The Influence of Monomer Composition and Surface-CrossLinking Condition on Biodegradation and Gel Strength of Super Absorbent Polymer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the CSAP
2.3. Preparation of the Surface-Crosslinked SAP (SSAP)
2.4. Measurement
2.4.1. Fourier-Transform Infrared (FT-IR) Spectroscopic Analysis
2.4.2. Free Absorbency (FA)
2.4.3. Gel Content
2.4.4. Centrifuge Retention Capacity (CRC)
2.4.5. Absorbency Under Load (AUL)
2.4.6. Permeability
2.4.7. Rheological Properties
2.4.8. Degree of Biodegradation
3. Results and Discussion
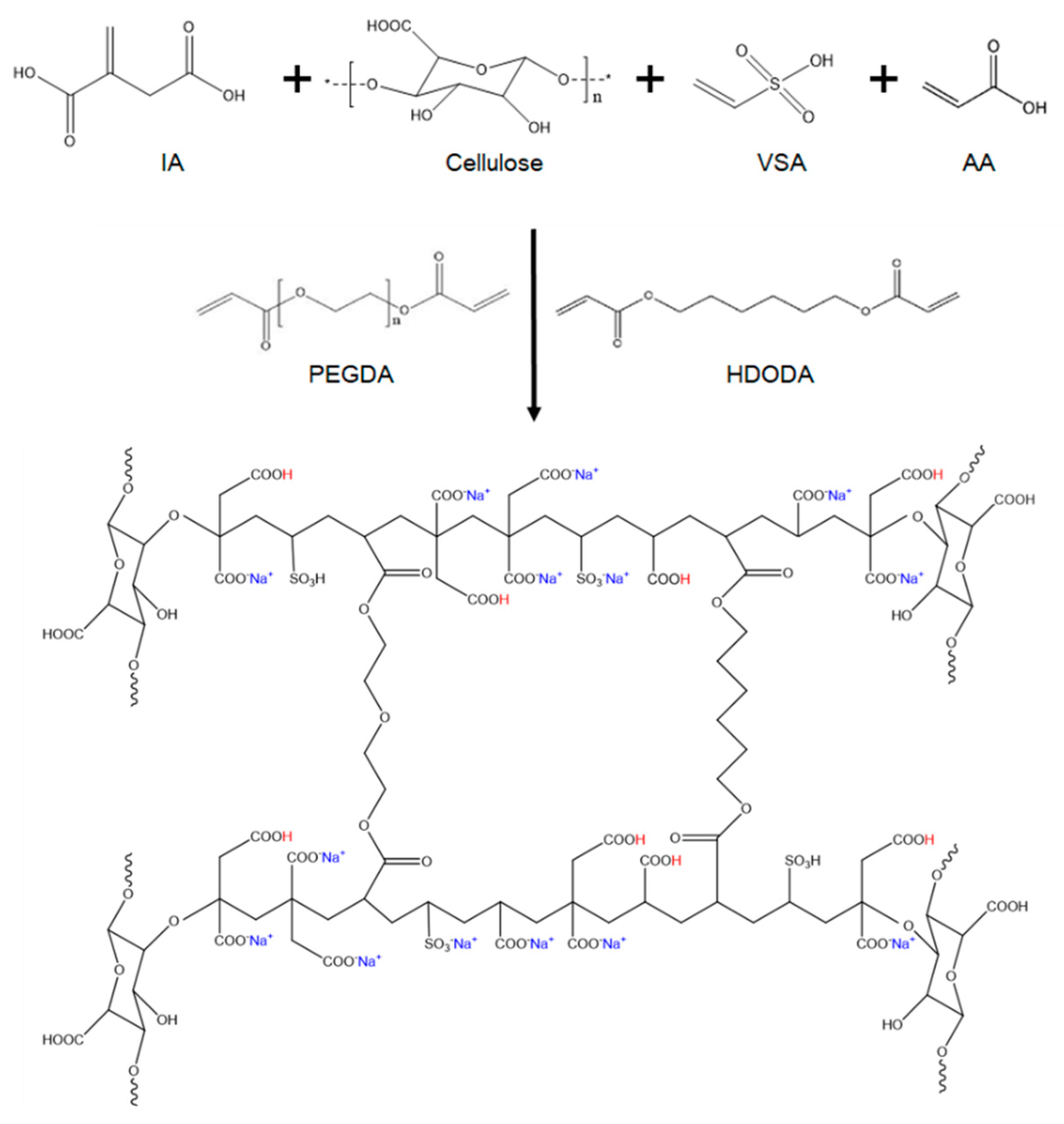
3.1. Synthesis of ICVA CSAP
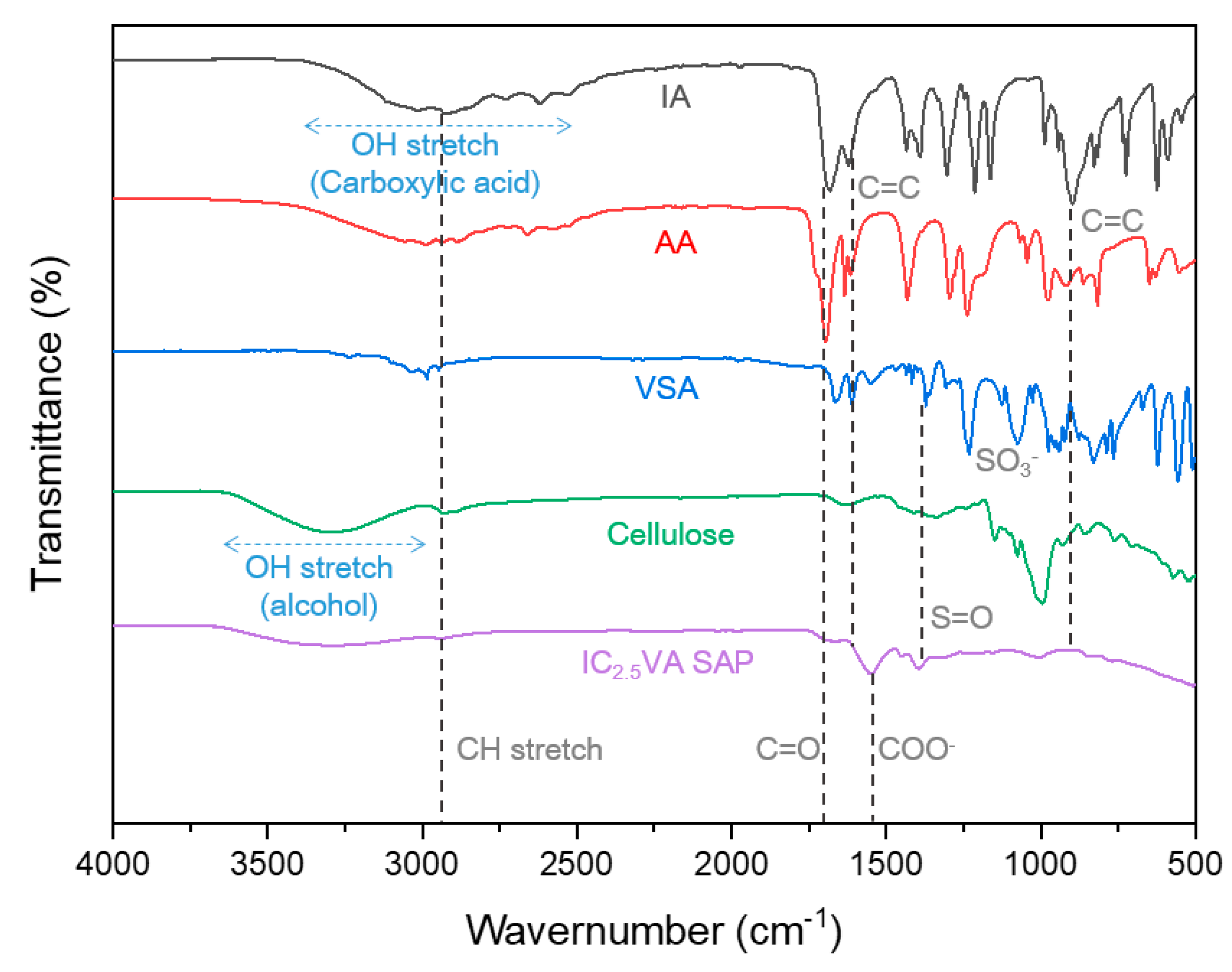
3.2. FT-IR Analysis of Monomers and IC2.5VA CSAP
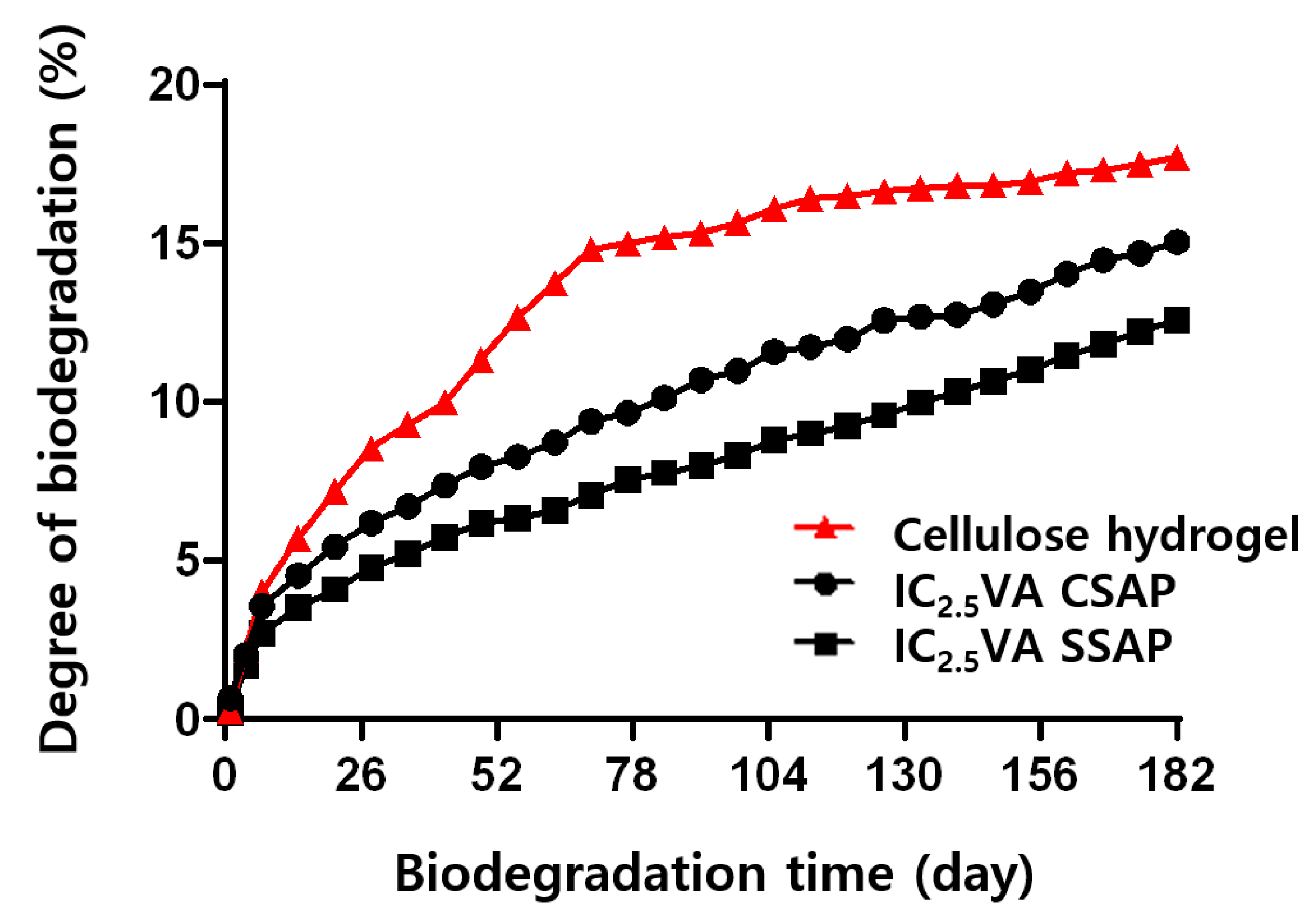
3.3. Properties of SAPs According to Composition of Cellulose and VSA
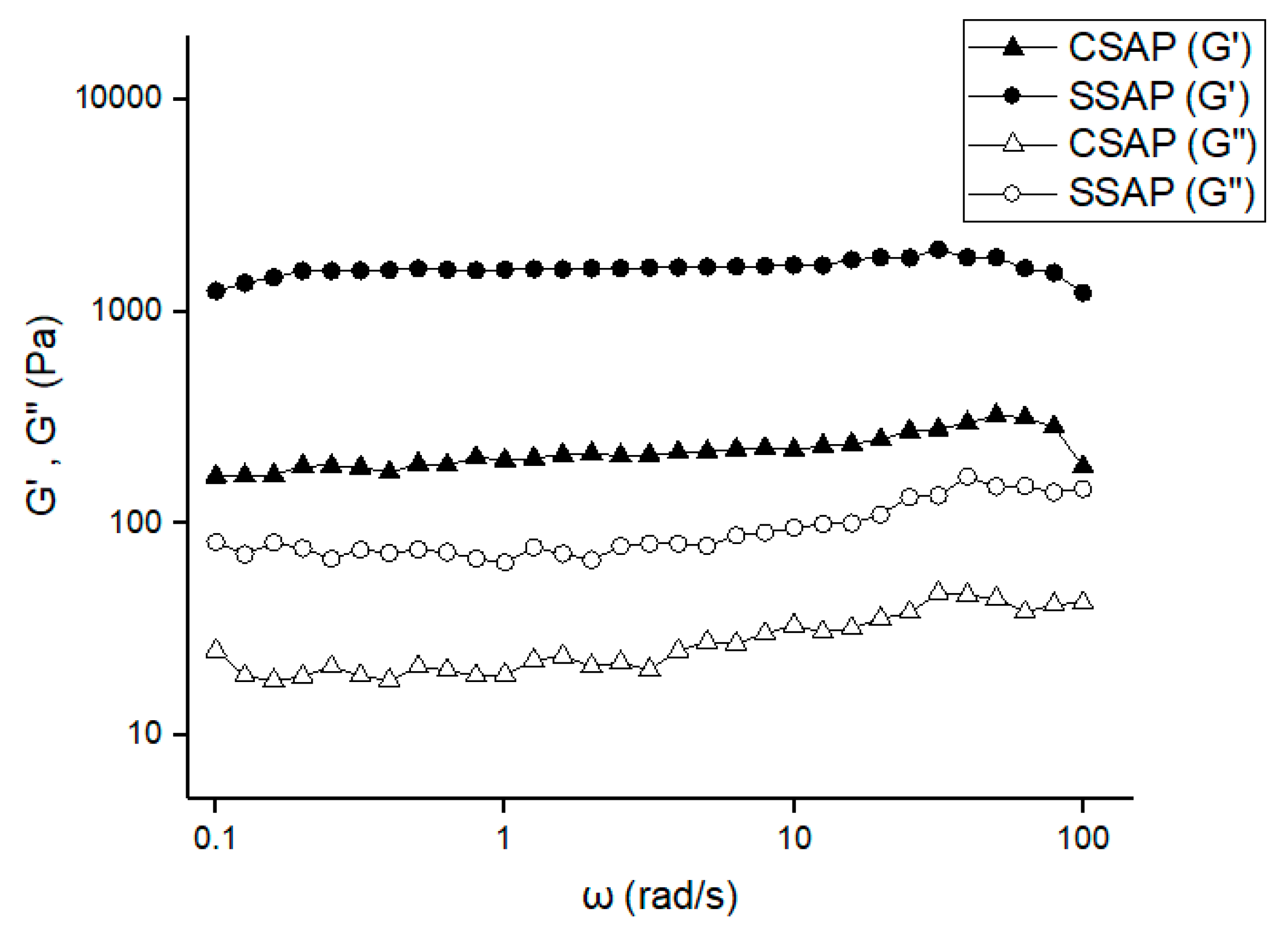
3.4. Properties of SAPs According to Surface-Crosslinking Time and Composition of Surface-Crosslinking Solution
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmed, E.M. Hydrogel: Preparation, characterization and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Cai, D.; He, L.; Zhong, N.; Yu, M.; Zhang, X.; Wu, Z. Fabrication of a high-performance fertilizer to control the loss of water and nutrient using micro/nano networks. ACS Sustain. Chem. Eng. 2015, 3, 645–653. [Google Scholar] [CrossRef]
- Schmaljohann, D. Thermo-and pH-responsive polymers in drug delivery. Adv. Drug Deliv. Rev. 2006, 58, 1655–1670. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.G.; Kim, M.Y.; Lee, J.M.; Jang, M.S. Superabsorbent Polymer. U.S. Patent No. 9,950,308, 24 April 2018. [Google Scholar]
- Hwang, K.S.; Jang, S.S.; Jung, Y.W.; Lee, S.H.; Ha, K.R. Studies on the Strength of Cement Mortars with Surface Crosslinked cPSA Absorbent. Polym. Korea 2012, 36, 208–215. [Google Scholar] [CrossRef] [Green Version]
- Azad, M.M.; Sandros, M.G. Microwave-assisted polymerization: Inert addition and surface coating of superabsorbent polymer for improved physical properties. J. Appl. Polym. Sci. 2016, 133, 43990. [Google Scholar] [CrossRef]
- Chang, S.; Kim, M.; Oh, S.; Min, J.H.; Kang, D.; Han, C.; Lee, H. Multi-scale characterization of surface-crosslinked superabsorbent polymer hydrogel spheres. Polymer 2015, 145, 174–183. [Google Scholar] [CrossRef]
- Mojarad-Jabali, S.; Kabiri, K.; Karami, Z.; Mastropietro, D.J.; Omidian, H. Surface cross-linked SAPs with improved swollen gel strength using diol compounds. J. Macromol. Sci. A 2020, 57, 62–71. [Google Scholar] [CrossRef]
- Xu, X.; Bai, B.; Ding, C.; Wang, H.; Suo, Y. Synthesis and properties of an ecofriendly superabsorbent composite by grafting the poly(acrylic acid) onto the surface of dopamine-coated sea buckthorn branches. Ind. Eng. Chem. Res. 2015, 54, 3268–3278. [Google Scholar] [CrossRef]
- Huang, Y.; Zeng, M.; Ren, J.; Wang, J.; Fan, L.; Xu, Q. Preparation and swelling properties of graphene oxide/poly (acrylic acid-co-acrylamide) super-absorbent hydrogel nanocomposites. Colloids Surf. A Physicochem. Eng. Asp. 2012, 401, 97–106. [Google Scholar] [CrossRef]
- Maitra, J.; Shukla, V.K. Cross-linking in hydrogels-a review. Am. J. Polym. Sci. 2014, 4, 25–31. [Google Scholar]
- Karadağ, E.; Saraydın, D.; Güven, O. Radiation induced superabsorbent hydrogels. Acrylamide/itaconic acid copolymers. Macromol. Mater. Eng. 2001, 286, 34–42. [Google Scholar]
- Dabbaghi, A.; Jahandideh, A.; Kabiri, K.; Ramazani, A.; Zohuriaan-Mehr, M.J. The synthesis and incorporation of a star-shaped bio-based modifier in the acrylic acid based superabsorbent: A strategy to enhance the absorbency under load. Polym.-Plast. Tech. Mat. 2019, 58, 1678–1690. [Google Scholar] [CrossRef]
- Bhuyan, M.; Chandra Dafader, N.; Hara, K.; Okabe, H.; Hidaka, Y.; Rahman, M.; Rahman, N. Synthesis of potato starch-acrylic-acid hydrogels by gamma radiation and their application in dye adsorption. Int. J. Polym. Sci. 2016, 2016, 1–11. [Google Scholar] [CrossRef]
- Ma, M.; Mukerabigwi, J.F.; Huang, R.; Lei, S.; Huang, X.; Cao, Y. Eco-Friendly Superabsorbent Synthesis Based on Polysaccharides. J. Polym. Environ. 2020, 28, 2801–2809. [Google Scholar] [CrossRef]
- Czarnecka, E.; Nowaczyk, J. Semi-Natural Superabsorbents Based on Starch-g-poly (acrylic acid): Modification, Synthesis and Application. Polymers 2020, 12, 1794. [Google Scholar] [CrossRef] [PubMed]
- Willke, T.; Vorlop, K.D. Biotechnological production of itaconic acid. Appl. Microbiol. Biotechnol. 2001, 56, 289–295. [Google Scholar] [CrossRef]
- Lanthong, P.; Nuisin, R.; Kiatkamjornwong, S. Graft copolymerization, characterization, and degradation of cassava starch-g-acrylamide/itaconic acid superabsorbents. Carbohydr. Polym. 2006, 66, 229–245. [Google Scholar] [CrossRef]
- Yang, J.; Li, F.; Li, M.; Zhang, S.; Liu, J.; Liang, C.; Xiong, L. Fabrication and characterization of hollow starch nanoparticles by gelation process for drug delivery application. Carbohydr. Polym. 2017, 173, 223–232. [Google Scholar] [CrossRef]
- Mylangam, C.K.; Beeravelli, S.; Medikonda, J.; Pidaparthi, J.S.; Kolapalli, V.R.M. Badam gum: A natural polymer in mucoadhesive drug delivery. Design, optimization, and biopharmaceutical evaluation of badam gum-based metoprolol succinate buccoadhesive tablets. Drug Deliv. 2016, 23, 195–206. [Google Scholar] [CrossRef]
- Riyajan, S.A. Robust and biodegradable polymer of cassava starch and modified natural rubber. Carbohydr. Polym. 2015, 134, 267–277. [Google Scholar] [CrossRef]
- Zhong, K.; Lin, Z.T.; Zheng, X.L.; Jiang, G.B.; Fang, Y.S.; Mao, X.Y.; Liao, Z.W. Starch derivative-based superabsorbent with integration of water-retaining and controlled-release fertilizers. Carbohydr. Polym. 2013, 92, 1367–1376. [Google Scholar] [CrossRef] [PubMed]
- Qiao, D.; Liu, H.; Yu, L.; Bao, X.; Simon, G.P.; Petinakis, E.; Chen, L. Preparation and characterization of slow-release fertilizer encapsulated by starch-based superabsorbent polymer. Carbohydr. Polym. 2016, 147, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Qiao, D.; Tu, W.; Wang, Z.; Yu, L.; Zhang, B.; Bao, X.; Lin, Q. Influence of crosslinker amount on the microstructure and properties of starch-based superabsorbent polymers by one-step preparation at high starch concentration. Int. J. Biol. Macromol. 2019, 129, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Park, S.J.; Kim, S.I. Properties of smart hydrogels composed of polyacrylic acid/poly (vinyl sulfonic acid) responsive to external stimuli. Smart Mater. Struct. 2004, 13, 317. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Rejeena, S.R. Poly (methacrylic acid-co-vinyl sulfonic acid)-grafted-magnetite/nanocellulose superabsorbent composite for the selective recovery and separation of immunoglobulin from aqueous solutions. Sep. Purif. Technol. 2013, 119, 82–93. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Ghasemzadeh, H. Carrageenan-g-poly (acrylamide)/poly (vinylsulfonic acid, sodium salt) as a novel semi-IPN hydrogel: Synthesis, characterization, and swelling behavior. Polym. Eng. Sci. 2007, 47, 1388–1395. [Google Scholar] [CrossRef]
- Hussain, T.; Ansari, M.; Ranjha, N.M.; Khan, I.U.; Shahzad, Y. Chemically cross-linked poly (acrylic-co-vinylsulfonic) acid hydrogel for the delivery of isosorbide mononitrate. Sci. World J. 2013, 2013, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Karami, Z.; Naderi, P.; Kabiri, K.; Zohuriaan-Mehr, M.J. Epoxidized and Cyclocarbonated Star-Shaped Macromolecules as Bio-Based Internal and External Crosslinkers for Superabsorbent Polymer Hydrogels. J. Polym. Environ. 2020, 28, 1684–1694. [Google Scholar] [CrossRef]
- Moini, N.; Kabiri, K.; Zohuriaan-Mehr, M.J. Practical improvement of SAP hydrogel properties via facile tunable cross-linking of the particles surface. Polym.-Plast. Tech. Eng. 2016, 55, 278–290. [Google Scholar] [CrossRef]
- Azizi, A.; Kabiri, K.; Zohuriaan-Mehr, M.J.; Bouhendi, H.; Karami, Z. Transamidation: A feasible approach of surface modification to improve absorbency under load of agricultural superabsorbent materials. J. Mater. Res. 2018, 33, 2327–2335. [Google Scholar] [CrossRef]
- Ghasri, M.; Bouhendi, H.; Kabiri, K.; Zohuriaan-Mehr, M.J.; Karami, Z.; Omidian, H. Superabsorbent polymers achieved by surface cross linking of poly (sodium acrylate) using microwave method. Iran. Polym. J. 2019, 28, 539–548. [Google Scholar] [CrossRef]
- Ha, J.; Kim, M.; Lee, W.; Lee, H.; Han, C.; Koh, W.G.; Ryu, D.Y. Direct measurement of crosslinked surface layer in superabsorbent poly (acrylic acid). Mater. Lett. 2018, 228, 33–36. [Google Scholar] [CrossRef]
- Lee, K.M.; Min, J.H.; Oh, S.; Lee, H.; Koh, W.G. Preparation and characterization of superabsorbent polymers (SAPs) surface-crosslinked with polycations. React. Funct. Polym. 2020, 157, 104774. [Google Scholar] [CrossRef]
- Yadav, M.; Srivastav, A.; Verma, S.K.; Behari, K. Graft (partially carboxymethylated guar gum-g-poly vinyl sulfonic acid) copolymer: From synthesis to applications. Carbohydr. Polym. 2013, 97, 597–603. [Google Scholar] [CrossRef]
- Chen, M.; Ni, Z.; Shen, Y.; Xiang, G.; Xu, L. Reinforced swelling and water-retention properties of super-absorbent hydrogel fabricated by a dual stretchable single network tactic. Colloids Surf. A Physicochem. Eng. Asp. 2020, 608, 125133. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Amini-Fazl, M.S.; Ayyari, M. Optimization of synthetic conditions CMC-g-poly (acrylic acid)/Celite composite superabsorbent by Taguchi method and determination of its absorbency under load. Express Polym. Lett. 2007, 1, 488–494. [Google Scholar] [CrossRef]
- Pulat, M.; Eksi, H. Determination of swelling behavior and morphological properties of poly (acrylamide-co-itaconic acid) and poly (acrylic acid-co-itaconic acid) copolymeric hydrogels. J. Appl. Polym. Sci. 2006, 102, 5994–5999. [Google Scholar] [CrossRef]
- Dabbaghi, A.; Kabiri, K.; Ramazani, A.; Zohuriaan-Mehr, M.J.; Jahandideh, A. Synthesis of bio-based internal and external cross-linkers based on tannic acid for preparation of antibacterial superabsorbents. Polym. Adv. Technol. 2019, 30, 2894–2905. [Google Scholar] [CrossRef]
- Amonpattaratkit, P.; Khunmanee, S.; Kim, D.H.; Park, H. Synthesis and characterization of gelatin-based crosslinkers for the fabrication of superabsorbent hydrogels. Materials 2017, 10, 826. [Google Scholar] [CrossRef] [Green Version]
- Olad, A.; Doustdar, F.; Gharekhani, H. Fabrication and characterization of a starch-based superabsorbent hydrogel composite reinforced with cellulose nanocrystals from potato peel waste. Colloid Surf. A-Physicochem. Eng. Asp. 2020, 601, 124962. [Google Scholar] [CrossRef]


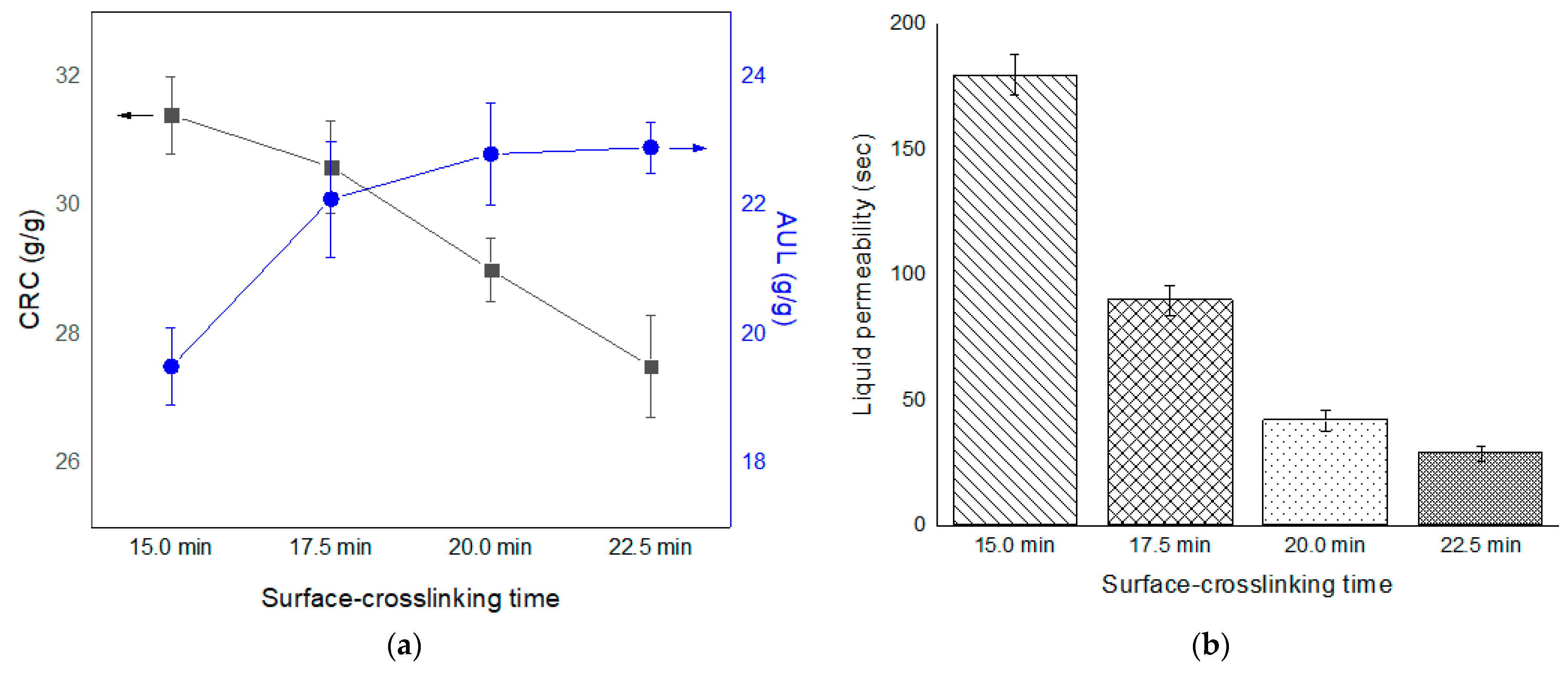
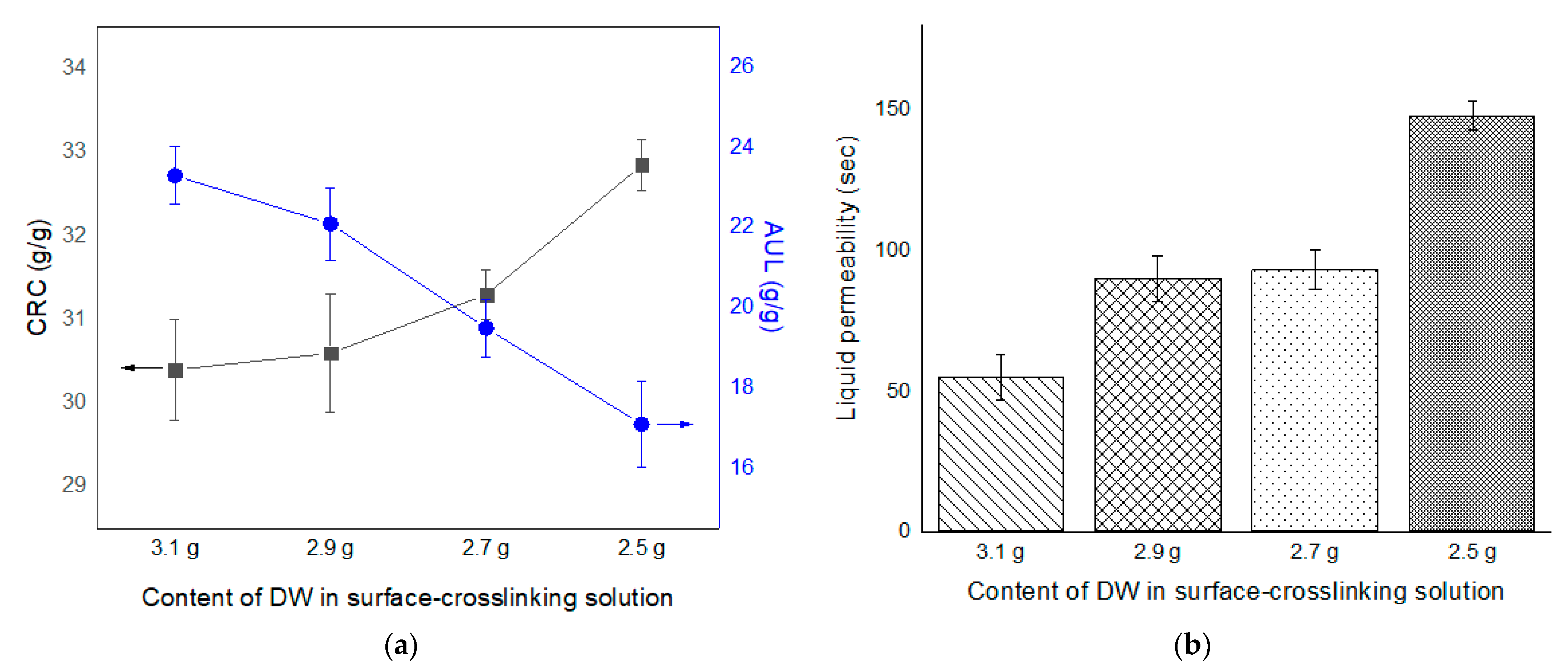
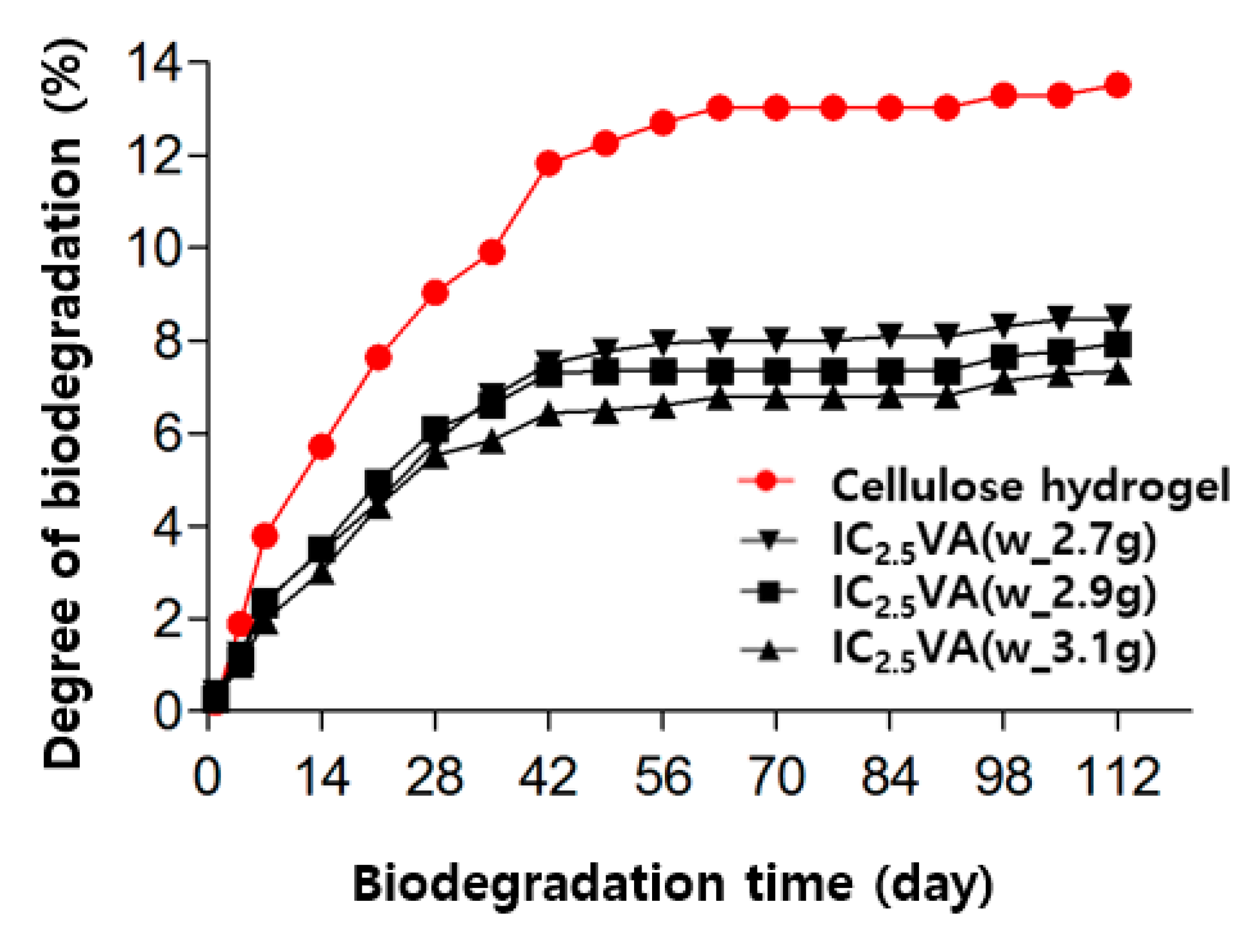
| NAME * | Monomer (g) | Crosslinker (g) | Initiator (g) | ||||
|---|---|---|---|---|---|---|---|
| IA | Cellulose | VSA | AA | HDODA | PEGDA | APS | |
| IVA | 50 | 0 | 10.0 | 40 | 0.25 | 0.25 | 0.5 |
| IC2.5VA | 2.5 | 7.5 | |||||
| IC5.0VA | 5.0 | 5.0 | |||||
| IC7.5VA | 7.5 | 2.5 | |||||
| ICA | 10.0 | 0 | |||||
| CSAP (g) | Surface-Crosslinking Solution Composition | Temperature (°C) | Time (min) | ||
|---|---|---|---|---|---|
| Methanol (g) | DW (g) | Surface-Crosslinker | |||
| BD (g) | |||||
| 3.0 | 6.1 | 2.9 | 1.0 | 170 | 15.0 |
| 17.5 | |||||
| 20.0 | |||||
| 22.5 | |||||
| 5.9 | 3.1 | 17.5 | |||
| 6.3 | 2.7 | ||||
| 6.5 | 2.5 | ||||
| NAME | CSAP | |
|---|---|---|
| CRC (g/g) | AUL (g/g) | |
| IVA | 55.8 | 6.6 |
| IC2.5VA | 52.3 | 7.6 |
| IC5.0VA | 51.3 | 7.6 |
| IC7.5VA | 49.8 | 7.3 |
| ICA | 49.6 | 7.8 |
| CSAP | SSAP * | |||
|---|---|---|---|---|
| CRC (g/g) | AUL (g/g) | Permeability (sec) | Gel Content (%) | |
| IVA | 30.6 | 17.4 | 126 | 90.0 |
| IC2.5VA | 29.0 | 22.8 | 42 | 90.6 |
| IC5.0VA | 27.0 | 22.9 | 35 | 90.1 |
| IC7.5VA | 25.5 | 24.1 | 25 | 89.2 |
| ICA | 24.4 | 23.6 | 28 | 89.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.S.; Kim, D.H.; Lee, Y.S. The Influence of Monomer Composition and Surface-CrossLinking Condition on Biodegradation and Gel Strength of Super Absorbent Polymer. Polymers 2021, 13, 663. https://doi.org/10.3390/polym13040663
Kim JS, Kim DH, Lee YS. The Influence of Monomer Composition and Surface-CrossLinking Condition on Biodegradation and Gel Strength of Super Absorbent Polymer. Polymers. 2021; 13(4):663. https://doi.org/10.3390/polym13040663
Chicago/Turabian StyleKim, Jung Soo, Dong Hyun Kim, and Youn Suk Lee. 2021. "The Influence of Monomer Composition and Surface-CrossLinking Condition on Biodegradation and Gel Strength of Super Absorbent Polymer" Polymers 13, no. 4: 663. https://doi.org/10.3390/polym13040663
APA StyleKim, J. S., Kim, D. H., & Lee, Y. S. (2021). The Influence of Monomer Composition and Surface-CrossLinking Condition on Biodegradation and Gel Strength of Super Absorbent Polymer. Polymers, 13(4), 663. https://doi.org/10.3390/polym13040663






