Isothermal Crystallization Kinetics of Poly(ethylene oxide)/Poly(ethylene glycol)-g-silica Nanocomposites
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Sample Preparation
2.3. Characterization
2.3.1. Thermogravimetric Analysis
2.3.2. Differential Scanning Calorimetry
2.3.3. Polarized Light Optical Microscope
3. Results and Discussion
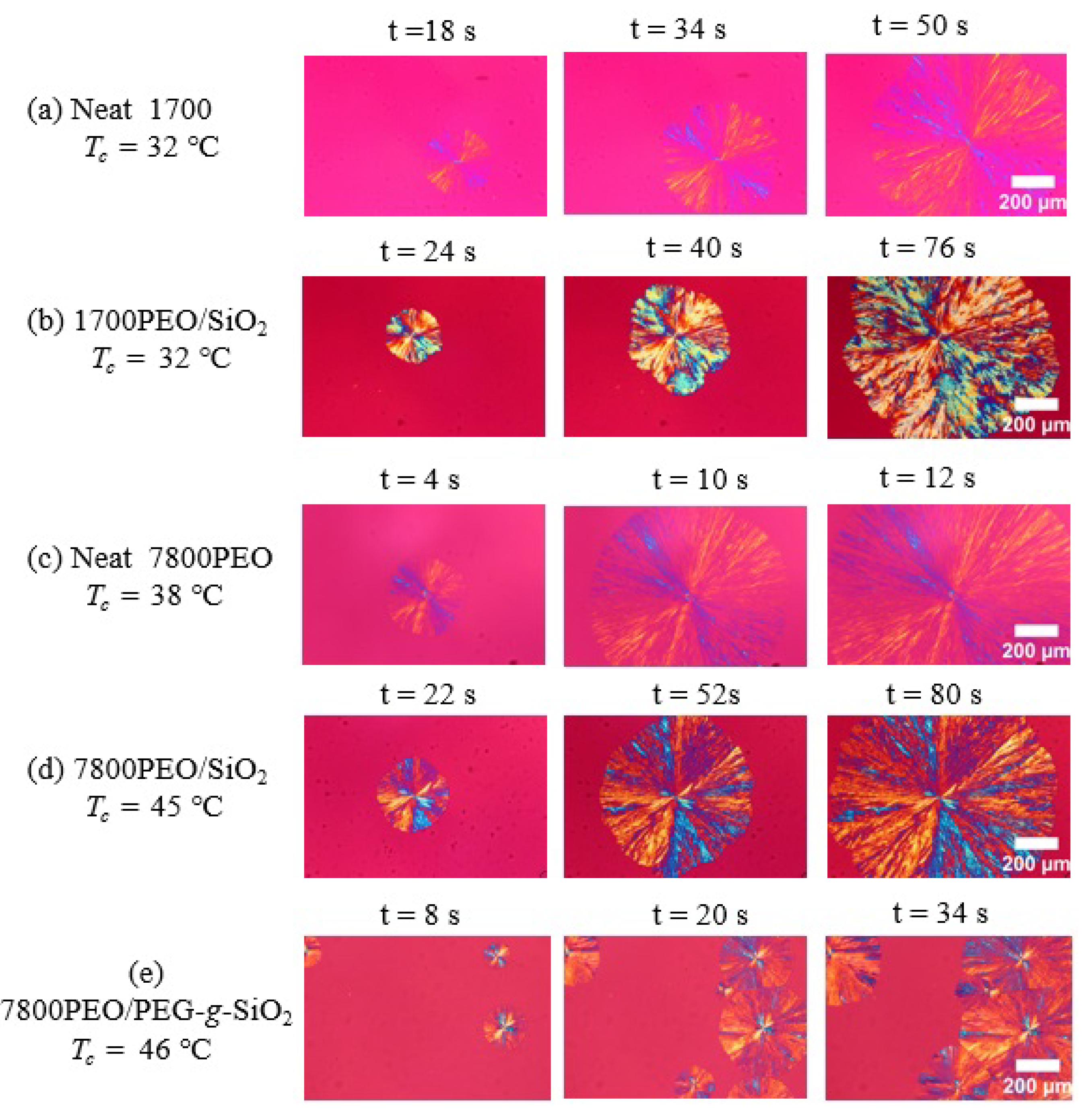
3.1. Nucleation Kinetics of PEO Nanocomposites Studied by PLOM: Primary Nucleation
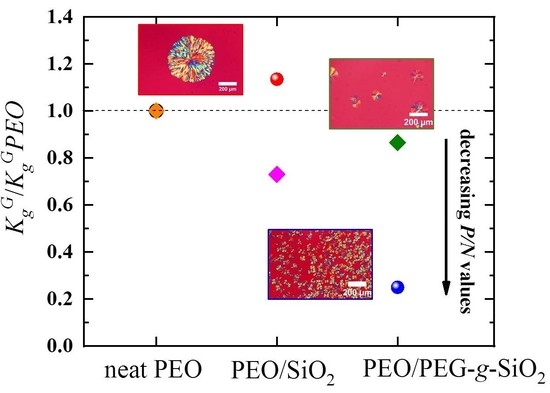
3.2. Growth Kinetics of PEO Nanocomposites Studied by PLOM: Secondary Nucleation
3.3. Overall Isothermal Crystallization Behavior
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Non-Isothermal Crystallization of PEO Nanocomposite

Appendix B. Nucleation Density of PEO Nanocomposites

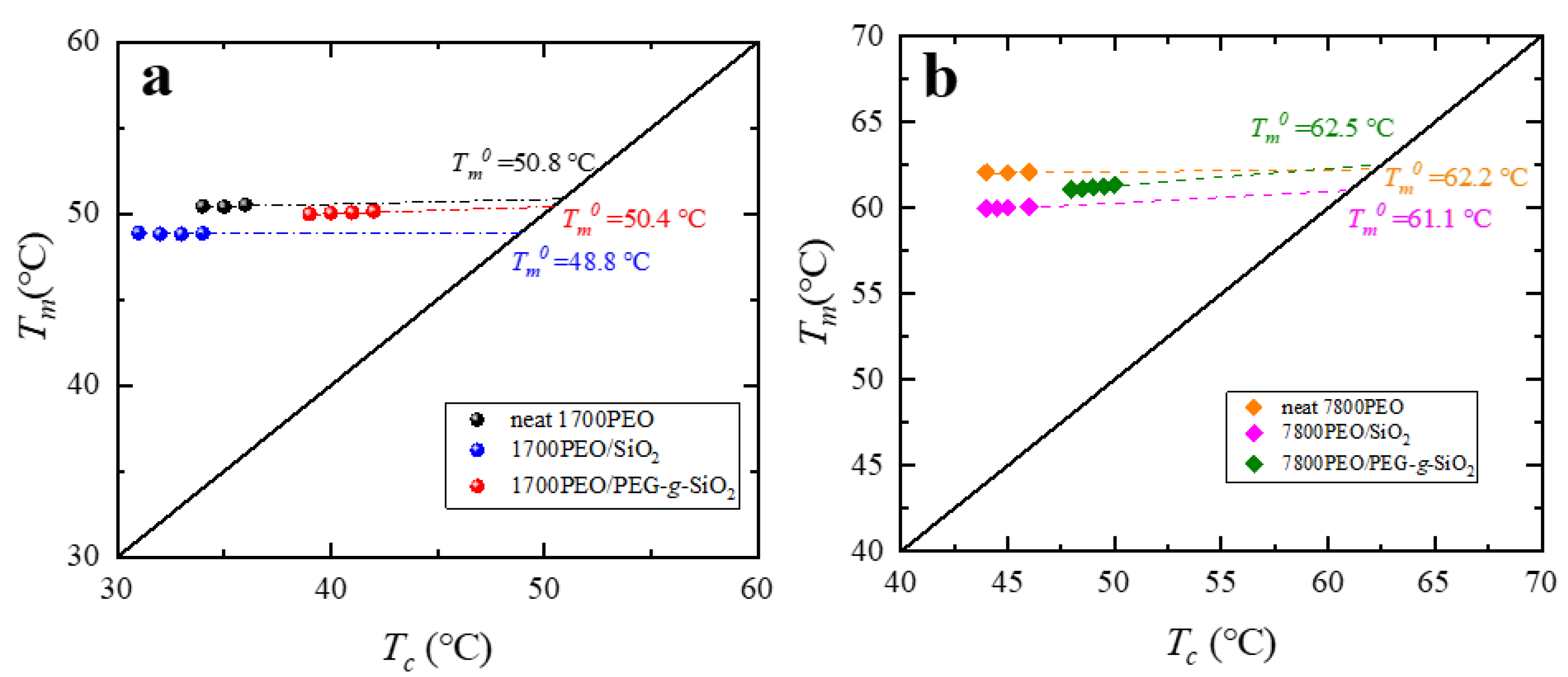
Appendix C. Equilibrium Melting Temperature of the PEO Nanocomposites
Appendix D. Nucleation Kinetics of PEO Nanocomposites Studied by PLOM
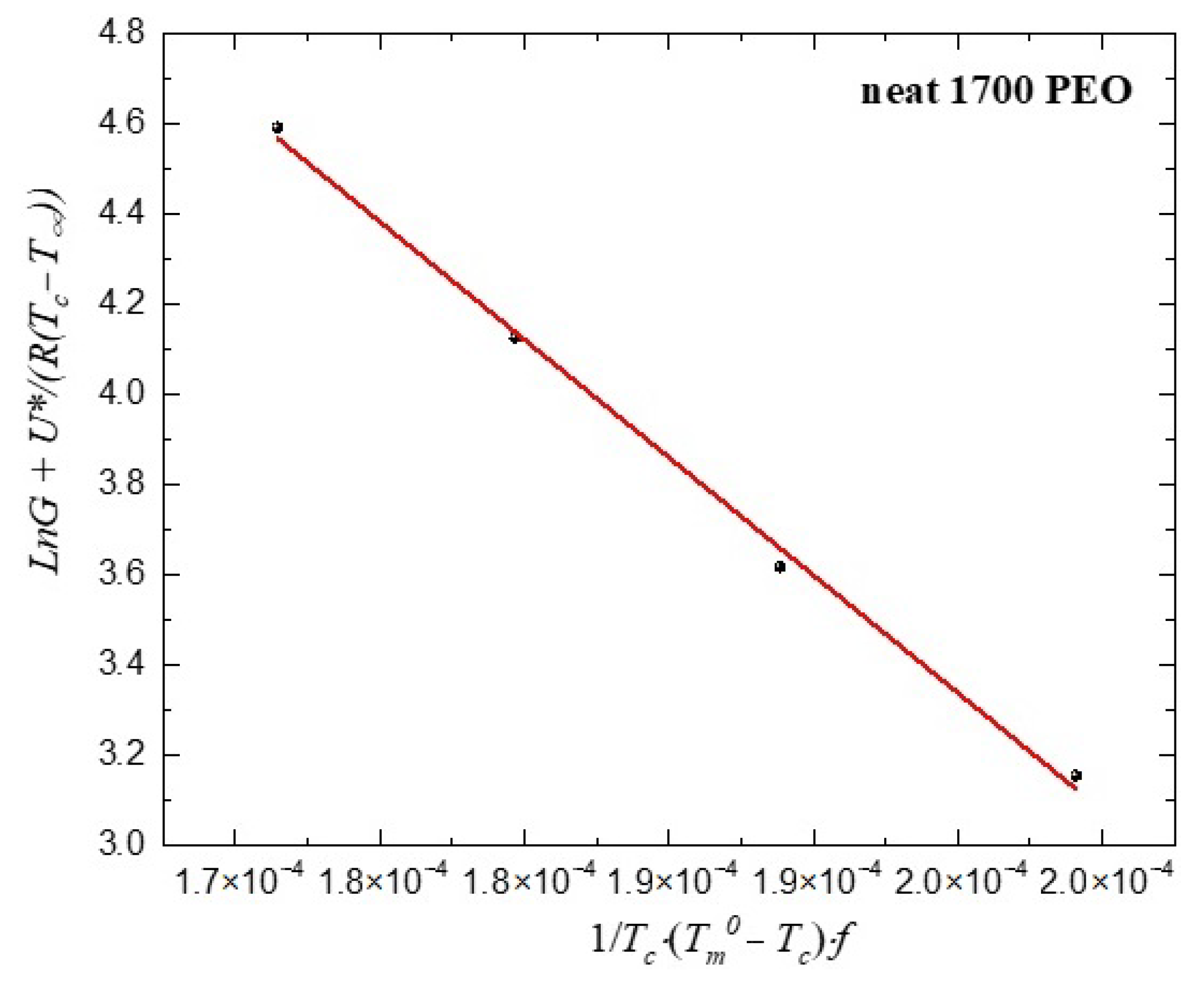
Appendix E. Lauritzen−Hoffman Fits
References
- Zhao, W.; Su, Y.; Wang, D. Synergetic effects of interfacial and spatial confinement in polymer nanocomposites. Mod. Phys. Lett. B 2017, 31, 1730003. [Google Scholar]
- Zou, H.; Wu, S.; Shen, J. Polymer/silica nanocomposites: Preparation, characterization, properties, and applications. Chem. Rev. 2008, 108, 3893–3957. [Google Scholar]
- Balazs, A.C.; Emrick, T.; Russell, T.P. Nanoparticle polymer composites: Where two small worlds meet. Science 2006, 314, 1107–1110. [Google Scholar]
- Liu, S.W.; Wang, L.; Lin, M.; Liu, Y.; Zhang, L.N.; Zhang, H. Tumor photothermal therapy employing photothermal inorganic nanoparticles/polymers nanocomposites. Chin. J. Polym. Sci. 2019, 37, 115–128. [Google Scholar]
- Pan, L.; Liu, Y.T.; Xie, X.M. Progress in high-performance graphene/polymer nanocomposites—design of filler/matrix interfacial interactions and their influences. Acta Polym. Sin. 2014, 6, 724–736. [Google Scholar]
- Akcora, P.; Kumar, S.K.; Moll, J.; Lewis, S.; Schadler, L.S.; Li, Y.; Benicewicz, B.C.; Sandy, A.; Narayanan, S.; Illavsky, J.; et al. “Gel-like” mechanical reinforcement in polymer nanocomposite melts. Macromolecules 2010, 43, 1003–1010. [Google Scholar]
- Toyonaga, M.; Chammingkwan, P.; Terano, M.; Taniike, T. Well-defined polypropylene/polypropylene-grafted silica nanocomposites: Roles of number and molecular weight of grafted chains on mechanistic reinforcement. Polymers 2016, 8, 300. [Google Scholar]
- Lai, X.L.; Yang, W.; Wang, Z.; Shi, D.W.; Liu, Z.Y.; Yang, M.B. Enhancing crystallization rate and melt strength of PLLA with four-arm PLLA grafted silica: The effect of molecular weight of the grafting PLLA chains. J. Appl. Polym. Sci. 2018, 135, 45675. [Google Scholar]
- Bieligmeyer, M.; Taheri, S.M.; German, I.; Boisson, C.; Probst, C.; Milius, W.; Altstadt, V.; Breu, J.; Schmidt, H.W.; D’Agosto, F.; et al. Completely miscible polyethylene nanocomposites. J. Am. Chem. Soc. 2012, 134, 18157–18160. [Google Scholar]
- Kumar, S.K.; Jouault, N.; Benicewicz, B.; Neely, T. Nanocomposites with polymer grafted nanoparticles. Macromolecules 2013, 46, 3199–3214. [Google Scholar]
- Akcora, P.; Liu, H.; Kumar, S.K.; Moll, J.; Li, Y.; Benicewicz, B.C.; Schadler, L.S.; Acehan, D.; Panagiotopoulos, A.Z.; Pryamitsyn, V.; et al. Anisotropic self-assembly of spherical polymer-grafted nanoparticles. Nat. Mater. 2009, 8, 354–359. [Google Scholar]
- Kojima, Y.; Usuki, A.; Kawasumi, M.; Okada, A.; Fukushima, Y.; Kurauchi, T.; Kamigaito, O. Mechanical-properties of nylon 6-clay hybrid. J. Mater. Res. 1993, 8, 1185–1189. [Google Scholar]
- Jimenez, A.M.; Krauskopf, A.A.; Perez-Camargo, R.A.; Zhao, D.; Pribyl, J.; Jestin, J.; Benicewicz, B.C.; Mueller, A.J.; Kumar, S.K. Effects of hairy nanoparticles on polymer crystallization kinetics. Macromolecules 2019, 52, 9186–9198. [Google Scholar]
- Wang, Z.; Lu, Z.; Mahoney, C.; Yan, J.; Ferebee, R.; Luo, D.; Matyjaszewski, K.; Bockstaller, M.R. Transparent and high refractive index thermoplastic polymer glasses using evaporative ligand exchange of hybrid particle fillers. ACS Appl. Mater. Interfaces 2017, 9, 7515–7522. [Google Scholar]
- Chen, X.C.; Green, P.F. Control of morphology and Its effects on the optical properties of polymer nanocomposites. Langmuir 2010, 26, 3659–3665. [Google Scholar]
- Grabowski, C.A.; Koerner, H.; Meth, J.S.; Dang, A.; Hui, C.M.; Matyjaszewski, K.; Bockstaller, M.R.; Durstock, M.F.; Vaia, R.A. Performance of dielectric nanocomposites: Matrix-free, hairy nanoparticle assemblies and amorphous polymer-nanoparticle blends. ACS Appl. Mater. Interfaces 2014, 6, 21500–21509. [Google Scholar]
- Kim, S.A.; Mangal, R.; Archer, L.A. Relaxation dynamics of nanoparticle-tethered polymer chains. Macromolecules 2015, 48, 6280–6293. [Google Scholar]
- McGrail, B.T.; Sehirlioglu, A.; Pentzer, E. Polymer composites for thermoelectric applications. Angew. Chem. Int. Edit. 2015, 54, 1710–1723. [Google Scholar]
- Sunday, D.F.; Green, D.L. Thermal and rheological behavior of polymer grafted nanoparticles. Macromolecules 2015, 48, 8651–8659. [Google Scholar]
- Green, P.F. The structure of chain end-grafted nanoparticle/homopolymer nanocomposites. Soft Matter 2011, 7, 7914–7926. [Google Scholar]
- Xu, C.; Ohno, K.; Ladmiral, V.; Composto, R.J. Dispersion of polymer-grafted magnetic nanoparticles in homopolymers and block copolymers. Polymer 2008, 49, 3568–3577. [Google Scholar]
- Khan, J.; Harton, S.E.; Akcora, P.; Benicewicz, B.C.; Kumar, S.K. Polymer crystallization in nanocomposites: Spatial reorganization of nanoparticles. Macromolecules 2009, 42, 5741–5744. [Google Scholar]
- Jog, J.P. Crystallisation in polymer nanocomposites. Mater. Sci. Technol. 2006, 22, 797–806. [Google Scholar]
- Sun, L.; Yang, J.-T.; Lin, G.-Y.; Zhong, M.-Q. Crystallization and thermal properties of polyamide 6 composites filled with different nanofillers. Mater. Lett. 2007, 61, 3963–3966. [Google Scholar]
- Wu, H.; Nagarajan, S.; Zhou, L.J.; Duan, Y.X.; Zhang, J.M. Synthesis and characterization of cellulose nanocrystal-graft-poly(D-lactide) and its nanocomposite with poly(L-lactide). Polymer 2016, 103, 365–375. [Google Scholar]
- Wu, H.; Nagarajan, S.; Shu, J.; Zhang, T.; Zhou, L.; Duan, Y.; Zhang, J. Green and facile surface modification of cellulose nanocrystal as the route to produce poly(lactic acid) nanocomposites with improved properties. Carbohydr. Polym. 2018, 197, 204–214. [Google Scholar]
- Perez, R.A.; Lopez, J.V.; Hoskins, J.N.; Zhang, B.Y.; Grayson, S.M.; Casas, M.T.; Puiggali, J.; Müller, A.J. Nucleation and antinucleation effects of functionalized carbon nanotubes on cyclic and linear poly(epsilon-caprolactones). Macromolecules 2014, 47, 3553–3566. [Google Scholar]
- Sun, Y.; He, C. Synthesis and stereocomplex crystallization of poly(lactide)-graphene oxide nanocomposites. ACS Macro Lett. 2012, 1, 709–713. [Google Scholar]
- Dukes, D.; Li, Y.; Lewis, S.; Benicewicz, B.; Schadler, L.; Kumar, S.K. Conformational transitions of spherical polymer brushes: Synthesis, characterization, and theory. Macromolecules 2010, 43, 1564–1570. [Google Scholar]
- Wen, X.N.; Su, Y.L.; Li, S.F.; Müller, A.J.; Kumar, S.K.; Wang, D.J. Direct relationship between dispersion and crystallization behavior in poly(ethylene oxide)/poly(ethylene glycol)-g-silica nanocomposites. Macromolecules 2021. [Google Scholar] [CrossRef]
- Altorbaq, A.; Jimenez, A.M.; Pribyl, J.; Benicewicz, B.C.; Müller, A.J.; Kumar, S.K. Polymer spherulitic growth kinetics mediated by nanoparticle assemblies. Macromolecules 2021, 54, 1063–1072. [Google Scholar]
- Hong, B.B.; Panagiotopoulos, A.Z. Molecular dynamics simulations of silica nanoparticles grafted with poly(ethylene oxide) oligomer chains. J. Phys. Chem. B 2012, 116, 2385–2395. [Google Scholar]
- Aranda, P.; Ruizhitzky, E. Poly(ethylene oxide)-silicate intercalation materials. Chem. Mater. 1992, 4, 1395–1403. [Google Scholar]
- Zhao, W.W.; Su, Y.L.; Wen, X.N.; Wang, D.J. Manipulating crystallization behavior of poly(ethylene oxide) by functionalized nanoparticle inclusion. Polymer 2019, 165, 28–38. [Google Scholar]
- Vassiliou, A.A.; Papageorgiou, G.Z.; Achilias, D.S.; Bikiaris, D.N. Non-isothermal crystallisation kinetics of in situ prepared poly(epsilon-caprolactone)/surface-treated SiO2 nanocomposites. Macromol. Chem. Phys. 2007, 208, 364–376. [Google Scholar]
- Wu, F.; Zhang, S.Y.; Zhang, B.; Yang, W.; Liu, Z.Y.; Yang, M.B. The effect of the grafted chains on the crystallization of PLLA/PLLA-grafted SiO2 nanocomposites. Colloid Polym. Sci. 2016, 294, 801–813. [Google Scholar]
- Taniike, T.; Toyonaga, M.; Terano, M. Polypropylene-grafted nanoparticles as a promising strategy for boosting physical properties of polypropylene-based nanocomposites. Polymer 2014, 55, 1012–1019. [Google Scholar]
- StÖber, W.; Fink, A. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar]
- Wen, X.N.; Su, Y.L.; Shui, Y.D.; Zhao, W.W.; Müller, A.J.; Wang, D.J. Correlation between grafting density and confined crystallization behavior of poly(ethylene glycol) grafted to silica. Macromolecules 2019, 52, 1505–1516. [Google Scholar]
- Shui, Y.D.; Su, Y.L.; Kuang, X.; Zhao, W.W.; Cai, Y.L.; Wang, D.J. Facile and controllable synthesis of hybrid silica nanoparticles densely grafted with poly(ethylene glycol). Polym. Int. 2017, 66, 1395–1401. [Google Scholar]
- Sasaki, T.; Yamamoto, Y.; Takahashi, T. Primary nucleation rate and radial growth rate of poly(ethylene oxide) spherulite in viscous solutions. Polym. J. 2000, 32, 263–268. [Google Scholar]
- Fernandez-d’Arlas, B.; Baumann, R.P.; Poselt, E.; Müller, A.J. Influence of composition on the isothermal crystallisation of segmented thermoplastic polyurethanes. CrystEngComm 2017, 19, 4720–4733. [Google Scholar]
- Martinez-Vazquez, D.G.; Medellin-Rodriguez, F.J.; Phillips, P.J.; Sanchez-Valdes, S. Heterogeneous nucleation of nylon 6 and PET with selected inorganic compounds. J. Appl. Polym. Sci. 2003, 88, 360–368. [Google Scholar]
- Martuscelli, E.; Silvestre, C.; Addonizio, M.L.; Amelino, L. Phase structure and compatibility studies in poly(ethylene oxide)/poly(methyl methacrylate) blends. Macromolecules 1986, 187, 1557–1571. [Google Scholar]
- Cheng, S.Z.D.; Zhang, A.Q.; Barley, J.S.; Chen, J.H.; Habenschuss, A.; Zschack, P.R. Isothermal thickening and thinning processes in low molecular weight 1. From nonintegral-folding to integral-folding chain crystal transitions. Macromolecules 1991, 24, 3937–3944. [Google Scholar]
- Xu, J.Z.; Zhang, Z.J.; Xu, H.; Chen, J.B.; Ran, R.; Li, Z.M. Highly enhanced crystallization kinetics of poly(l-lactic acid) by poly(ethylene glycol) grafted graphene oxide simultaneously as heterogeneous nucleation agent and chain mobility promoter. Macromolecules 2015, 48, 4891–4900. [Google Scholar]
- Lorenzo, A.T.; Muller, A.J. Estimation of the nucleation and crystal growth contributions to the overall crystallization energy barrier. J. Polym. Sci. Part B Polym. Phys. 2008, 46, 1478–1487. [Google Scholar]
- Sabino, M.A.; Ronca, G.; Müller, A.J. Heterogeneous nucleation and self-nucleation of poly(p-dioxanone). J. Mater. Sci. 2000, 35, 5071–5084. [Google Scholar]
- Hoffman, J.D.; Miller, R.L.; Marand, H.; Roitman, D.B. Relationship between the lateral surface free energy-sigma and the chain structure of melt-crystallized polymers. Macromolecules 1992, 25, 2221–2229. [Google Scholar]
- Lee, E.; Hong, J.Y.; Ungar, G.; Jang, J. Crystallization of poly(ethylene oxide) embedded with surface-modified SiO2 nanoparticles. Polym. Int. 2013, 62, 1112–1122. [Google Scholar]
- Waddon, A.J.; Petrovic, Z.S. Spherulite crystallization in poly(ethylene oxide)-silica nanocomposites. Retardation of growth rates through reduced molecular mobility. Polym. J. 2002, 34, 876–881. [Google Scholar]
- Krikorian, V.; Pochan, D.J. Unusual crystallization behavior of organoclay reinforced poly(l-lacticacid) nanocomposites. Macromolecules 2004, 37, 6480–6491. [Google Scholar]
- Strawhecker, K.E.; Manias, E. Crystallization behavior of poly(ethylene oxide) in the presence of Na plus montmorillonite fillers. Chem. Mat. 2003, 15, 844–849. [Google Scholar]
- Siqueira, G.; Fraschini, C.; Bras, J.; Dufresne, A.; Prud’homme, R.; Laborie, M.P. Impact of the nature and shape of cellulosic nanoparticles on the isothermal crystallization kinetics of poly(epsilon-caprolactone). Eur. Polym. J. 2011, 47, 2216–2227. [Google Scholar]
- Jimenez, A.M.; Al-Torbaq, A.S.; Müller, A.J.; Kumar, S.K. Polymer crystallization under confinement by well-dispersed nanoparticles. Macromolecules 2020, 53, 10256–10266. [Google Scholar]
- Chatterjee, T.; Lorenzo, A.T.; Krishnamoorti, R. Poly(ethylene oxide) crystallization in single walled carbon nanotube based nanocomposites: Kinetics and structural consequences. Polymer 2011, 52, 4938–4946. [Google Scholar]
- Habel, C.; Maiz, J.; Olmedo-Martinez, J.L.; Lopez, J.V.; Breu, J.; Müller, A.J. Competition between nucleation and confinement in the crystallization of poly(ethylene glycol)/large aspect ratio hectorite nanocomposites. Polymer 2020, 202, 122734. [Google Scholar]
- Chen, K.Y.; Kuo, J.F. Influence of fluorocarbon chains on the crystallization behaviors of aliphatic polyurethanes. J. Appl. Polym. Sci. 2009, 111, 371–379. [Google Scholar]
- Müller, A.J.; Balsamo, V.; Arnal, M.L. Nucleation and crystallization in diblock and triblock copolymers. Adv. Polym. Sci. 2005, 190, 1–63. [Google Scholar]
- Lorenzo, A.T.; Arnal, M.L.; Albuerne, J.; Müller, A.J. DSC isothermal polymer crystallization kinetics measurements and the use of the Avrami equation to fit the data: Guidelines to avoid common problems. Polym. Test 2007, 26, 222–231. [Google Scholar]
- Trujillo, M.; Arnal, M.L.; Müller, A.J.; Mujica, M.A.; de Navarro, C.U.; Ruelle, B.; Dubois, P. Supernucleation and crystallization regime change provoked by MWNT addition to poly(epsilon-caprolactone). Polymer 2012, 53, 832–841. [Google Scholar]
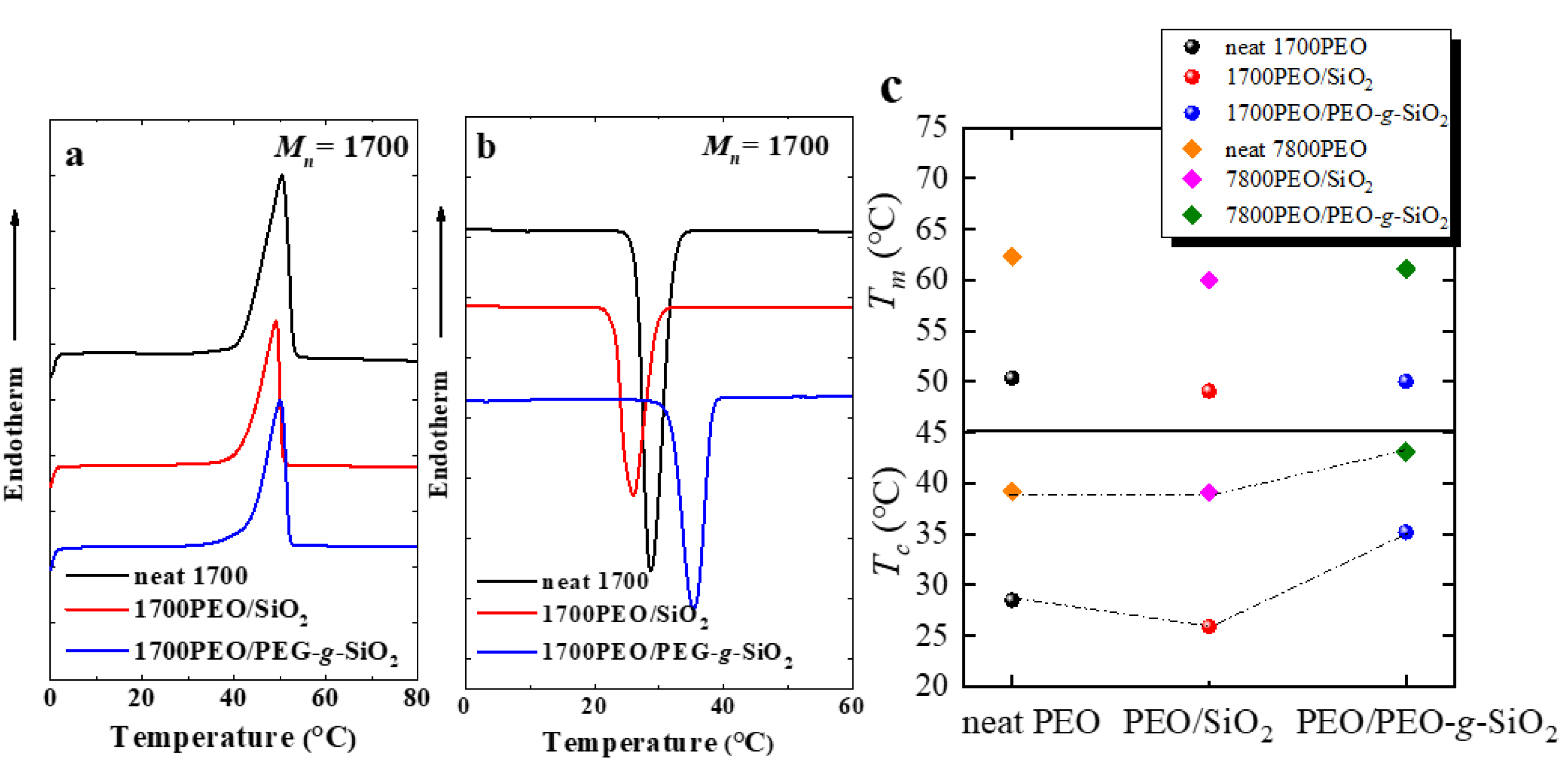


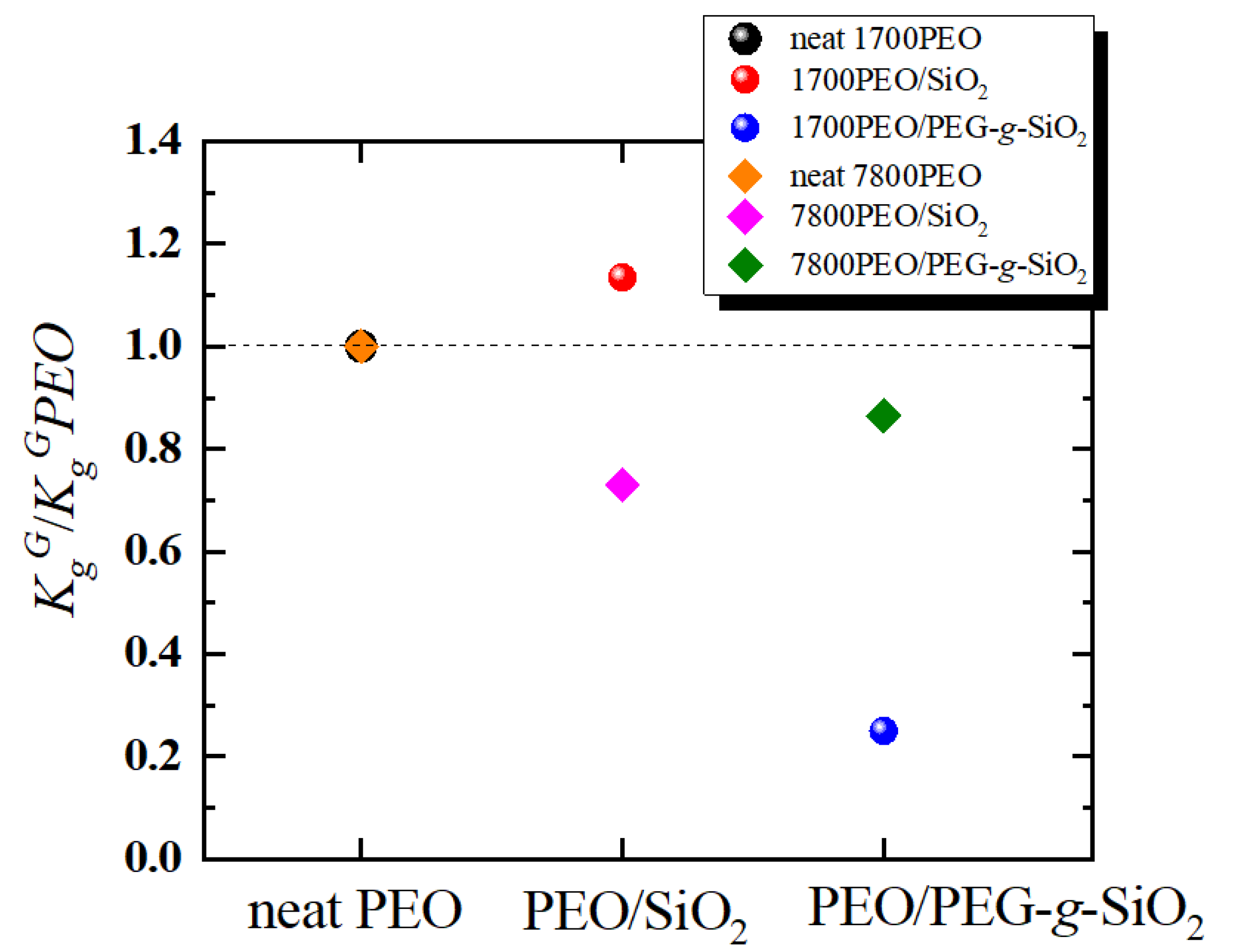
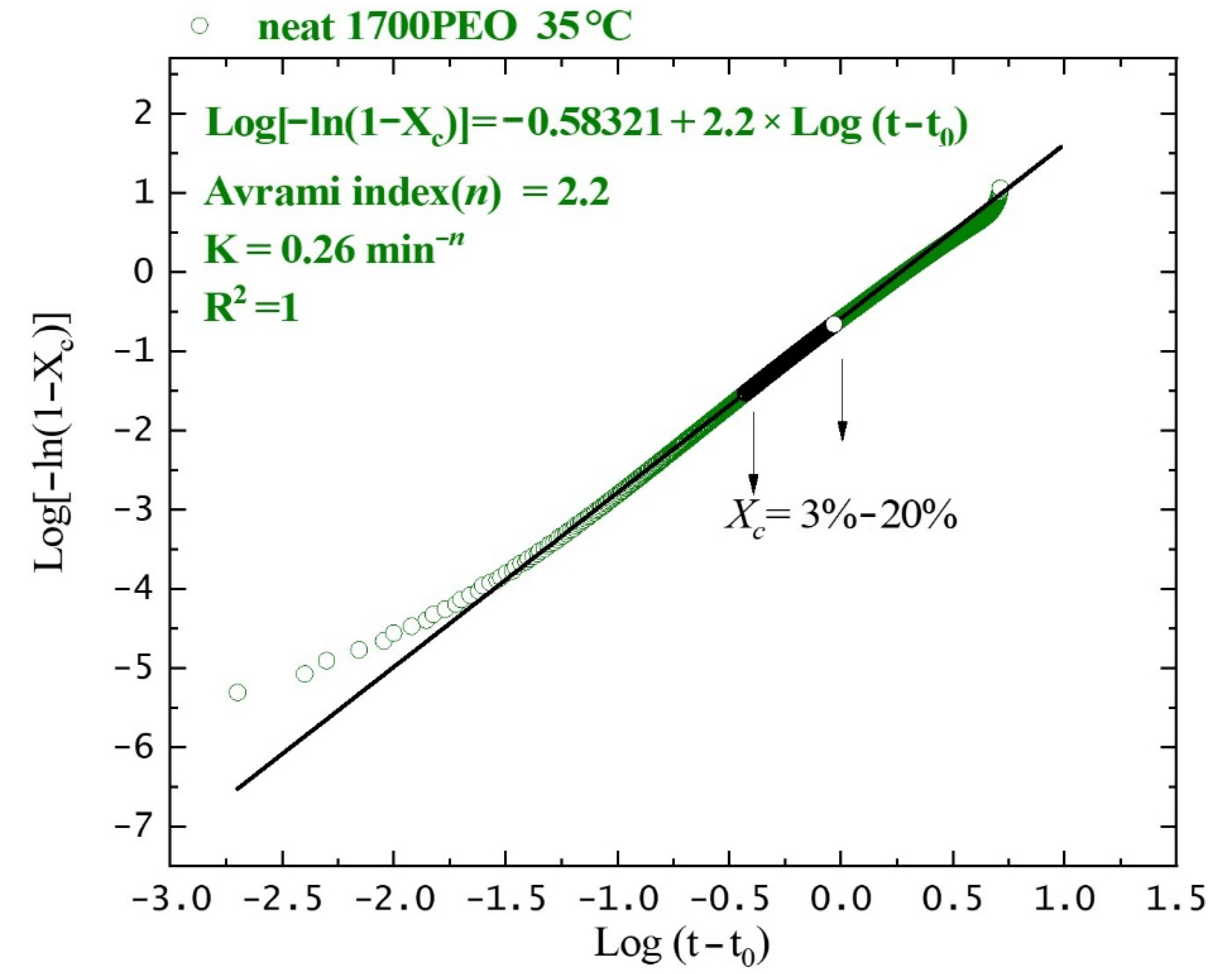
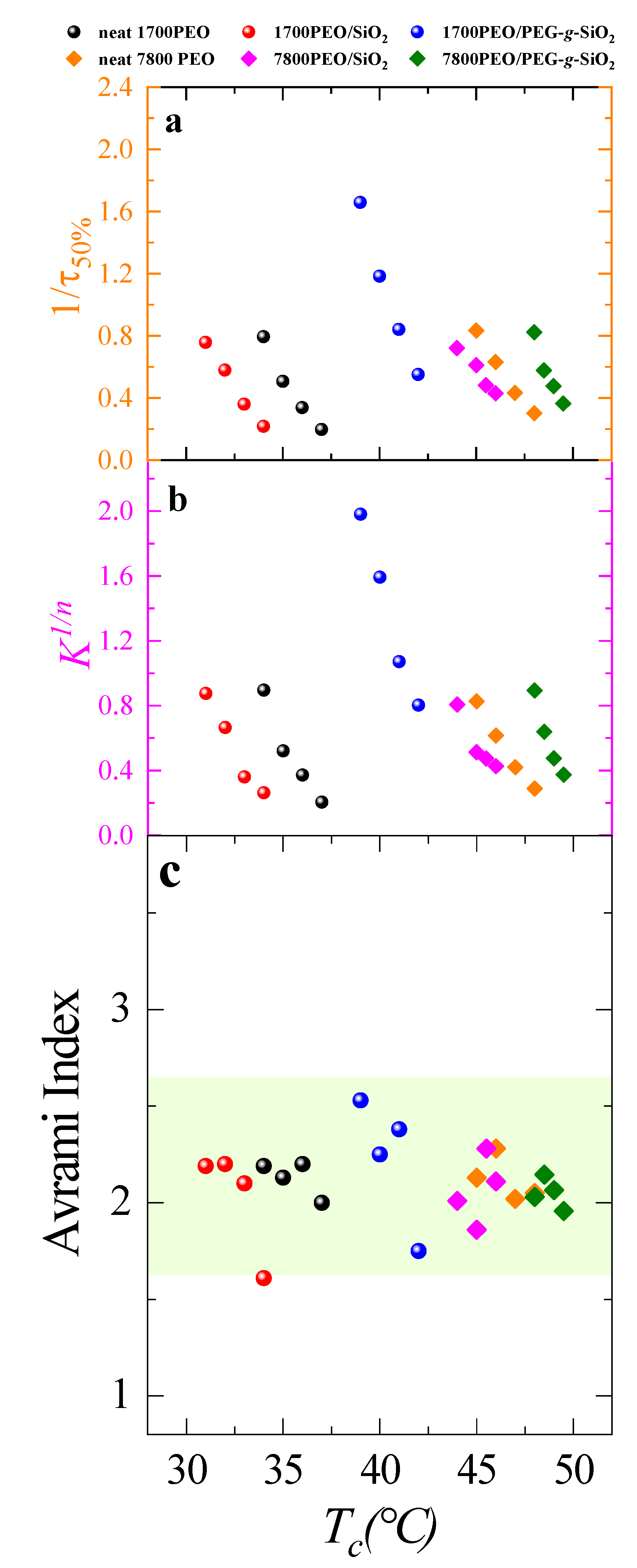
| Sample | Tc (°C) | Tm (°C) | a ΔHm (J g−1) | Xc (%) | Tm0 (°C) | Tg (°C) | (°C) | bKgG (K2) | cR2 |
|---|---|---|---|---|---|---|---|---|---|
| neat 1700PEO | 28.5 | 50.4 | 150.1 | 73.2 | 50.8 | −45.9 | −75.9 | 5.2 × 104 | 0.99 |
| 1700PEO/SiO2 | 25.9 | 49.1 | 159.2 | 77.6 | 48.8 | −35.7 | −65.7 | 5.9 × 104 | 0.99 |
| 1700PEO/PEG-g-SiO2 | 35.1 | 50.4 | 182.9 | 89.2 | 50.4 | −43.6 | −73.6 | 1.3 × 104 | 0.99 |
| neat 7800PEO | 39.2 | 62.3 | 171.9 | 83.8 | 62.2 | −40.3 | −70.3 | 3.7 × 104 | 0.99 |
| 7800PEO/SiO2 | 39.1 | 60.0 | 163.9 | 79.5 | 61.1 | −36.7 | −66.7 | 2.7 × 104 | 0.99 |
| 7800PEO/PEG-g-SiO2 | 43.1 | 61.1 | 170.4 | 83.1 | 62.5 | −37.3 | −67.3 | 3.2 × 104 | 0.96 |
| System 1 [55] | PEO | aVPEO/SANP = 78 | VPEO/SANP = 16 | VPEO/SANP = 139 |
|---|---|---|---|---|
| KgG (104 K2) | 13.0 | 13.1 | 13.1 | 12.2 |
| System 2 [50] | PEO | 1 wt% SiO2/PEO | 5 wt% SiO2/PEO | 9 wt% SiO2/PEO |
| KgG (104 K2) | 3.65 | 3.53 | 3.49 | 3.36 |
| System 3 [54] | PCL | b PCL–CNW | c PCL–MFC | |
| KgG (104 K2) | 14 | 6.3 | 5.5 | |
| System 4 [27] | d L-PCL | e L-PCL/SWNT-ODA | f L-PCL/MWNT-g-PCL | |
| KgG (104 K2) | 11.2 | 10.4 | 8.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, X.; Su, Y.; Li, S.; Ju, W.; Wang, D. Isothermal Crystallization Kinetics of Poly(ethylene oxide)/Poly(ethylene glycol)-g-silica Nanocomposites. Polymers 2021, 13, 648. https://doi.org/10.3390/polym13040648
Wen X, Su Y, Li S, Ju W, Wang D. Isothermal Crystallization Kinetics of Poly(ethylene oxide)/Poly(ethylene glycol)-g-silica Nanocomposites. Polymers. 2021; 13(4):648. https://doi.org/10.3390/polym13040648
Chicago/Turabian StyleWen, Xiangning, Yunlan Su, Shaofan Li, Weilong Ju, and Dujin Wang. 2021. "Isothermal Crystallization Kinetics of Poly(ethylene oxide)/Poly(ethylene glycol)-g-silica Nanocomposites" Polymers 13, no. 4: 648. https://doi.org/10.3390/polym13040648
APA StyleWen, X., Su, Y., Li, S., Ju, W., & Wang, D. (2021). Isothermal Crystallization Kinetics of Poly(ethylene oxide)/Poly(ethylene glycol)-g-silica Nanocomposites. Polymers, 13(4), 648. https://doi.org/10.3390/polym13040648