Bio-Based Hotmelt Adhesives with Well-Adhesion in Water
Abstract
:1. Introduction
2. Experiment Section
2.1. Materials
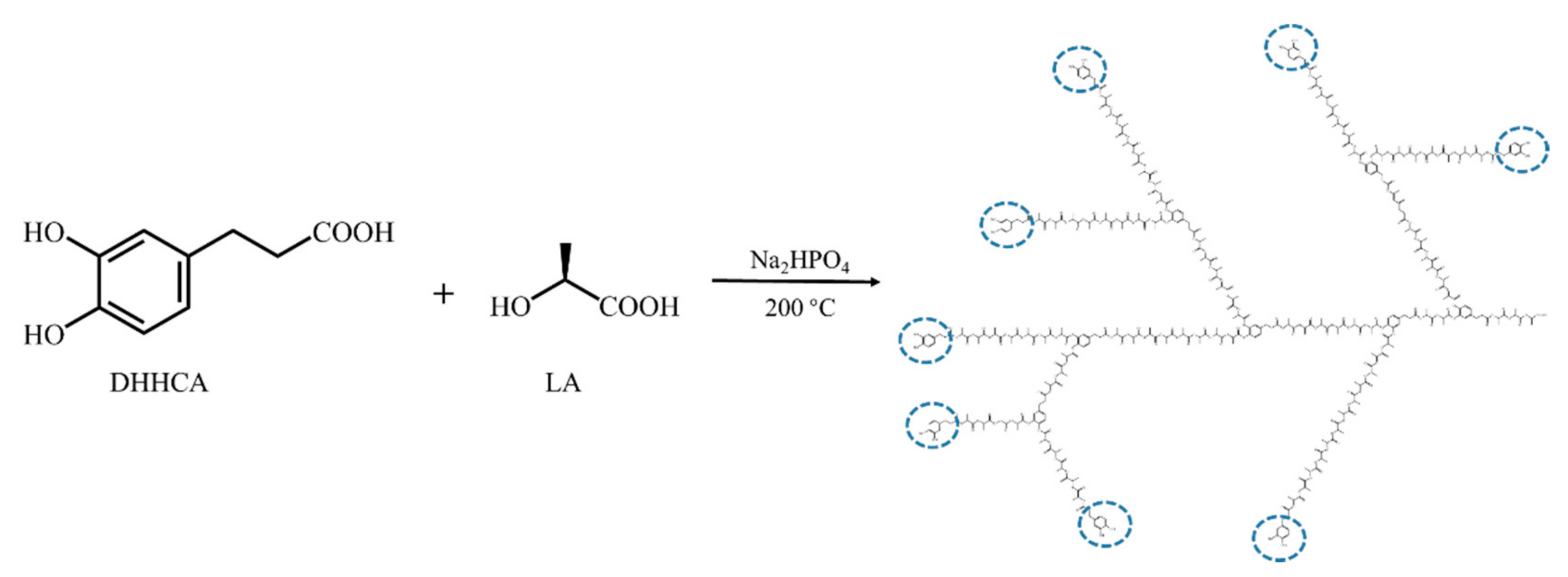
2.2. Synthesis of Poly(DHHCA-co-LA)
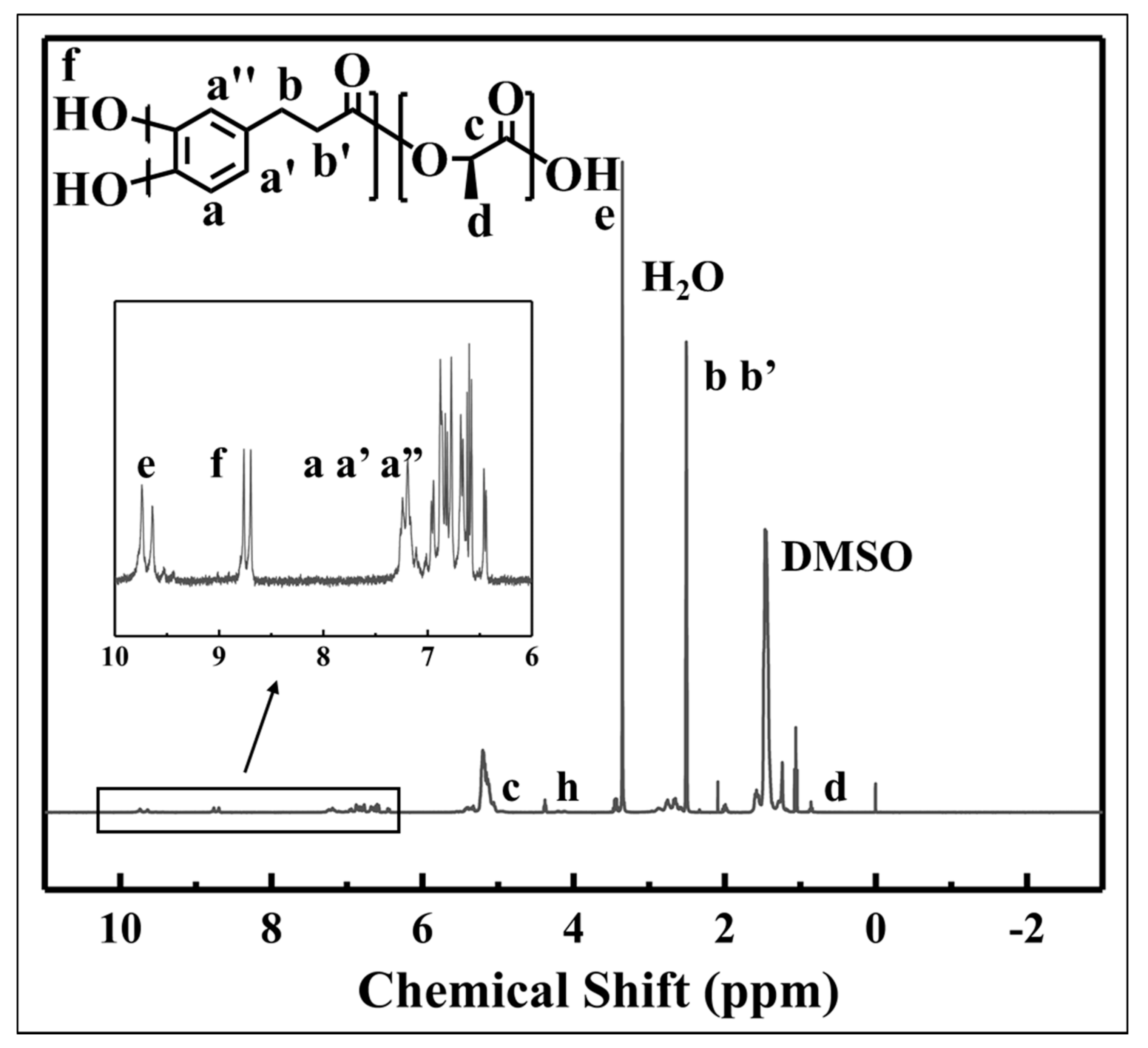
2.3. Characterization
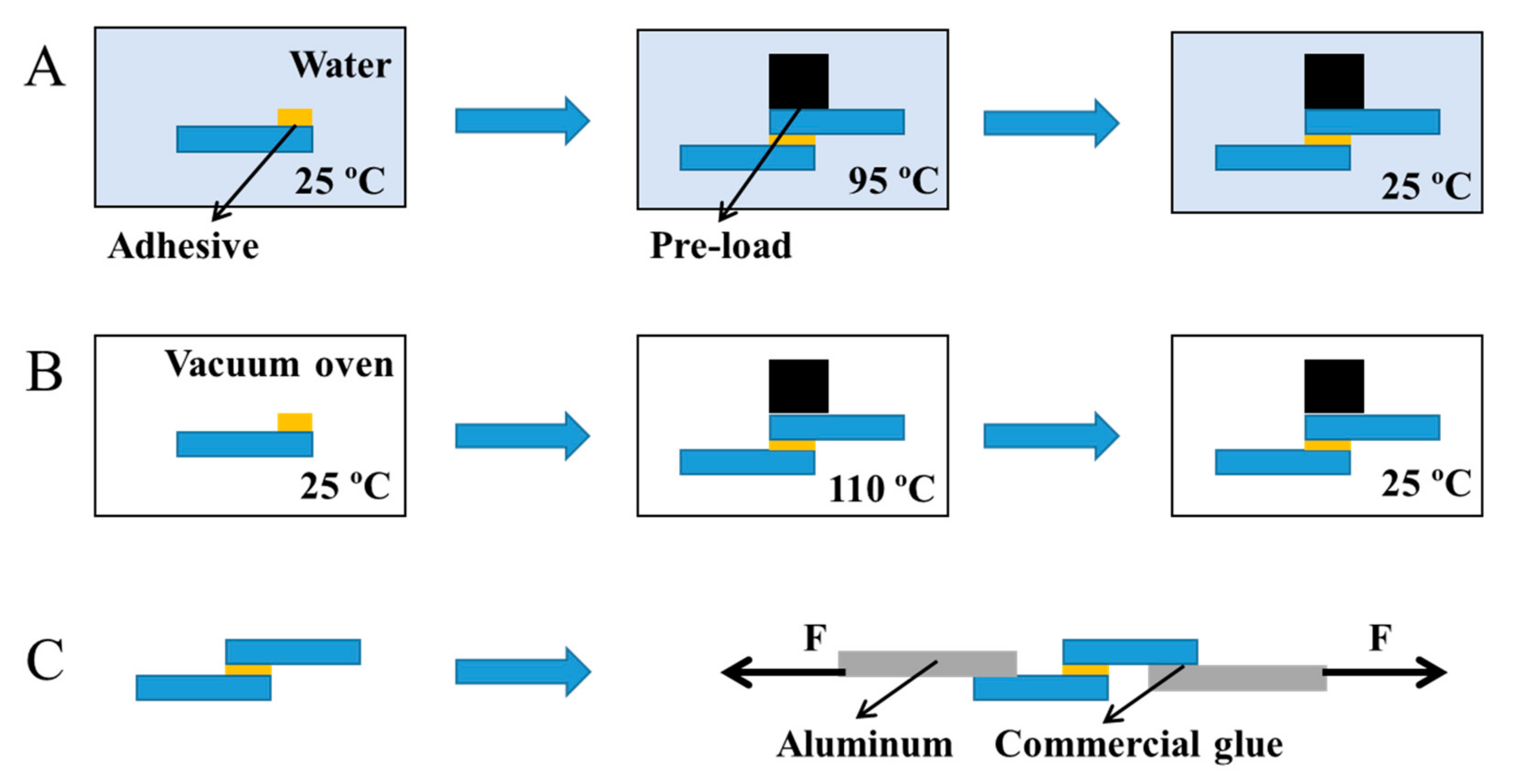
2.4. Lap Shear Test
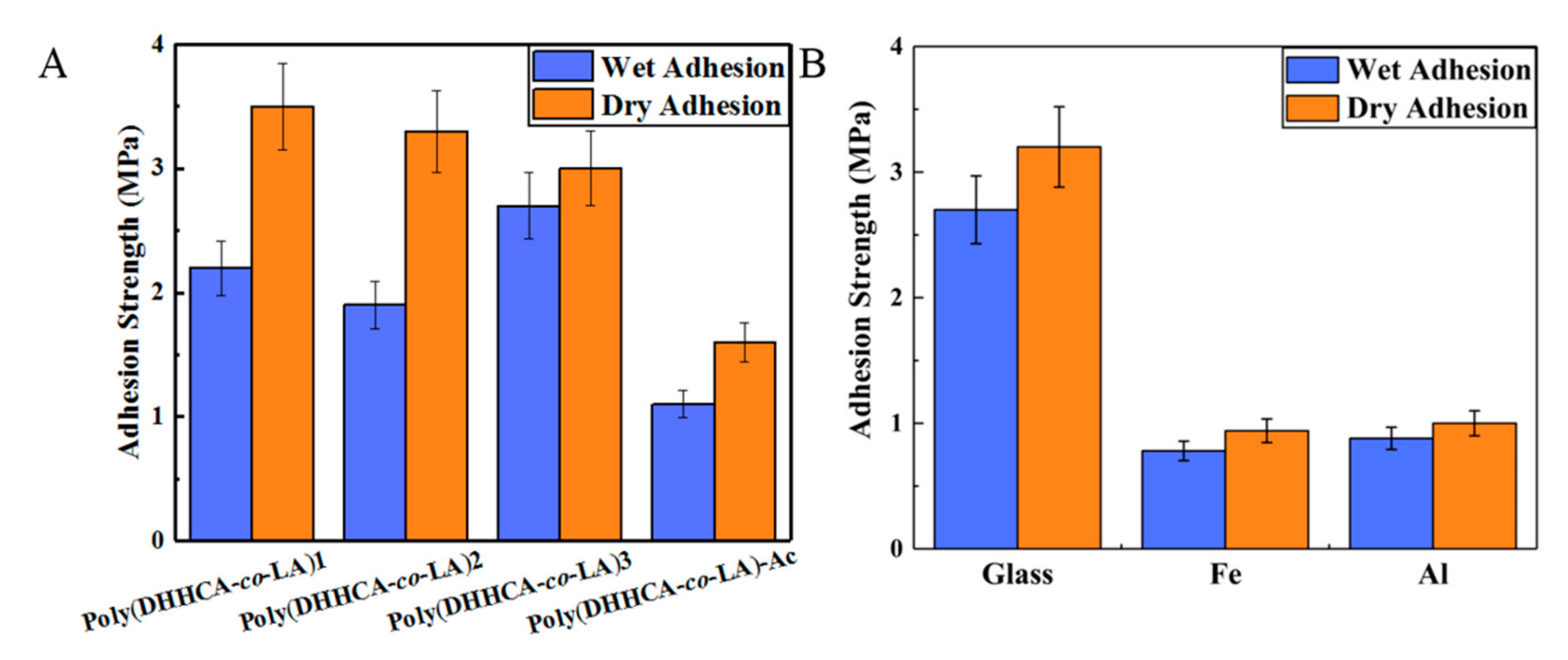
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- White, J.D.; Wilker, J.J. Underwater Bonding with charged polymer mimics of marine mussel adhesive proteins. Macromolecules 2011, 44, 5085–5088. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, Z.; Wang, Y.; Guo, L.; Yin, C.; Zhang, X.; Hao, J.; Zhang, G.; Chen, L. Eco-friendly, self-healing hydrogels for adhesive and elastic strain sensors, circuit repairing, and flexible electronic devices. Macromolecules 2019, 52, 2531–2541. [Google Scholar] [CrossRef]
- Zhang, H.; Bre, L.P.; Zhao, T.; Zheng, Y.; Newland, B.; Wang, W. Mussel-inspired hyperbranched poly(amino ester) polymer as strong wet tissue adhesive. Biomaterials 2014, 35, 711–719. [Google Scholar] [CrossRef]
- Yang, Y.J.; Kwon, Y.; Choi, B.H.; Jung, D.; Seo, J.H.; Lee, K.H.; Cha, H.J. Multifunctional adhesive silk fibroin with blending of RGD-bioconjugated mussel adhesive protein. Biomacromolecules 2014, 15, 1390–1398. [Google Scholar] [CrossRef]
- Ohzono, T.; Saed, M.O.; Terentjev, E.M. Enhanced dynamic adhesion in nematic liquid crystal elastomers. Adv. Mater. 2019, 31, e1902642. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Q.; Gao, G. Bioinspired adhesive hydrogels tackified by nucleobases. Adv. Funct. Mater. 2017, 27, 1703132. [Google Scholar] [CrossRef]
- Cui, C.; Fan, C.; Wu, Y.; Xiao, M.; Wu, T.; Zhang, D.; Chen, X.; Liu, B.; Xu, Z.; Qu, B.; et al. Water-triggered hyperbranched polymer universal adhesives: From strong underwater adhesion to rapid sealing hemostasis. Adv. Mater. 2019, 31, e1905761. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, Y.; Wang, L.; Zhang, M.; Chen, X.; Liu, M.; Fan, J.; Liu, J.; Zhou, F.; Wang, Z. Bio-inspired reversible underwater adhesive. Nat. Commun. 2017, 8, 2218. [Google Scholar] [CrossRef]
- Tiu, B.D.B.; Delparastan, P.; Ney, M.R.; Gerst, M.; Messersmith, P.B. Enhanced adhesion and cohesion of bioinspired dry/wet pressure-sensitive adhesives. ACS Appl. Mater. Interfaces 2019, 11, 28296–28306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mogaki, R.; Okuro, K.; Ueki, R.; Sando, S.; Aida, T. Molecular glue that spatiotemporally turns on protein-protein interactions. J. Am. Chem. Soc. 2019, 141, 8035–8040. [Google Scholar] [CrossRef]
- Meredith, H.J.; Wilker, J.J. The interplay of modulus, strength, and ductility in adhesive design using biomimetic polymer chemistry. Adv. Funct. Mater. 2015, 25, 5057–5065. [Google Scholar] [CrossRef]
- Dompe, M.; Cedano-Serrano, F.J.; Heckert, O.; van den Heuvel, N.; van der Gucht, J.; Tran, Y.; Hourdet, D.; Creton, C.; Kamperman, M. Thermoresponsive complex coacervate-based underwater adhesive. Adv. Mater. 2019, 31, e1808179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baik, S.; Kim, D.W.; Park, Y.; Lee, T.J.; Ho Bhang, S.; Pang, C. A wet-tolerant adhesive patch inspired by protuberances in suction cups of octopi. Nature 2017, 546, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Ahmed, M.; Long, L.; Khashab, N.M.; Huang, F.; Sessler, J.L. Adhesive supramolecular polymeric materials constructed from macrocycle-based host-guest interactions. Chem. Soc. Rev. 2019, 48, 2682–2697. [Google Scholar] [CrossRef] [PubMed]
- Tamesue, S.; Yasuda, K.; Endo, T. Adhesive hydrogel system based on the intercalation of anionic substituents into layered double hydroxides. ACS Appl. Mater. Interfaces 2018, 10, 29925–29932. [Google Scholar] [CrossRef]
- Ryu, J.H.; Kim, H.J.; Kim, K.; Yoon, G.; Wang, Y.; Choi, G.S.; Lee, H.; Park, J.S. Multipurpose intraperitoneal adhesive patches. Adv. Funct. Mater. 2019, 29, 1900945. [Google Scholar] [CrossRef]
- Qiao, H.; Qi, P.; Zhang, X.; Wang, L.; Tan, Y.; Luan, Z.; Xia, Y.; Li, Y.; Sui, K. Multiple weak h-bonds lead to highly sensitive, stretchable, self-adhesive, and self-healing ionic sensors. ACS Appl. Mater. Interfaces 2019, 11, 7755–7763. [Google Scholar] [CrossRef] [PubMed]
- Panchireddy, S.; Grignard, B.; Thomassin, J.-M.; Jerome, C.; Detrembleur, C. Catechol containing polyhydroxyurethanes as high-performance coatings and adhesives. ACS Sustain. Chem. Eng. 2018, 6, 14936–14944. [Google Scholar] [CrossRef]
- Jia, Z.; Zeng, Y.; Tang, P.; Gan, D.; Xing, W.; Hou, Y.; Wang, K.; Xie, C.; Lu, X. Conductive, tough, transparent, and self-healing hydrogels based on catechol–metal ion dual self-catalysis. Chem. Mater. 2019, 31, 5625–5632. [Google Scholar] [CrossRef]
- Cholewinski, A.; Yang, F.; Zhao, B. Algae–mussel-inspired hydrogel composite glue for underwater bonding. Mater. Horizons 2019, 6, 285–293. [Google Scholar] [CrossRef]
- Liang, C.; Ye, Z.; Xue, B.; Zeng, L.; Wu, W.; Zhong, C.; Cao, Y.; Hu, B.; Messersmith, P.B. Self-assembled nanofibers for strong underwater adhesion: The trick of barnacles. ACS Appl. Mater. Interfaces 2018, 10, 25017–25025. [Google Scholar] [CrossRef] [PubMed]
- Kamprad, N.; Witt, H.; Schroder, M.; Kreis, C.T.; Baumchen, O.; Janshoff, A.; Tarantola, M. Adhesion strategies of Dictyostelium discoideum—A force spectroscopy study. Nanoscale 2018, 10, 22504–22519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, B.; Zhang, G.; Lian, J.; Zhang, Q.; Zhan, X.; Chen, F. Boron nitride nanosheet embedded bio-inspired wet adhesives with switchable adhesion and oxidation resistance. J. Mater. Chem. A 2019, 7, 12266–12275. [Google Scholar] [CrossRef]
- Jeon, E.Y.; Hwang, B.H.; Yang, Y.J.; Kim, B.J.; Choi, B.H.; Jung, G.Y.; Cha, H.J. Rapidly light-activated surgical protein glue inspired by mussel adhesion and insect structural crosslinking. Biomaterials 2015, 67, 11–19. [Google Scholar] [CrossRef]
- Gan, D.; Xing, W.; Jiang, L.; Fang, J.; Zhao, C.; Ren, F.; Fang, L.; Wang, K.; Lu, X. Plant-inspired adhesive and tough hydrogel based on Ag-Lignin nanoparticles-triggered dynamic redox catechol chemistry. Nat. Commun. 2019, 10, 1487. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Hou, J.; Yuan, Q.; Xin, P.; Cheng, H.; Gu, Z.; Wu, J. Arginine derivatives assist dopamine-hyaluronic acid hybrid hydrogels to have enhanced antioxidant activity for wound healing. Chem. Eng. J. 2020, 392, 123775. [Google Scholar] [CrossRef]
- Xie, T.; Ding, J.; Han, X.; Jia, H.; Yang, Y.; Liang, S.; Wang, W.; Liu, W.; Wang, W. Wound dressing change facilitated by spraying zinc ions. Mater. Horizons 2020, 7, 605–614. [Google Scholar] [CrossRef]
- Han, L.; Lu, X.; Liu, K.; Wang, K.; Fang, L.; Weng, L.T.; Zhang, H.; Tang, Y.; Ren, F.; Zhao, C.; et al. Mussel-Inspired adhesive and tough hydrogel based on nanoclay confined dopamine polymerization. ACS Nano 2017, 11, 2561–2574. [Google Scholar] [CrossRef]
- Saiz-Poseu, J.; Mancebo-Aracil, J.; Nador, F.; Busque, F.; Ruiz-Molina, D. The chemistry behind catechol-based adhesion. Angew. Chem. Int. Ed. Engl. 2019, 58, 696–714. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cao, Y. The molecular mechanisms underlying mussel adhesion. Nanoscale Adv. 2019, 1, 4246–4257. [Google Scholar] [CrossRef] [Green Version]
- Hwang, D.S.; Sim, S.B.; Cha, H.J. Cell adhesion biomaterial based on mussel adhesive protein fused with RGD peptide. Biomaterials 2007, 28, 4039–4046. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.S.; Gim, Y.; Yoo, H.J.; Cha, H.J. Practical recombinant hybrid mussel bioadhesive fp-151. Biomaterials 2007, 28, 3560–3568. [Google Scholar] [CrossRef] [PubMed]
- Baik, S.; Lee, H.J.; Kim, D.W.; Kim, J.W.; Lee, Y.; Pang, C. Bioinspired Adhesive architectures: From skin patch to integrated bioelectronics. Adv. Mater. 2019, 31, e1803309. [Google Scholar] [CrossRef]
- Zhu, W.; Peck, Y.; Iqbal, J.; Wang, D.A. A novel DOPA-albumin based tissue adhesive for internal medical applications. Biomaterials 2017, 147, 99–115. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Dai, B.; Qiao, J.B.; Li, W.; Fortner, J.D.; Zhang, F. Microbially synthesized repeats of mussel foot protein display enhanced underwater adhesion. ACS Appl. Mater. Interfaces 2018, 10, 43003–43012. [Google Scholar] [CrossRef]
- Yang, J.; Bai, R.; Chen, B.; Suo, Z. Hydrogel adhesion: A Supramolecular synergy of chemistry, topology, and mechanics. Adv. Funct. Mater. 2019, 30, 1901693. [Google Scholar] [CrossRef]
- Yang, B.; Jin, S.; Park, Y.; Jung, Y.M.; Cha, H.J. Coacervation of interfacial adhesive proteins for initial mussel adhesion to a wet surface. Small 2018, 14, e1803377. [Google Scholar] [CrossRef]
- Wei, K.; Chen, X.; Zhao, P.; Feng, Q.; Yang, B.; Li, R.; Zhang, Z.Y.; Bian, L. Stretchable and bioadhesive supramolecular hydrogels activated by a one-stone-two-bird postgelation functionalization method. ACS Appl. Mater. Interfaces 2019, 11, 16328–16335. [Google Scholar] [CrossRef] [PubMed]
- Sedo, J.; Saiz-Poseu, J.; Busque, F.; Ruiz-Molina, D. Catechol-based biomimetic functional materials. Adv. Mater. 2013, 25, 653–701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Zeng, H. Marine mussel adhesion and bio-inspired wet adhesives. Biotribology 2016, 5, 44–51. [Google Scholar] [CrossRef]
- Lee, H.; Lee, Y.; Statz, A.R.; Rho, J.; Park, T.G.; Messersmith, P.B. Substrate-independent layer-by-layer assembly by using mussel-adhesive-inspired polymers. Adv. Mater. 2008, 20, 1619–1623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, S.; Lee, D.W.; Ahn, J.S.; Cunha, K.; Filippidi, E.; Ju, S.W.; Shin, E.; Kim, B.-S.; Levine, Z.A.; Lins, R.D.; et al. Significant Performance Enhancement of Polymer Resins by Bioinspired Dynamic Bonding. Adv. Mater. 2017, 29, 1703026. [Google Scholar] [CrossRef] [Green Version]
- Kizilkan, E.; Gorb, S.N. Bioinspired further enhanced dry adhesive by the combined effect of the microstructure and surface free-energy increase. ACS Appl. Mater. Interfaces 2018, 10, 26752–26758. [Google Scholar] [CrossRef]
- Hwang, D.G.; Trent, K.; Bartlett, M.D. Kirigami-inspired structures for smart adhesion. ACS Appl. Mater. Interfaces 2018, 10, 6747–6754. [Google Scholar] [CrossRef] [PubMed]
- Singla, S.; Amarpuri, G.; Dhopatkar, N.; Blackledge, T.A.; Dhinojwala, A. Hygroscopic compounds in spider aggregate glue remove interfacial water to maintain adhesion in humid conditions. Nat. Commun. 2018, 9, 1890. [Google Scholar] [CrossRef]
- Li, A.; Jia, Y.; Sun, S.; Xu, Y.; Minsky, B.B.; Stuart, M.A.C.; Colfen, H.; von Klitzing, R.; Guo, X. Mineral-enhanced polyacrylic acid hydrogel as an oyster-inspired organic-inorganic hybrid adhesive. ACS Appl. Mater. Interfaces 2018, 10, 10471–10479. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, L.; Xiao, H.; Cong, H.; Wang, S. A reversible underwater glue based on photo- and thermo-responsive dynamic covalent bonds. Mater. Horiz. 2020, 7, 282–288. [Google Scholar] [CrossRef]
- Kislyak, A.; Kodura, D.; Frisch, H.; Feist, F.; Van Steenberge, P.H.M.; Barner-Kowollik, C.; D’Hooge, D.R. A holistic approach for anthracene photochemistry kinetics. Chem. Eng. J. 2020, 402, 126259. [Google Scholar] [CrossRef]
- Jenkins, C.L.; Siebert, H.M.; Wilker, J.J. Integrating mussel chemistry into a bio-based polymer to create degradable adhesives. Macromolecules 2017, 50, 561–568. [Google Scholar] [CrossRef]
- Heinrich, L.A. Future opportunities for bio-based adhesives–Advantages beyond renewability. Green Chem. 2019, 21, 1866–1888. [Google Scholar] [CrossRef] [Green Version]
- Korde, J.M.; Kandasubramanian, B. Biocompatible alkyl cyanoacrylates and their derivatives as bio-adhesives. Biomater. Sci. 2018, 6, 1691–1711. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Lu, S.; Zhang, Z.; Zhu, L.; Wen, Y.; Zhang, T.; Ji, Z. Mussel-inspired copolymer-coated polypropylene mesh with anti-adhesion efficiency for abdominal wall defect repair. Biomater. Sci. 2019, 7, 1323–1334. [Google Scholar] [CrossRef]
- Hong, S.; Yang, K.; Kang, B.; Lee, C.; Song, I.T.; Byun, E.; Park, K.I.; Cho, S.-W.; Lee, H. Hyaluronic acid catechol: A biopolymer exhibiting a pH-dependent adhesive or cohesive property for human neural stem cell engineering. Adv. Funct. Mater. 2013, 23, 1774–1780. [Google Scholar] [CrossRef]
- Han, L.; Wang, M.; Li, P.; Gan, D.; Yan, L.; Xu, J.; Wang, K.; Fang, L.; Chan, C.W.; Zhang, H.; et al. Mussel-Inspired tissue-adhesive hydrogel based on the polydopamine-chondroitin sulfate complex for growth-factor-free cartilage regeneration. ACS Appl. Mater. Interfaces 2018, 10, 28015–28026. [Google Scholar] [CrossRef] [PubMed]
- Hiraishi, N.; Kaneko, D.; Taira, S.; Wang, S.; Otsuki, M.; Tagami, J. Mussel-mimetic, bioadhesive polymers from plant-derived materials. J. Investig. Clin. Dent. 2015, 6, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, D.; Wang, S.; Matsumoto, K.; Kinugawa, S.; Yasaki, K.; Chi, D.H.; Kaneko, T. Mussel-mimetic strong adhesive resin from bio-base polycoumarates. Polymer J. 2011, 43, 855–858. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Kitamura, Y.; Hiraishi, N.; Taira, S.; Tsuge, A.; Kaneko, T.; Kaneko, D. Preparation of mussel-inspired biopolyester adhesive and comparative study of effects of meta- or para-hydroxyphenylpropionic acid segments on their properties. Polymer 2019, 165, 152–162. [Google Scholar] [CrossRef]
- Kaur, S.; Narayanan, A.; Dalvi, S.; Liu, Q.; Joy, A.; Dhinojwala, A. Direct observation of the interplay of catechol binding and polymer hydrophobicity in a mussel-inspired elastomeric adhesive. ACS Cent. Sci. 2018, 4, 1420–1429. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Sample Name | CDHHCA (%) | CLA (%) | Mn (g/mol) | Mw (g/mol) | Tg (°C) |
|---|---|---|---|---|---|
| Poly(DHHCA-co-LA)1 | 5.0 | 95.0 | 7.7 × 103 | 1.2 × 104 | 40.6 |
| Poly(DHHCA-co-LA)2 | 3.3 | 96.7 | 6.9 × 103 | 8.9 × 103 | 39.2 |
| Poly(DHHCA-co-LA)3 a | 1.2 | 98.8 | 6.3 × 103 | 1.0 × 104 | 37.3 |
| Poly(DHHCA-co-LA)8 b | 5.0 | 95.0 | 4.2 × 103 | 6.8 × 103 | 35.5 |
| Poly(DHHCA-co-LA)190 c | 5.0 | 95.0 | 2.8 × 103 | 4.7 × 103 | 22.8 |
| Poly(DHHCA-co-LA)-Ac | 5.0 | 95.0 | 7.8 × 103 | 1.1 × 104 | 40.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, X.; Dong, C.; Zhuang, W.; Shi, D.; Dong, W.; Chen, M.; Kaneko, D. Bio-Based Hotmelt Adhesives with Well-Adhesion in Water. Polymers 2021, 13, 666. https://doi.org/10.3390/polym13040666
Yu X, Dong C, Zhuang W, Shi D, Dong W, Chen M, Kaneko D. Bio-Based Hotmelt Adhesives with Well-Adhesion in Water. Polymers. 2021; 13(4):666. https://doi.org/10.3390/polym13040666
Chicago/Turabian StyleYu, Xi, Chuang Dong, Wei Zhuang, Dongjian Shi, Weifu Dong, Mingqing Chen, and Daisaku Kaneko. 2021. "Bio-Based Hotmelt Adhesives with Well-Adhesion in Water" Polymers 13, no. 4: 666. https://doi.org/10.3390/polym13040666





