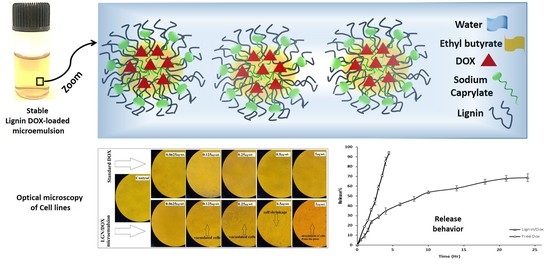
Lignin-Stabilized Doxorubicin Microemulsions: Synthesis, Physical Characterization, and In Vitro Assessments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cell Lines
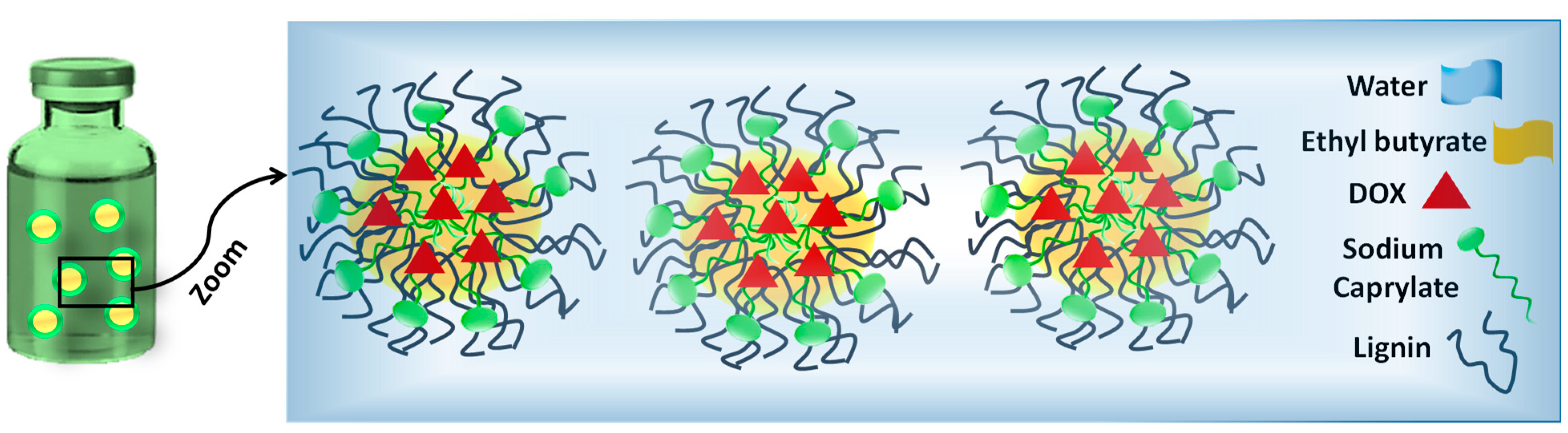
2.3. Formulation of DOX-Loaded Microemulsions
2.4. Characterization of DOX-Loaded Microemulsions by DLS
2.5. Entrapment Efficiency of DOX
2.6. Release Study
2.7. Cell Viability Assay and Evaluation of Cell Morphology
2.8. Membrane Integrity
2.9. Statistical Analysis
3. Results
3.1. Characterization of DOX-Loaded Microemulsions by DLS
3.2. Entrapment Efficiency
3.3. In Vitro Release Experiment
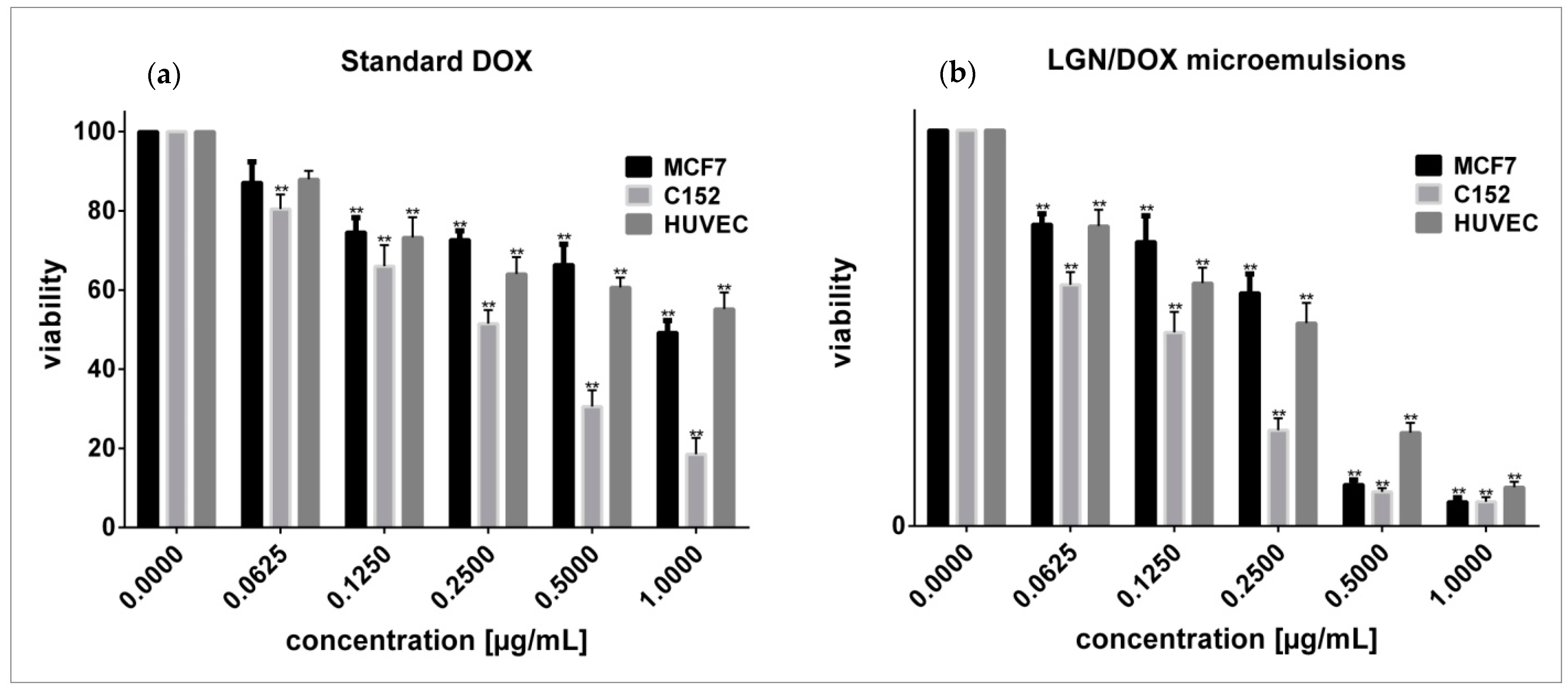
3.4. Effects of Standard and Encapsulated DOX on Cells Viability and Morphology
3.5. LDH-Based Cytotoxicity Assay
3.6. Practical Applications and Future Research Perspectives
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, N.; Woodle, M.C.; Mixson, A.J. Advances in delivery systems for doxorubicin. J. Nanomed. Nanotechnol. 2018, 9, 519. [Google Scholar] [CrossRef]
- Renu, K. Molecular mechanism of doxorubicin-induced cardiomyopathy–An update. Eur. J. Pharmacol. 2018, 818, 241–253. [Google Scholar] [CrossRef]
- Shafei, A. A review on the efficacy and toxicity of different doxorubicin nanoparticles for targeted therapy in metastatic breast cancer. Biomed. Pharmacother. 2017, 95, 1209–1218. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, A.M. New green surfactants for emulsion polymerization. Progr. Organ. Coat. 2005, 53, 246–255. [Google Scholar] [CrossRef]
- Trouet, A. Extracellularly tumor-activated prodrugs for the selective chemotherapy of cancer: Application to doxorubicin and preliminary in vitro and in vivo studies. Cancer Res. 2001, 61, 2843–2846. [Google Scholar] [PubMed]
- Rahmani, S. Synthesis of mesoporous silica nanoparticles and nanorods: Application to doxorubicin delivery. Solid State Sci. 2017, 68, 25–31. [Google Scholar] [CrossRef]
- Ghosh, S. Biomedical application of doxorubicin coated hydroxyapatite—poly (lactide-co-glycolide) nanocomposite for controlling osteosarcoma therapeutics. J. Nanosci. Nanotechnol. 2020, 20, 3994–4004. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, T. Clinical application of drug delivery systems in cancer chemotherapy: Review of the efficacy and side effects of approved drugs. Biol. Pharm. Bullet. 2013, 36, 715–718. [Google Scholar] [CrossRef] [Green Version]
- Pugazhendhi, A. Toxicity of Doxorubicin (Dox) to different experimental organ systems. Life Sci. 2018, 200, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.L.; Choong, P.F.; Dass, C.R. Doxorubicin delivery systems based on chitosan for cancer therapy. J. Pharm. Pharmacol. 2009, 61, 131–142. [Google Scholar] [CrossRef]
- Alavi, M.; Nokhodchi, A. Microformulations and Nanoformulations of Doxorubicin for Improvement of Its Therapeutic Efficiency. Crit. Rev. Ther. Drug Carrier Syst. 2020, 37. [Google Scholar] [CrossRef]
- Formariz, T. Doxorubicin biocompatible O/W microemulsion stabilized by mixed surfactant containing soya phosphatidylcholine. Colloids Surf. B Biointerf. 2006, 51, 54–61. [Google Scholar] [CrossRef]
- Sedyakina, N.E. Formulation, drug release features and in vitro cytotoxic evaluation of nonionic mixed surfactant stabilized water-in-oil microemulsion loaded with doxorubicin. Mendel. Commun. 2019, 29, 320–322. [Google Scholar] [CrossRef]
- Aboudzadeh, M.A. Low-Energy Encapsulation of α-Tocopherol Using Fully Food Grade Oil-in-Water Microemulsions. ACS Omega 2018, 3, 10999–11008. [Google Scholar] [CrossRef]
- Candido, C.D. Biocompatible microemulsion modifies the tissue distribution of doxorubicin. J. Pharm. Sci. 2014, 103, 3297–3301. [Google Scholar] [CrossRef] [PubMed]
- Bera, A.; Mandal, A. Microemulsions: A novel approach to enhanced oil recovery: A review. J. Petrol. Exp. Product. Technol. 2015, 5, 255–268. [Google Scholar] [CrossRef] [Green Version]
- Muzaffar, F.; Singh, U.; Chauhan, L. Review on microemulsion as futuristic drug delivery. Int. J. Pharm. Pharm. Sci. 2013, 5, 39–53. [Google Scholar]
- Grampurohit, N.; Ravikumar, P.; Mallya, R. Microemulsions for topical use–a review. Ind. J. Pharm. Ed. Res. 2011, 45, 100–107. [Google Scholar]
- Rebello, S. Surfactants: Toxicity, remediation and green surfactants. Environ. Chem. Lett. 2014, 12, 275–287. [Google Scholar] [CrossRef]
- Wenan, L. Application and research advance of green surfactants. J. Anhui Agric. Sci. 2007, 35, 5691. [Google Scholar]
- Kandasamy, R. New Trends in the Biomanufacturing of Green Surfactants: Biobased Surfactants and Biosurfactants, in Next Generation Biomanufacturing Technologies. Am. Chem. Soc. 2019, 1329, 243–260. [Google Scholar]
- Saratale, R.G. A comprehensive overview and recent advances on polyhydroxyalkanoates (PHA) production using various organic waste streams. Bioresour. Technol. 2021, 325, 124685. [Google Scholar] [CrossRef] [PubMed]
- Shulga, G.; Shakels, V.; Skudra, S.; Bogdanovs, V. Modified lignin as an environmentally friendly surfactant. In Proceedings of the 8th International Scientific and Practical Conference, Rēzekne, Latvia, 20–22 June 2011; pp. 276–281. [Google Scholar]
- Figueiredo, P. In vitro evaluation of biodegradable lignin-based nanoparticles for drug delivery and enhanced antiproliferation effect in cancer cells. Biomaterials 2017, 121, 97. [Google Scholar] [CrossRef]
- Li, H. Preparation of nanocapsules via the self-assembly of kraft lignin: A totally green process with renewable resources. ACS Sustain. Chem. Eng. 2016, 4, 1946. [Google Scholar] [CrossRef]
- Zhou, Y. Preparation of targeted lignin–based hollow nanoparticles for the delivery of doxorubicin. Nanomaterials 2019, 9, 188. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y. Effects of Lignin-Based Hollow Nanoparticle Structure on the Loading and Release Behavior of Doxorubicin. Materials 2019, 12, 1694. [Google Scholar] [CrossRef] [Green Version]
- Sargazi, S. Hydro-alcoholic Extract of Achillea Wilhelmsii, C. Koch Reduces the Expression of Cell Death-Associated Genes while Inducing DNA Damage in HeLa Cervical Cancer Cells. Ir. J. Med. Sci. 2020, 45, 359. [Google Scholar]
- Varshney, M. Pluronic microemulsions as nanoreservoirs for extraction of bupivacaine from normal saline. J. Am. Chem. Soc. 2004, 126, 5108–5112. [Google Scholar] [CrossRef] [Green Version]
- Rahdar, A. Dynamic light scattering and zeta potential measurements: Effective techniques to characterize therapeutic nanoparticles. J. Nanoanal. 2019. [Google Scholar] [CrossRef]
- Rahdar, A. Effect of ion exchange in NaAOT surfactant on droplet size and location of dye within Rhodamine B (RhB)-containing microemulsion at low dye concentration. J. Mol. Liq. 2018, 252, 506–513. [Google Scholar] [CrossRef]
- Rahdar, A. Deferasirox-loaded pluronic nanomicelles: Synthesis, characterization, in vitro and in vivo studies. J. Mol. Liq. 2020, 323, 114605. [Google Scholar] [CrossRef]
- Barani, M. A new formulation of hydrophobin-coated niosome as a drug carrier to cancer cells. Mater. Sci. Eng. C 2020, 113, 110975. [Google Scholar] [CrossRef]
- Hajizadeh, M.R. In vitro cytotoxicity assay of D-limonene niosomes: An efficient nanocarrier for enhancing solubility of plant-extracted agents. Res. Pharm. Sci. 2019, 14, 448. [Google Scholar]
- Gerlier, D.; Thomasset, N. Use of MTT colorimetric assay to measure cell activation. J. Immunol. Methods 1986, 94, 57. [Google Scholar] [CrossRef]
- Acosta, E. Bioavailability of nanoparticles in nutrient and nutraceutical delivery. Curr. Opin. Colloid Interf. Sci. 2009, 14, 3. [Google Scholar] [CrossRef]
- Rahdar, A. Effect of tocopherol on the properties of Pluronic F127 microemulsions: Physico-chemical characterization and in vivo toxicity. J. Mol. Liquids 2019, 277, 624–630. [Google Scholar] [CrossRef]
- Pandey, M. Hyaluronic acid-modified betamethasone encapsulated polymeric nanoparticles: Fabrication, characterisation, in vitro release kinetics, and dermal targeting. Drug Deliv. Trans. Res. 2019, 9, 520–533. [Google Scholar] [CrossRef]
- Anwer, M.K. Preparation of sustained release apremilast-loaded PLGA nanoparticles: In vitro characterization and in vivo pharmacokinetic study in rats. Int. J. Nanomed. 2019, 14, 1587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y. pH-responsive lignin-based complex micelles: Preparation, characterization and application in oral drug delivery. Chem. Eng. J. 2017, 327, 1176. [Google Scholar] [CrossRef]
- Deng, Y.H. Hollow lignin azo colloids encapsulated avermectin with high anti-photolysis and controlled release performance. Ind. Crop. Prod. 2016, 87, 191. [Google Scholar] [CrossRef]
- Li, Y. Lignin-based microsphere: Preparation and performance on encapsulating the pesticide avermectin. ACS Sustain. Chem. Eng. 2017, 5, 3321. [Google Scholar] [CrossRef]
- Heggannavar, G.B. Development of doxorubicin-loaded magnetic silica–pluronic F-127 nanocarriers conjugated with transferrin for treating glioblastoma across the blood–brain barrier using an in vitro model. ACS Omega 2018, 3, 8017. [Google Scholar] [CrossRef] [PubMed]
- Siegal, T.; Horowitz, A.; Gabizon, A. Doxorubicin encapsulated in sterically stabilized liposomes for the treatment of a brain tumor model: Biodistribution and therapeutic efficacy. J. Neurosurg. 1995, 83, 1029–1037. [Google Scholar] [CrossRef] [Green Version]
- Mohan, P.; Rapoport, N. Doxorubicin as a molecular nanotheranostic agent: Effect of doxorubicin encapsulation in micelles or nanoemulsions on the ultrasound-mediated intracellular delivery and nuclear trafficking. Mol. Pharm. 2010, 7, 1959–1973. [Google Scholar] [CrossRef] [Green Version]
- Working, P.K.; Newman, M.S.; Huang, S.K.; Mayhew, E.; Vaage, J.; Lasic, D.D. Pharmacokinetics, biodistribution and therapeutic efficacy of doxorubicin encapsulated in Stealth® liposomes (Doxil®). J. Lip. Res. 1994, 4, 667–687. [Google Scholar] [CrossRef]
- Saini, A.; Panesar, P.S.; Bera, M.B. Valorization of fruits and vegetables waste through green extraction of bioactive compounds and their nanoemulsions-based delivery system. Bioresour. Bioproc. 2019, 6, 26. [Google Scholar] [CrossRef]
- Kaur, P.; Garg, T.; Rath, G.; Murthy, R.S.; Goyal, A.K. Surfactant-based drug delivery systems for treating drug-resistant lung cancer. Drug Deliv. 2016, 23, 717–728. [Google Scholar] [CrossRef]
- Shakeel, F.; Haq, N.; Al-Dhfyan, A.; Alanazi, F.K.; Alsarra, I.A. Chemoprevention of skin cancer using low HLB surfactant nanoemulsion of 5-fluorouracil: A preliminary study. Drug Deliv. 2015, 22, 573–580. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Zhang, W.; Huang, Y.; Gao, F.; Sha, X.; Fang, X. Pluronic-based functional polymeric mixed micelles for co-delivery of doxorubicin and paclitaxel to multidrug resistant tumor. Int. J. Pharm. 2015, 488, 44–58. [Google Scholar] [CrossRef] [PubMed]
- Abasian, P.; Radmansouri, M.; Jouybari, M.H.; Ghasemi, M.V.; Mohammadi, A.; Irani, M.; Jazi, F.S. Incorporation of magnetic NaX zeolite/DOX into the PLA/chitosan nanofibers for sustained release of doxorubicin against carcinoma cells death in vitro. Int. J. Biol. Macromol. 2019, 121, 398–406. [Google Scholar] [CrossRef]
- Hassett, M.J.; O’Malley, A.J.; Pakes, J.R.; Newhouse, J.P.; Earle, C.C. Frequency and cost of chemotherapy-related serious adverse effects in a population sample of women with breast cancer. J. Nat. Cancer Inst. 2006, 98, 1108–1117. [Google Scholar] [CrossRef]
- Lee, T.; Lau, T.; Ng, I. Doxorubicin-induced apoptosis and chemosensitivity in hepatoma cell lines. Cancer Chemother. Pharmacol. 2002, 49, 78–86. [Google Scholar] [CrossRef]
- Dhingra, R.; Margulets, V.; Chowdhury, S.R.; Thliveris, J.; Jassal, D.; Fernyhough, P.; Dorn, G.W.; Kirshenbaum, L.A. Bnip3 mediates doxorubicin-induced cardiac myocyte necrosis and mortality through changes in mitochondrial signaling. Proc. Nat. Acad. Sci. USA 2014, 111, E5537–E5544. [Google Scholar] [CrossRef] [Green Version]
- Shin, H.J.; Kwon, H.K.; Lee, J.H.; Gui, X.; Achek, A.; Kim, J.H.; Choi, S. Doxorubicin-induced necrosis is mediated by poly-(ADP-ribose) polymerase 1 (PARP1) but is independent of p53. Sci. Rep. 2015, 5, 15798. [Google Scholar] [CrossRef] [Green Version]
- Krysko, D.V.; Berghe, T.V.; Parthoens, E.; D’Herde, K.; Vandenabeele, P. Methods for distinguishing apoptotic from necrotic cells and measuring their clearance. Methods Enzymol. 2008, 442, 307–341. [Google Scholar] [PubMed]
- Golstein, P.; Kroemer, G. Cell death by necrosis: Towards a molecular definition. Trends Biochem. Sci. 2007, 32, 37–43. [Google Scholar] [CrossRef]
- Pilco-Ferreto, N.; Calaf, G.M. Influence of doxorubicin on apoptosis and oxidative stress in breast cancer cell lines. Int. J. Oncol. 2016, 49, 753–762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharifi, S.; Barar, J.; Hejazi, M.S.; Samadi, N. Doxorubicin changes Bax/Bcl-xL ratio, caspase-8 and 9 in breast cancer cells. Adv. Pharm. Bullet. 2015, 5, 351. [Google Scholar] [CrossRef] [Green Version]
- Eom, Y.W.; Kim, M.A.; Park, S.S.; Goo, M.J.; Kwon, H.J.; Sohn, S.; Kim, W.H.; Yoon, G.; Choi, K.S. Two distinct modes of cell death induced by doxorubicin: Apoptosis and cell death through mitotic catastrophe accompanied by senescence-like phenotype. Oncogene. 2005, 24, 4765–4777. [Google Scholar] [CrossRef] [Green Version]
- Narang, A.S.; Delmarre, D.; Gao, D. Stable drug encapsulation in micelles and microemulsions. Int. J. Pharm. 2007, 345, 9–25. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahdar, A.; Sargazi, S.; Barani, M.; Shahraki, S.; Sabir, F.; Aboudzadeh, M.A. Lignin-Stabilized Doxorubicin Microemulsions: Synthesis, Physical Characterization, and In Vitro Assessments. Polymers 2021, 13, 641. https://doi.org/10.3390/polym13040641
Rahdar A, Sargazi S, Barani M, Shahraki S, Sabir F, Aboudzadeh MA. Lignin-Stabilized Doxorubicin Microemulsions: Synthesis, Physical Characterization, and In Vitro Assessments. Polymers. 2021; 13(4):641. https://doi.org/10.3390/polym13040641
Chicago/Turabian StyleRahdar, Abbas, Saman Sargazi, Mahmood Barani, Sheida Shahraki, Fakhara Sabir, and M. Ali Aboudzadeh. 2021. "Lignin-Stabilized Doxorubicin Microemulsions: Synthesis, Physical Characterization, and In Vitro Assessments" Polymers 13, no. 4: 641. https://doi.org/10.3390/polym13040641
APA StyleRahdar, A., Sargazi, S., Barani, M., Shahraki, S., Sabir, F., & Aboudzadeh, M. A. (2021). Lignin-Stabilized Doxorubicin Microemulsions: Synthesis, Physical Characterization, and In Vitro Assessments. Polymers, 13(4), 641. https://doi.org/10.3390/polym13040641