7, 23301–23336. [Google Scholar] [CrossRef]
- ISO/ASTM DIS 52900:2018, Additive Manufacturing—General Principles—Terminology; ISO: Geneva, Switzerland, 2018.
- Goodridge, R.; Tuck, C.; Hague, R. Laser sintering of polyamides and other polymers. Prog. Mater. Sci. 2012, 57, 229–267. [Google Scholar] [CrossRef]
- Schmid, M. Laser Sintering with Plastics: Technology, Processes, and Materials; Hanser: Munich, Germany, 2018. [Google Scholar]
- Wegner, A.; Witt, G. Design rules for laser sintering. J. Plast. Technol. 2012, 8, 253–277. [Google Scholar]
- Adam, G.A.O.; Zimmer, D. On design for additive manufacturing: Evaluating geometrical limitations. Rapid Prototyp. J. 2015, 21, 662–670. [Google Scholar] [CrossRef]
- Tasch, D.; Mad, A.; Stadlbauer, R.; Schagerl, M. Thickness dependency of mechanical properties of laser-sintered polyamide lightweight structures. Addit. Manuf. 2018, 23, 25–33. [Google Scholar] [CrossRef]
- Wörz, A.; Drummer, D. Understanding hatch-dependent part properties in SLS. In Proceedings of the 29th Annual International Solid Freeform Fabrication Symposium—An Additive Manufacturing Conference, Austin, TX, USA, 13–15 August 2018; pp. 1360–1369. [Google Scholar]
- Wörz, A.; Wudy, K.; Drummer, D. Understanding the influence of energy-density on the layer dependent part properties in laser-sintering of PA12. In Proceedings of the 30th Annual International Solid Freeform Fabrication Symposium—An Additive Manufacturing Conference, Austin, TX, USA, 12–14 August 2019; pp. 835–842. [Google Scholar]
- Sindinger, S.-L.; Kralovec, C.; Tasch, D.; Schagerl, M. Thickness dependent anisotropy of mechanical properties and inhomogeneous porosity characteristics in laser-sintered polyamide 12 specimens. Addit. Manuf. 2020, 33, 101141. [Google Scholar] [CrossRef]
- Liebrich, A.; Langowski, H.-C.; Pinzer, B.R. Effect of thickness and build orientation on the water vapor and oxygen permeation properties of laser-sintered polyamide 12 sheets. Rapid Prototyp. J. To be published.
- Gogolewski, S.; Czerntawska, K.; Gastorek, M. Effect of annealing on thermal properties and crystalline structure of polyamides. Nylon 12 (polylaurolactam). Colloid Polym. Sci. 1980, 258, 1130–1136. [Google Scholar] [CrossRef]
- Liebrich, A.; Langowski, H.; Schreiber, R.; Pinzer, B. Porosity distribution in laser-sintered polymeric thin sheets as revealed by X-ray micro tomography. Polym. Test. 2019, 76, 286–297. [Google Scholar] [CrossRef]
- Müller, K.; Scheuerer, Z.; Florian, V.; Skutschik, T.; Sängerlaub, S. Comparison of test methods for oxygen permeability: Optical method versus carrier gas method. Polym. Test. 2017, 63, 126–132. [Google Scholar] [CrossRef]
- Barrer, R.M. Diffusion in and through Solids; The University Press: Cambridge, UK, 1941. [Google Scholar]
- Coulson, C.A.; Crank, J. The Mathematics of Diffusion. Math. Gaz. 1958, 42, 165. [Google Scholar] [CrossRef] [Green Version]
- Hiramatsu, N.; Haraguchi, K.; Hirakawa, S. Study of Transformations among α, γ and γ’Forms in Nylon 12 by X-Ray and DSC. Jpn. J. Appl. Phys. 1983, 22, 335–339. [Google Scholar] [CrossRef]
- Fischer, C.; Seefried, A.; Drummer, D. Crystallization and Component Properties of Polyamide 12 at Processing-Relevant Cooling Conditions. Polym. Eng. Sci. 2016, 57, 450–457. [Google Scholar] [CrossRef]
- Zarringhalam, H.; Majewski, C.; Hopkinson, N. Degree of particle melt in Nylon-12 selective laser-sintered parts. Rapid Prototyp. J. 2009, 15, 126–132. [Google Scholar] [CrossRef]
- Kruth, J.-P.; Levy, G.; Klocke, F.; Childs, T. Consolidation phenomena in laser and powder-bed based layered manufacturing. CIRP Ann. 2007, 56, 730–759. [Google Scholar] [CrossRef]
- Zhao, M.; Wudy, K.; Drummer, D. Crystallization Kinetics of Polyamide 12 during Selective Laser Sintering. Polymers 2018, 10, 168. [Google Scholar] [CrossRef] [Green Version]
- Amado, A.; Wegener, K.; Schmid, M.; Levy, G. Characterization and modeling of non-isothermal crystallization of Polyamide 12 and co-Polypropylene during the SLS process. In Proceedings of the 5th International Polymers & Moulds Innovations Conference, Ghent, Belgium, 12–14 September 2012; pp. 207–216. [Google Scholar]
- Balemans, C.; Looijmans, S.F.; Grosso, G.; Hulsen, M.A.; Anderson, P.D. Numerical analysis of the crystallization kinetics in SLS. Addit. Manuf. 2020, 33, 101126. [Google Scholar] [CrossRef]
- Drummer, D.; Greiner, S.; Zhao, M.; Wudy, K. A novel approach for understanding laser sintering of polymers. Addit. Manuf. 2019, 27, 379–388. [Google Scholar] [CrossRef]
- Stichel, T.; Frick, T.; Laumer, T.; Tenner, F.; Hausotte, T.; Merklein, M.; Schmidt, M. A Round Robin study for Selective Laser Sintering of polyamide 12: Microstructural origin of the mechanical properties. Opt. Laser Technol. 2017, 89, 31–40. [Google Scholar] [CrossRef]
- Stichel, T.; Frick, T.; Laumer, T.; Tenner, F.; Hausotte, T.; Merklein, M.; Schmidt, M. A Round Robin study for selective laser sintering of polymers: Back tracing of the pore morphology to the process parameters. J. Mater. Process. Technol. 2018, 252, 537–545. [Google Scholar] [CrossRef]
- Dupin, S.; Lame, O.; Barrès, C.; Charmeau, J.-Y. Microstructural origin of physical and mechanical properties of polyamide 12 processed by laser sintering. Eur. Polym. J. 2012, 48, 1611–1621. [Google Scholar] [CrossRef]
- Dewulf, W.; Pavan, M.; Craeghs, T.; Kruth, J.-P. Using X-ray computed tomography to improve the porosity level of polyamide-12 laser sintered parts. CIRP Ann. 2016, 65, 205–208. [Google Scholar] [CrossRef]
- Bain, E.D.; Garboczi, E.J.; Seppala, J.E.; Parker, T.C.; Migler, K.B. AMB2018-04: Benchmark Physical Property Measurements for Powder Bed Fusion Additive Manufacturing of Polyamide 12. Integr. Mater. Manuf. Innov. 2019, 8, 335–361. [Google Scholar] [CrossRef]
- Rüsenberg, S.; Schmidt, L.; Schmid, H. Mechanical and Physical Properties–A Way to assess quality of Laser Sintered Parts. In Proceedings of the 22nd International Solid Freeform Fabrication Symposium—An Additive Manufacturing Conference, Austin, TX, USA, 3–5 August 2011; pp. 239–251. [Google Scholar]
- Pavan, M.; Craeghs, T.; Verhelst, R.; Ducatteeuw, O.; Kruth, J.-P.; Dewulf, W. CT-based quality control of Laser Sintering of Polymers. Case Stud. Nondestruct. Test. Eval. 2016, 6, 62–68. [Google Scholar] [CrossRef] [Green Version]
- Rogers, C. Permeation of Gases and Vapours in Polymers. In Polymer Permeability; Comyn, J., Ed.; Springer: Dordrecht, The Netherlands, 1985; pp. 11–73. [Google Scholar]
- Van Krevelen, D.W.; Te Nijenhuis, K. Properties of Polymers: Their Correlation with Chemical Structure; Their Numerical Estimation and Prediction from Additive Group Contributions; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]





| Number of Layers | 2 | 4 | 6 | 8 | |
|---|---|---|---|---|---|
| Crystallinity/% | PA2201 powder | -------------------------------- 50.8 (4.6) -------------------------------- | |||
| Quenched LS sheets | 23.8 (1.7) | 24.0 (1.0) | 24.4 (0.2) | 24.5 (1.2) | |
| Standard LS sheets | 34.5 (0.7) | 35.2 (2.5) | 37.0 (0.6) | 37.0 (0.7) | |
| Recrystallization temperature/°C | PA2201 powder | ----------------------------- Not observed ----------------------------- | |||
| Quenched LS sheets | 161.7 (0.6) | 162.4 (0.0) | 162.2 (0.0) | 163.5 (1.0) | |
| Standard LS sheets | Not observed | Not observed | Not observed | Not observed | |
| Melting temperature/°C | PA2201 powder | ------------------------------ 185.5 (0.6) ------------------------------- | |||
| Quenched LS sheets | 178.7 (2.0) | 178.9 (0.2) | 178.8 (0.9) | 178.7 (0.4) | |
| Standard LS sheets | 180.8 (0.4) | 180.7 (0.1) | 180.0 (0.5) | 181.1 (0.9) | |
| Number of Layers | 2 | 4 | 6 | 8 | ||
|---|---|---|---|---|---|---|
| Porosity/% | Quenched LS sheets | 0.32 | 0.45 | 0.56 | 0.60 | |
| Standard LS sheets | 0.31 | 0.47 | 0.78 | 1.04 | ||
| Pore number density/mm−3 | Quenched LS sheets | 220 | 144 | 152 | 115 | |
| Standard LS sheets | 266 | 231 | 182 | 212 | ||
| Pore volume/10−4 mm3 | Quenched LS sheets | 0.14 | 0.31 | 0.37 | 0.52 | |
| Standard LS sheets | 0.12 | 0.20 | 0.43 | 0.49 | ||
| s | Quenched LS sheets | 0.43 | 0.69 | 0.71 | 1.01 | |
| Standard LS sheets | 0.83 | 1.26 | 1.80 | 2.84 | ||
| max * | Quenched LS sheets | 7.48 | 8.51 | 6.09 | 17.96 | |
| Standard LS sheets | 15.29 | 23.14 | 20.43 | 38.87 | ||
| Number of Layers | 2 | 4 | 6 | 8 | ||
|---|---|---|---|---|---|---|
| Thickness/µm | (=leff) | Quenched LS sheets | 269 | 506 | 723 | 956 |
| Standard LS sheets | 301 | 500 | 726 | 932 | ||
| s | Quenched LS sheets | 44 | 37 | 37 | 36 | |
| Standard LS sheets | 41 | 46 | 43 | 49 | ||
| Number of Layers | 2 | 4 | 6 | 8 | |
|---|---|---|---|---|---|
| DO2/10−12 m2 s−1 | Quenched LS sheets | - | 3.4 | 3.1 | 3.0 |
| Standard LS sheets | 3.6 | 2.8 | 2.4 | 2.3 | |
| DHe/10−10 m2 s−1 | Quenched LS sheets | - | 2.2 | 1.8 | 1.6 |
| Standard LS sheets | - | 2.0 | 1.2 | 1.1 | |
| SO2/10−5 mol m−3 Pa−1 | Quenched LS sheets | - | 2.0 | 2.1 | 2.0 |
| Standard LS sheets | 1.3 | 1.6 | 1.8 | 2.1 | |
| SHe/10−6 mol m−3 Pa−1 | Quenched LS sheets | - | 1.8 | 2.0 | 2.0 |
| Standard LS sheets | - | 1.7 | 2.5 | 2.6 | |
| PO2/10−17 mol m m−2 s−1 Pa−1 | Quenched LS sheets | - | 6.6 | 6.6 | 6.0 |
| Standard LS sheets | 4.8 | 4.6 | 4.4 | 4.7 | |
| PHe/10−16 mol m m−2 s−1 Pa−1 | Quenched LS sheets | - | 3.9 | 3.5 | 3.3 |
| Standard LS sheets | 3.6 | 3.4 | 3.0 | 2.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liebrich, A.; Langowski, H.-C.; Pinzer, B.R. Thickness-Dependent Permeation Properties of Quenched and Standard Laser-Sintered Polyamide 12 Sheets. Polymers 2021, 13, 603. https://doi.org/10.3390/polym13040603
Liebrich A, Langowski H-C, Pinzer BR. Thickness-Dependent Permeation Properties of Quenched and Standard Laser-Sintered Polyamide 12 Sheets. Polymers. 2021; 13(4):603. https://doi.org/10.3390/polym13040603
Chicago/Turabian StyleLiebrich, Anna, Horst-Christian Langowski, and Bernd R. Pinzer. 2021. "Thickness-Dependent Permeation Properties of Quenched and Standard Laser-Sintered Polyamide 12 Sheets" Polymers 13, no. 4: 603. https://doi.org/10.3390/polym13040603