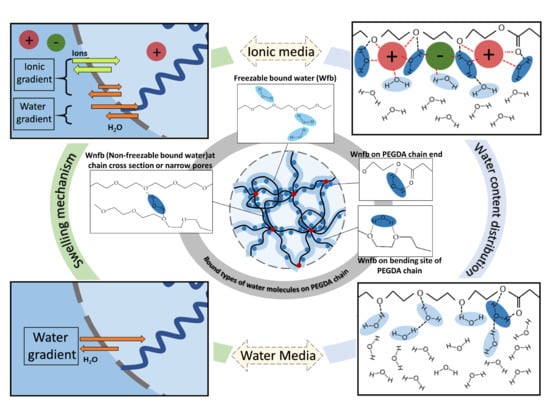
Elucidating the Molecular Mechanisms for the Interaction of Water with Polyethylene Glycol-Based Hydrogels: Influence of Ionic Strength and Gel Network Structure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. PEGDA Hydrogel Preparation
2.3. Dynamic Gravimetric Swelling Ratio
2.4. Dynamic Volumetric Swelling Ratio
2.5. Thermal Analysis and Water Content Determination
2.6. Statistical Analysis
3. Results and Discussion
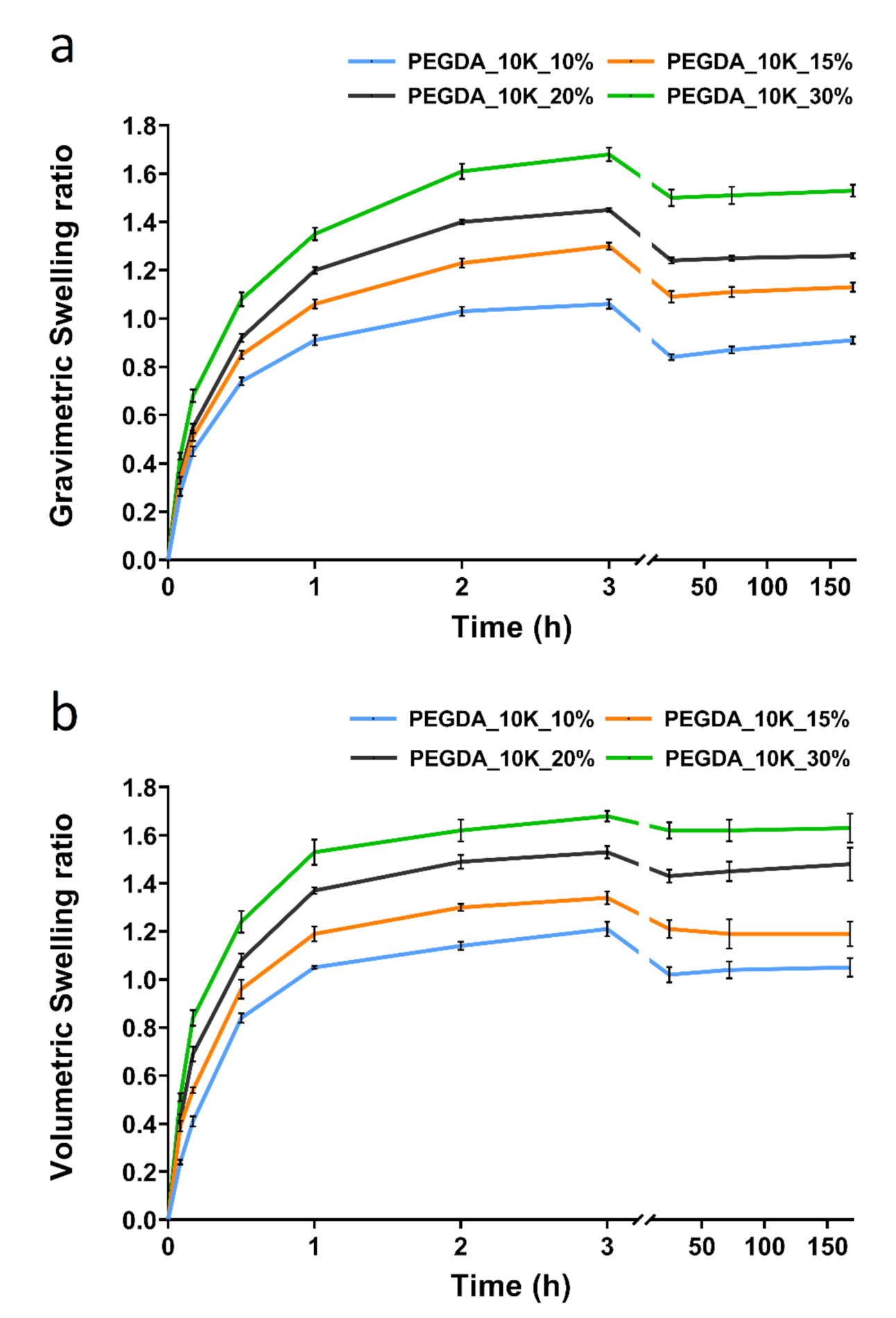
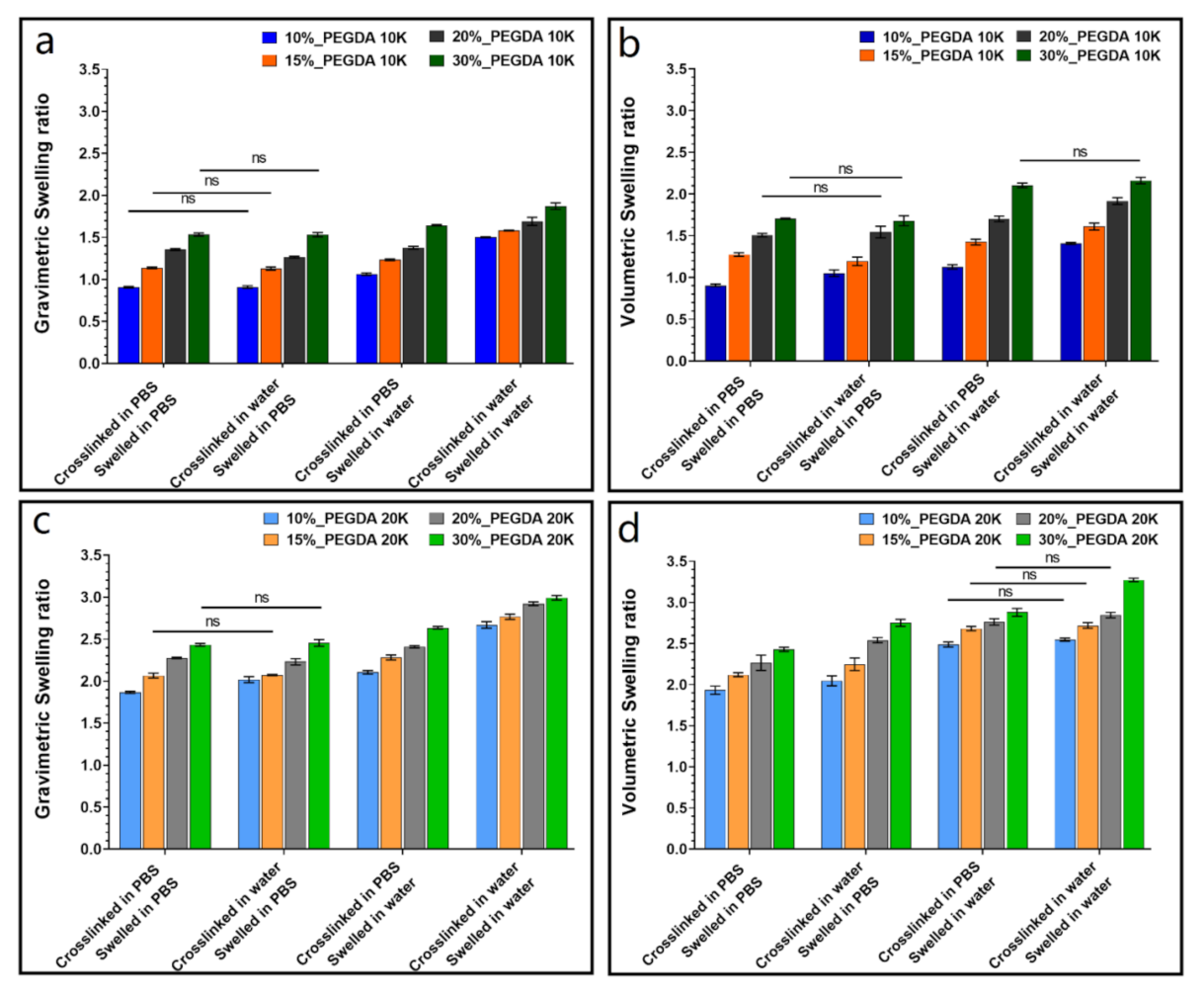
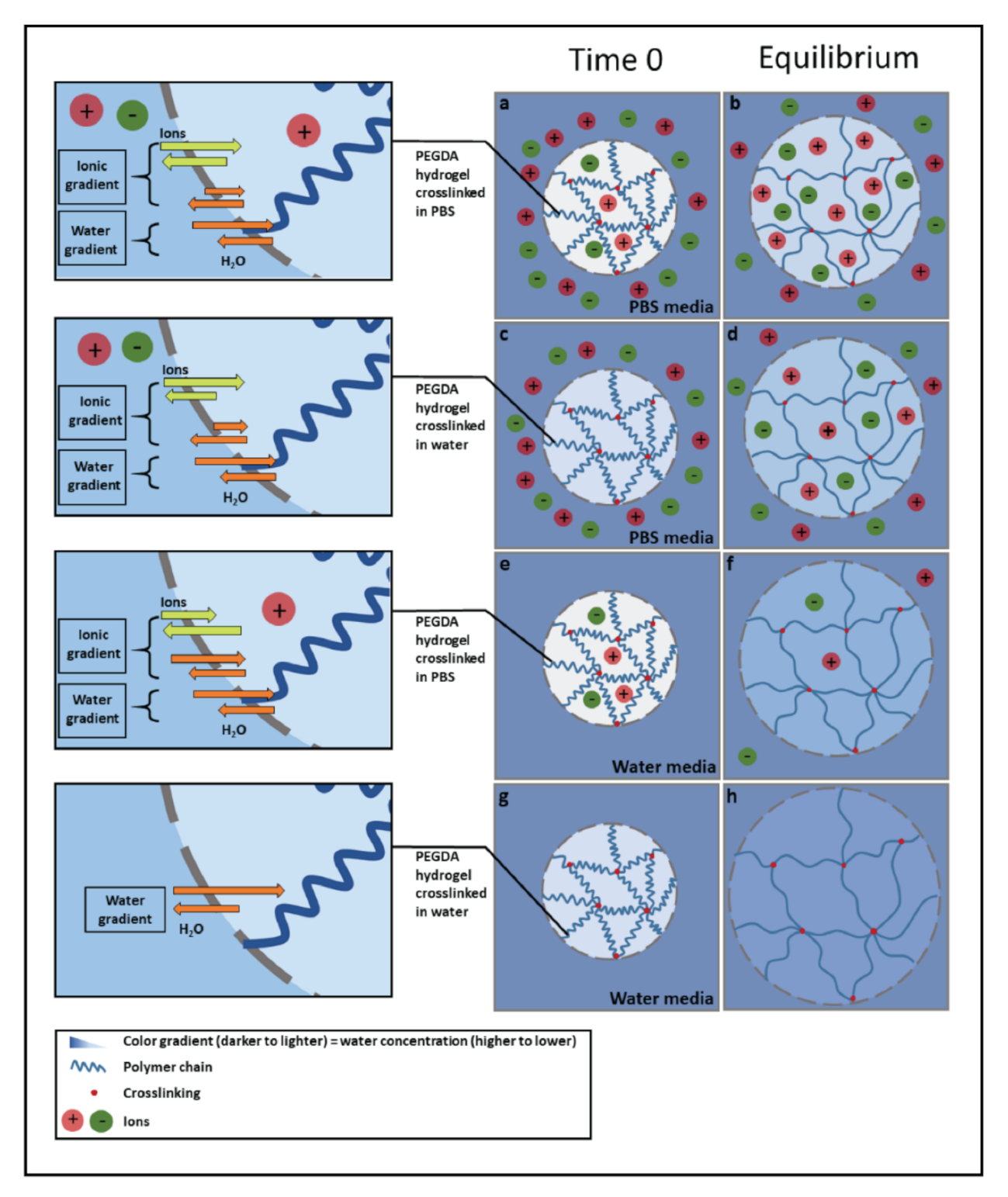
3.1. Dynamic Swelling Studies
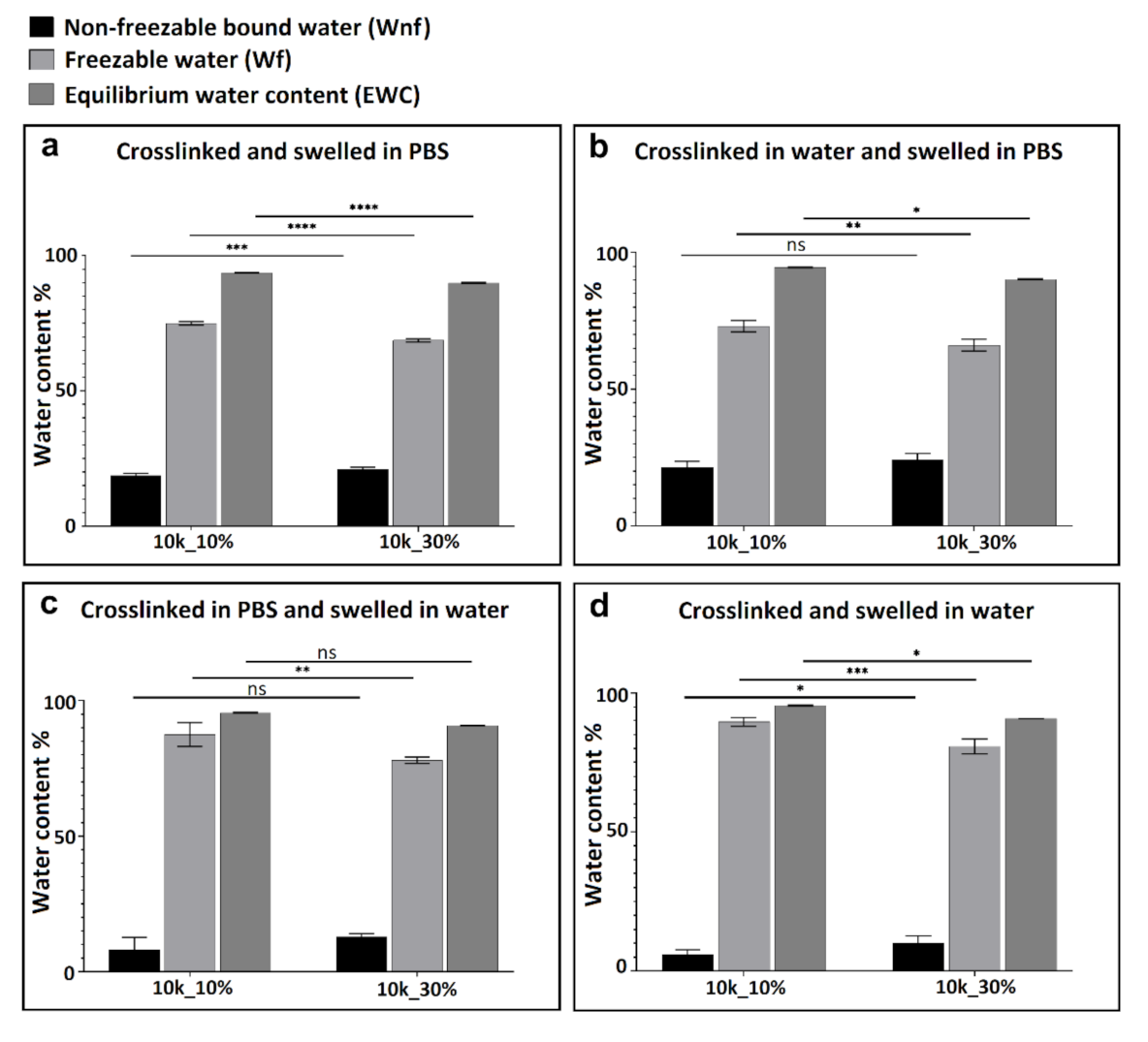
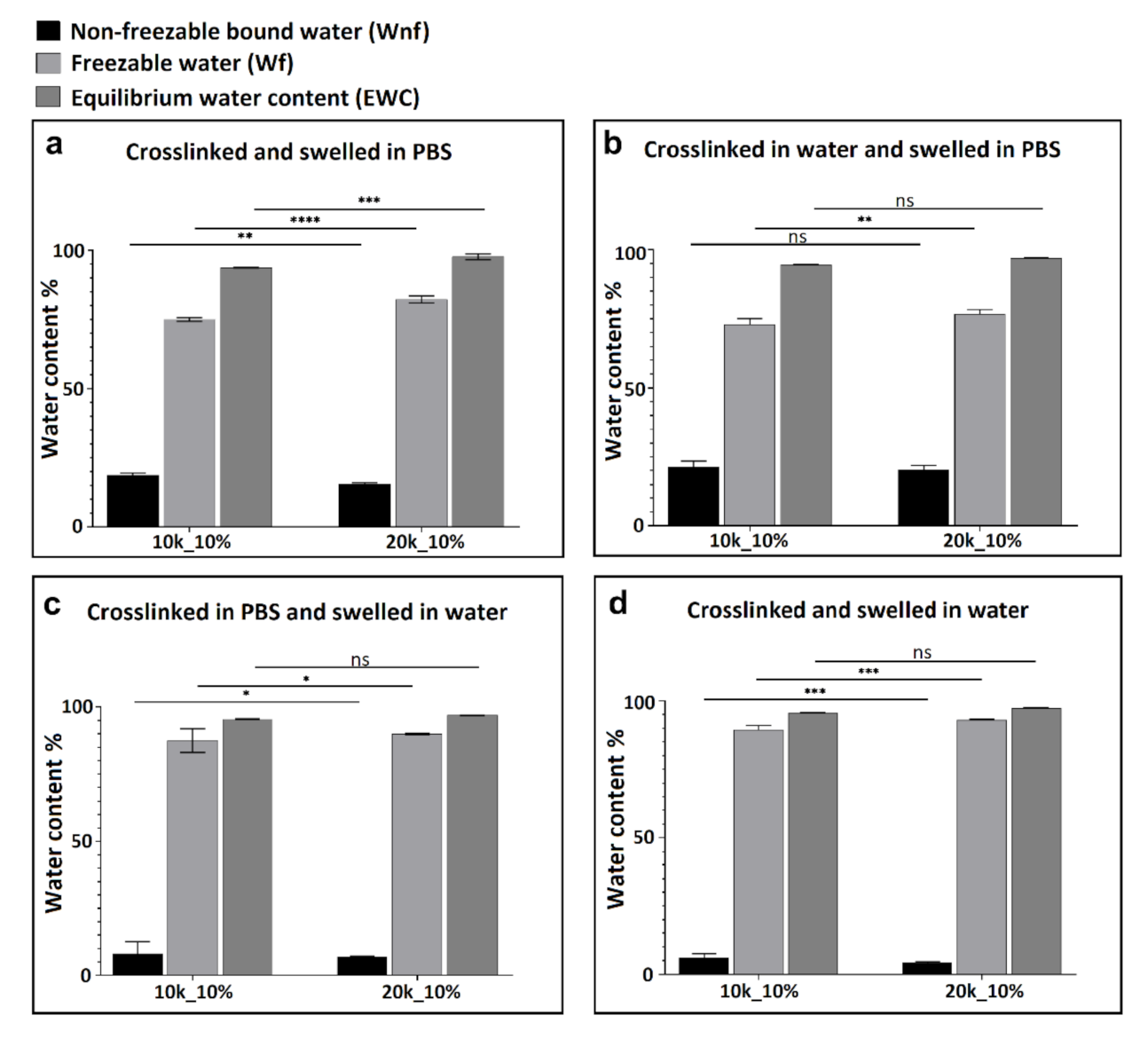
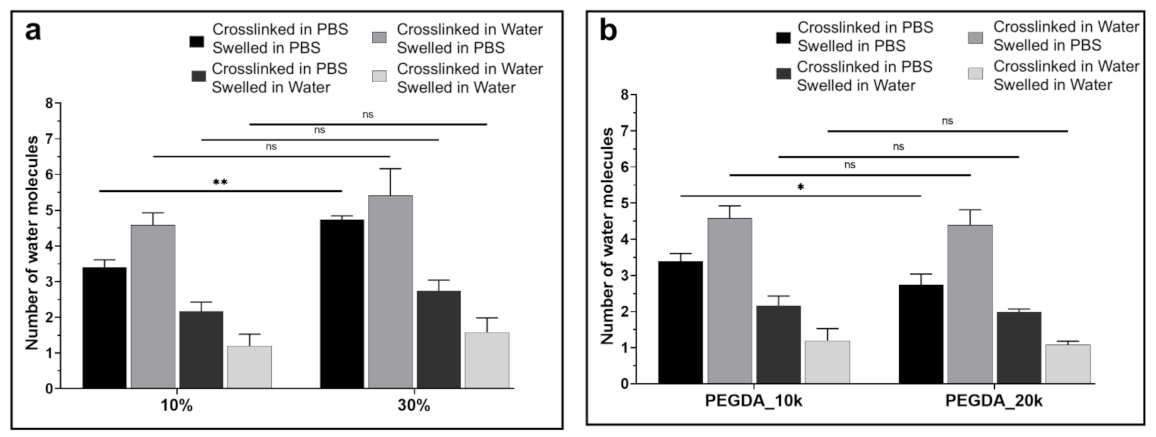
3.2. Thermal Analysis (DSC) and Water Content Determination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ratner, B.D. Biomaterials Science: An Introduction to Materials in Medicine; Ratner, B.D., Ed.; Academic Press: Cambridge, MA, USA, 2012; pp. 55–59. [Google Scholar]
- Tanaka, M.; Hayashi, T.; Morita, S. The Roles of Water Molecules at the Biointerface of Medical Polymers. Polym. J. 2013, 45, 701–710. [Google Scholar] [CrossRef]
- Mantha, S.; Pillai, S.; Khayambashi, P.; Upadhyay, A.; Zhang, Y.; Tao, O.; Pham, H.M.; Tran, S.D. Smart hydrogels in tissue engineering and regenerative medicine. Materials 2019, 12, 3323. [Google Scholar] [CrossRef] [Green Version]
- Erol, O.; Pantula, A.; Liu, W.; Gracias, D.H. Transformer Hydrogels: A Review. Adv. Mater. Technol. 2019, 4, 1900043–1900069. [Google Scholar] [CrossRef] [Green Version]
- Bu, Y.; Zhang, L.; Sun, G.; Sun, F.; Liu, J.; Yang, F.; Tang, P.; Wu, D. Tetra-Peg Based Hydrogel Sealants for in Vivo Visceral Hemostasis. Adv. Mater. 2019, 31, 1901580–1901589. [Google Scholar] [CrossRef]
- Zhao, S.; Tseng, P.; Grasman, J.; Wang, Y.; Li, W.; Napier, B.; Yavuz, B.; Chen, Y.; Howell, L.; Rincon, J.; et al. Programmable Hydrogel Ionic Circuits for Biologically Matched Electronic Interfaces. Adv. Mater. 2018, 30, 1800598–1800607. [Google Scholar] [CrossRef]
- Lee, Y.; Song, W.J.; Sun, J.-Y. Hydrogel soft robotics. Mater. Today Phys. 2020, 15, 100258–100281. [Google Scholar] [CrossRef]
- Narayanaswamy, R.; Torchilin, V.P. Hydrogels and their applications in targeted drug delivery. Molecules 2019, 24, 603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, H.; Zheng, M.; Yu, X.; Than, A.; Seeni, R.Z.; Kang, R.; Tian, J.; Khanh, D.P.; Liu, L.; Chen, P.; et al. A swellable microneedle patch to rapidly extract skin interstitial fluid for timely metabolic analysis. Adv. Mater. 2017, 29, 1702243–1702250. [Google Scholar] [CrossRef]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental concepts of hydrogels: Synthesis, properties, and their applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef]
- Catoira, M.C.; Fusaro, L.; Francesco, D.D.; Ramella, M.; Boccafoschi, F. Overview of natural hydrogels for regenerative medicine applications. J. Mater. Sci. Mater. Med. 2019, 30, 115–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez-Puyana, V.; Jiménez-Rosado, M.; Romero, A.; Guerrero, A. Fabrication and characterization of hydrogels based on gelatinised collagen with potential application in tissue engineering. Polymers 2020, 12, 1146. [Google Scholar] [CrossRef] [PubMed]
- Gibas, I.; Janik, H. Review: Synthetic polymer hydrogels for biomedical applications. Chem. Chem. Technol. 2010, 4, 297–304. [Google Scholar] [CrossRef]
- Yang, P.; Mather, P.T. Thermal Analysis to Determine Various Forms of Water Present in Hydrogels, TA Instruments Report. Available online: http://www.tainstruments.com/pdf/literature/TA384.pdf (accessed on 20 July 2020).
- Bag, M.A.; Valenzuela, L.M. Impact of the Hydration States of Polymers on their Hemocompatibility for Medical Applications: A Review. Int. J. Mol. Sci. 2017, 18, 1422. [Google Scholar] [CrossRef] [Green Version]
- Islam, A.; Yasin, T.; Gull, N.; Khan, S.M.; Munawar, M.A.; Shafiq, M.; Sabir, A.; Jamil, T. Evaluation of selected properties of biocompatible chitosan/poly(vinyl alcohol) blends. Int. J. Biomed. Macromol. 2016, 82, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, A.; Iijima, T.D.S.C. Investigation of the States of Water in Poly(Vinyl Alcohol) Membranes. Polymer 1985, 26, 1207–1211. [Google Scholar] [CrossRef]
- Kishi, A.; Tanaka, M.; Mochizuki, A. Comparative Study on Water Structures in PolyHEMA and PolyMEA by XRD-DSC Simultaneous Measurement. J. App. Pol. Sci. 2009, 111, 476–481. [Google Scholar] [CrossRef]
- Zhao, Y.; Ma, L.; Zeng, R.; Tu, M.; Zhao, J. Preparation, characterization and protein sorption of photo-crosslinked cell membrane-mimicking chitosan-based hydrogels. Carbohydr. Polym. 2016, 151, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Motomura, T.; Ishii, N.; Shimura, K.; Onishi, M.; Mochizuki, A.; Hatakeyama, T. Cold Crystallization of Water in Hydrated Poly(2-Methoxyethyl Acrylate) (PMEA). Polym. Int. 2000, 49, 1709–1713. [Google Scholar] [CrossRef]
- Miwa, Y.; Ishida, H.; Saito, H.; Tanaka, M.; Mochizuki, A. Network Structures and Dynamics of Dry and Swollen Poly(acrylate)s. Characterization of High- and Low-Frequency Motions as Revealed by Suppressed or Recovered Intensities (SRI) Analysis of C-13 NMR. Polymer 2009, 50, 6091–6099. [Google Scholar] [CrossRef]
- Munćan, J.; Mileusnić, I.; Šakota Rosić, J.; Vasić-Milovanović, A.; Matija, L. Water properties of soft contact lenses: A comparative near-infrared study of two hydrogel materials. Int. J. Polym. Sci. 2016, 2016, 3737916. [Google Scholar] [CrossRef] [Green Version]
- Cavallo, A.; Madaghiele, M.; Masullo, U.; Lionetto, M.G.; Sannino, A. Photo-Crosslinked Poly(Ethylene Glycol) Diacrylate (PEGDA) Hydrogels from Low Molecular Weight Prepolymer: Swelling and Permeation Studies. J. App. Pol. Sci. 2017, 134, 44380–44388. [Google Scholar] [CrossRef]
- Brogan, A.P.S.; Clarke, C.J.; Charalambidou, A.; Loynachan, C.N.; Norman, S.E.; Doutch, J.; Hallett, J.P. Expanding the Design Space of Gel Materials through Ionic Liquid Mediated Mechanical and Structural Tuneability. Mater. Horiz. 2020, 7, 820–826. [Google Scholar] [CrossRef]
- Yang, D.; Tao, K.; Chen, G.; Zhang, L.; He, Q.; Xu, H. Randomized controlled trial of polyethylene glycol versus oral sodium phosphate for bowel preparation in unsedated colonoscopy. Gastroent. Res. Pract. 2020, 2020, 6457079. [Google Scholar] [CrossRef] [PubMed]
- Knop, K.; Hoogenboom, R.; Fischer, D.; Schubert, U.S. Poly(Ethylene Glycol) in Drug Delivery: Pros and Cons as well as Potential Alternatives. Angew. Chem. Int. Ed. Engl. 2010, 49, 6288–6308. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhao, X.L.; Li, Z.H.; Zhu, Z.G.; Qian, S.H.; Flewitt, A.J. Current and Emerging Technology for Continuous Glucose Monitoring. Sensors 2017, 17, 182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; An, D.; Pardo, Y.; Chiu, A.; Song, W.; Liu, Q.; Zhou, F.; McDonough, S.P.; Ma, M. High water-content and resilient PEG-containing hydrogels with low fibrotic response. Acta Biomater. 2017, 53, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Konieczynska, M.D.; Villa-Camacho, J.C.; Ghobril, C.; Perez-Viloria, M.; Blessing, W.A.; Nazarian, A.; Rodriguez, E.K. A Hydrogel Sealant for the Treatment of Severe Hepatic and Aortic Trauma with a Dissolution Feature for Post-Emergent Care. Mater. Horiz. 2017, 4, 222–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sgambato, A.; Cipolla, L.; Russo, L. Bioresponsive hydrogels: Chemical strategies and perspectives in tissue engineering. Gels 2016, 2, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gun’ko, V.M.; Savina, I.N.; Mikhalovsky, S.V. Properties of Water Bound in Hydrogels. Gels 2017, 3, 37. [Google Scholar] [CrossRef]
- Della Sala, F.; Biondi, M.; Guarnieri, D.; Borzacchiello, A.; Ambrosio, L.; Mayol, L. Mechanical behavior of bioactive poly(ethylene glycol) diacrylate matrices for biomedical application. J. Mech. Behav. Biomed. Mater. 2020, 110, 103885–103893. [Google Scholar] [CrossRef]
- Choi, J.R.; Yong, K.W.; Choi, J.Y.; Cowie, A.C. Recent Advances in Photo-Crosslinked Hydrogels for Biomedical Applications. Biotechniques 2019, 66, 40–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chapla, R.; Abed, M.A.; West, J. Modulating functionalized poly(ethylene glycol) diacrylate hydrogel mechanical properties through competitive crosslinking mechanics for soft tissue applications. Polymers 2020, 12, 3000. [Google Scholar] [CrossRef] [PubMed]
- Zinkovska, N.; Smilek, J.; Pekar, M. Gradient hydrogels—the state of the art in preparation methods. Polymers 2020, 12, 966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yilmaz, Y.; Gelir, A.; Salehli, F.; Nigmatullin, R.R.; Arbuzov, A.A. Dielectric Study of Neutral and Charged Hydrogels During the Swelling Process. J. Chem. Phys. 2006, 125, 234705–234716. [Google Scholar] [CrossRef] [PubMed]
- Antonsen, K.P.; Hoffman, A.S. Poly(Ethylene Glycol) Chemistry. Topics in Applied Chemistry; Harris, J.M., Ed.; Springer: Boston, MA, USA, 1992; pp. 15–28. [Google Scholar]
- Yamauchi, T.; Tamai, N. A Novel Approach using Differential Scanning Calorimetry to Investigate the Dissolved State in Aqueous Solutions of Polymers used for Papermaking. J. App. Pol. Sci. 2003, 89, 2798–2807. [Google Scholar] [CrossRef]
- Kitano, H.; Ichikawa, K.; Ide, I.; Fukuda, M.; Mizuno, W. Fourier Transform Infrared Study on the State of Water Sorbed to Poly(Ethylene Glycol) films. Langmuir 2001, 17, 1889–1895. [Google Scholar] [CrossRef]
- Park, H.; Guo, X.; Temenoff, J.S.; Tabata, Y.; Caplan, A.I.; Kasper, F.K.; Mikos, A.G. Effect of Swelling Ratio of Injectable Hydrogel Composites on Chondrogenic Differentiation of Encapsulated Rabbit Marrow Mesenchymal Stem Cells in Vitro. Biomacromolecules 2009, 10, 541–546. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.P.; Gitsov, I. Synthesis and Physical Properties of Reactive Amphiphilic Hydrogels Based on Poly(p-Chloromethylstyrene) and Poly(Ethylene Glycol): Effects of Composition and Molecular Architecture. Macromolecules 2010, 43, 3256–3267. [Google Scholar] [CrossRef]
- Hatakeyama, T.; Tanaka, M.; Hatakeyama, H. Studies on Bound Water Restrained by Poly(2-Methacryloyloxyethyl Phosphorylcholine): Comparison with Polysaccharide-Water Systems. Acta Biomater. 2010, 6, 2077–2082. [Google Scholar] [CrossRef] [PubMed]
- Kankala, R.K.; Wang, S.-B.; Chen, A.-Z.; Zhang, Y.S. Handbook of Nanomaterials for Cancer Theranostics; Conde, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 33–62. [Google Scholar]
- Marcus, Y. Effect of Ions on the Structure of Water: Structure Making and Breaking. Chem. Rev. 2009, 109, 1346–1370. [Google Scholar] [CrossRef]
- Bouwstra, J.A.; Salomonsdevries, M.A.; Vanmiltenburg, J.C. The Thermal-Behavior of Water in Hydrogels. Thermochim. Acta 1995, 248, 319–327. [Google Scholar] [CrossRef]
- Shibukawa, M.; Ohta, N.; Onda, N. Investigation of the States of Water in Water-Swollen Hydrogels by Liquid-Chromatography and Differential Scanning Calorimetry. Bull. Chem. Soc. Jpn. 1990, 63, 3490–3494. [Google Scholar] [CrossRef] [Green Version]
- Hatakeyama, T.; Quinn, F.X. (Eds.) Thermal Analysis: Fundamentals and Applications and Polymer Science (Second Edition); Wiley: Chichester, UK; New York, NY, USA; Weinheim, Germany; Brisbane, Australia; Toronto, ON, Canada, 1999. [Google Scholar]
- Ping, Z.H.; Nguyen, Q.T.; Chen, S.M.; Zhou, J.Q.; Ding, Y.D. States of Water in Different Hydrophilic Polymers—DSC and FTIR studies. Polymer 2001, 42, 8461–8467. [Google Scholar] [CrossRef]
- Hirata, Y.; Miura, Y.; Nakagawa, T. Oxygen Permeability and the State of Water in Nafion Membranes with Alkalai Metal and Amino Sugar Counterions. J. Membr. Sci. 1999, 163, 357–366. [Google Scholar] [CrossRef]
- Lusse, S.; Arnold, K. The Interaction of Poly(Ethylene Glycol) with Water Studied by H-1 and H-2 NMR Relaxation Time Measurements. Macromolecules 1996, 29, 4251–4257. [Google Scholar] [CrossRef]
- Huang, L.; Nishinari, K. Interaction between Poly(Ethylene Glycol) and Water as Studied by Differential Scanning Calorimetry. J. Polym. Sci. Part B Polym. Phys. 2001, 39, 496–506. [Google Scholar] [CrossRef]
- Venohr, H.; Fraaije, V.; Strunk, H.; Borchard, W. Static and Dynamic Light Scattering From Aqueous Poly(Ethylene Oxide) Solutions. Eur. Polym. J. 1998, 34, 723–732. [Google Scholar]
- Devanand, K.; Selser, J.C. Polyethylene Oxide Does Not Necessarily Aggregate in Water. Nature 1990, 343, 739–741. [Google Scholar] [CrossRef]
- Zhao, C.; Li, X.; Li, L.; Cheng, G.; Gong, X.; Zheng, J. Dual Functionality of Antimicrobial and Antifouling of Poly(N-hydroxyethylacrylamide)/Salicylate Hydrogels. Langmuir 2013, 29, 1517–1524. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Dargaville, B.L.; Hutmacher, D.W. Elucidating the Molecular Mechanisms for the Interaction of Water with Polyethylene Glycol-Based Hydrogels: Influence of Ionic Strength and Gel Network Structure. Polymers 2021, 13, 845. https://doi.org/10.3390/polym13060845
Yang X, Dargaville BL, Hutmacher DW. Elucidating the Molecular Mechanisms for the Interaction of Water with Polyethylene Glycol-Based Hydrogels: Influence of Ionic Strength and Gel Network Structure. Polymers. 2021; 13(6):845. https://doi.org/10.3390/polym13060845
Chicago/Turabian StyleYang, Xin, Bronwin L. Dargaville, and Dietmar W. Hutmacher. 2021. "Elucidating the Molecular Mechanisms for the Interaction of Water with Polyethylene Glycol-Based Hydrogels: Influence of Ionic Strength and Gel Network Structure" Polymers 13, no. 6: 845. https://doi.org/10.3390/polym13060845
APA StyleYang, X., Dargaville, B. L., & Hutmacher, D. W. (2021). Elucidating the Molecular Mechanisms for the Interaction of Water with Polyethylene Glycol-Based Hydrogels: Influence of Ionic Strength and Gel Network Structure. Polymers, 13(6), 845. https://doi.org/10.3390/polym13060845