Removing Acrylic Conformal Coating with Safer Solvents for Re-Manufacturing Electronics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. General Procedures for Optimization of Solvent Blends
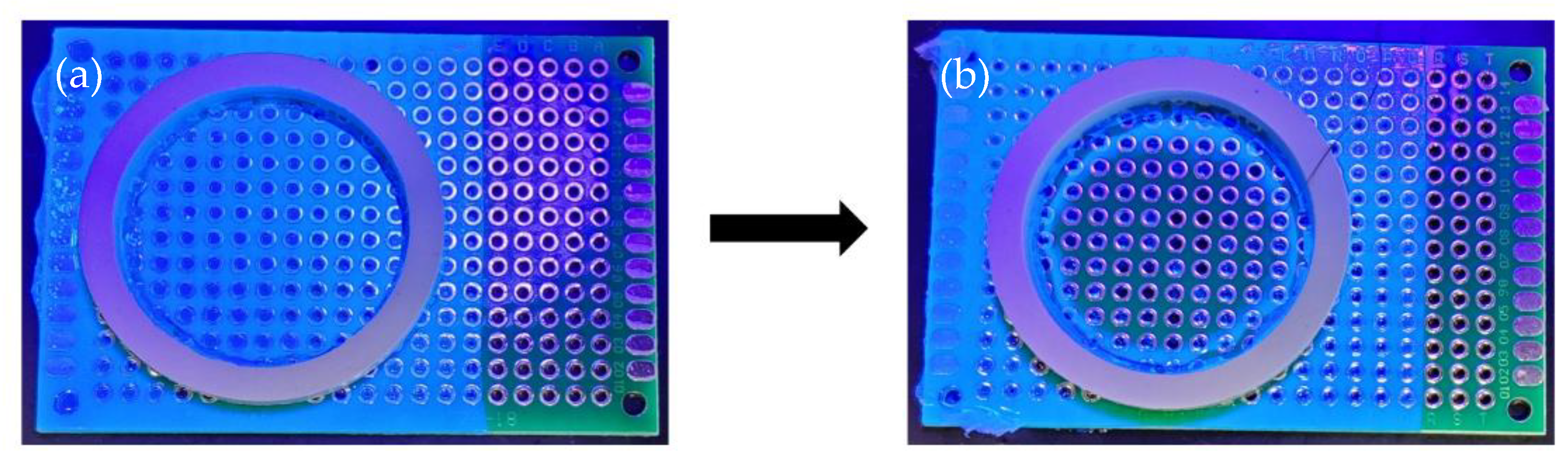
2.3. Dwell Time Test
3. Results and Discussion
3.1. Determination of the HSP Solubility of Acrylic Conformal Coatings
3.2. Model Verification and Improvement
3.3. Solvent Optimization
3.4. Implications for Similar Conformal Coatings
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhan, S.; Azarian, M.H.; Pecht, M.G. Surface Insulation Resistance of Conformally Coated Printed Circuit Boards Processed with No-Clean Flux. IEEE Trans. Electron. Packag. Manuf. 2006, 29, 217–223. [Google Scholar] [CrossRef]
- Morose, G.; Marshall, J.; McCarthy, A.; Harripersaud, V.; Giarrosso, A. Assessment of Safer and Effective Alternatives for Coating Removal Products; Toxics Use Reduction Institute: Lowell, MA, USA, 2020. [Google Scholar]
- Conformal Coatings Market Size & Share. Available online: https://www.grandviewresearch.com/industry-analysis/conformal-coating-market (accessed on 16 June 2020).
- Dobriyal, P.; Ramalingam, S.; Lim, S.L.; Kurella, A. Conformal coating challenges: Detection, rework and failure analysis. In Proceedings of the 2016 Pan Pacific Microelectronics Symposium (Pan Pacific), Big Island, HI, USA, 25–28 January 2016; pp. 1–6. [Google Scholar]
- Li, Z.; Hogan, K.A.; Cai, C.; Rieth, S. Human Health Effects of Biphenyl: Key Findings and Scientific Issues. Environ. Health Perspect. 2016, 124, 703–712. [Google Scholar] [CrossRef] [Green Version]
- USEPA. Scope of the Risk Evaluation for Methylene Chloride; USEPA: Washington, DC, USA, 2017.
- World Health Organization. Air Quality Guidelines for Europe; WHO Regional Office for Europe: Copenhagen, Denmark, 2000. [Google Scholar]
- Roelofs, C.R.; Ellenbecker, M.J. Source reduction for prevention of methylene chloride hazards: Cases from four industrial sectors. Environ. Health 2003, 2, 9. [Google Scholar] [CrossRef] [Green Version]
- Cseri, L.; Razali, M.; Pogany, P.; Szekely, G. Organic Solvents in Sustainable Synthesis and Engineering. In Green Chemistry; Elsevier: Amsterdam, The Netherlands, 2018; pp. 513–553. [Google Scholar]
- Paint and Varnish Strippers. Available online: https://saferchemicals.org/wp-content/uploads/2019/12/paint_strippers_safer_alternatives_december_2019-updated.pdf (accessed on 8 March 2021).
- Taygerly, J.P.; Miller, L.M.; Yee, A.; Peterson, E.A. A convenient guide to help select replacement solvents for dichloromethane in chromatography. Green Chem. 2012, 14. [Google Scholar] [CrossRef]
- Jhamb, S.; Liang, X.; Dam-Johansen, K.; Kontogeorgis, G.M. A model-based solvent selection and design framework for organic coating formulations. Prog. Org. Coatings 2020, 140. [Google Scholar] [CrossRef]
- Giarrosso, A. Investigating the Effective Removal of Gel Nail Polish with the Hansen Solubility Parameters; University of Massachusetts: Lowell, MA, USA, 2019. [Google Scholar]
- Weeden, G.S., Jr.; Soepriatna, N.H.; Wang, N.H. Method for efficient recovery of high-purity polycarbonates from electronic waste. Environ. Sci. Technol. 2015, 49, 2425–2433. [Google Scholar] [CrossRef]
- Sanchez-Lengeling, B.; Roch, L.M.; Perea, J.D.; Langner, S.; Brabec, C.J.; Aspuru-Guzik, A. A Bayesian Approach to Predict Solubility Parameters. Adv. Theory Simul. 2019, 2, 1800069. [Google Scholar] [CrossRef]
- Abbott, S.; Hansen, C.M.; Yamamoto, H. Hansen Solubility Parameters in Practice—Complete with eBook, Software and Data. 2015. Available online: www.hansen-solubility.com (accessed on 14 January 2020).
- Ma, J.; Zhou, L. A new procedure for calculating Hansen solubility parameters of carbon nanotube/polymer composites. Polymer Bull. 2012, 68, 1053–1063. [Google Scholar] [CrossRef]
- Díaz de los Ríos, M.; Hernández Ramos, E. Determination of the Hansen solubility parameters and the Hansen sphere radius with the aid of the solver add-in of Microsoft Excel. SN Appl. Sci. 2020, 2. [Google Scholar] [CrossRef] [Green Version]
- Jankovic, S.; Tsakiridou, G.; Ditzinger, F.; Koehl, N.J.; Price, D.J.; Ilie, A.-R.; Kalantzi, L.; Kimpe, K.; Holm, R.; Nair, A.; et al. Application of the solubility parameter concept to assist with oral delivery of poorly water-soluble drugs—A PEARRL review. J. Pharm. Pharmacol. 2019, 71, 441–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcus, Y. Are solubility parameters relevant to supercritical fluids? J. Supercrit. Fluids 2006, 38, 7–12. [Google Scholar] [CrossRef]
- Hazard Assessments. Available online: https://pharosproject.net/ (accessed on 18 June 2020).
- Scriven, L.E. Physics and Applications of DIP Coating and Spin Coating. MRS Proc. 1988, 121, 717. [Google Scholar] [CrossRef]
- Andecochea Saiz, C.; Darvishmanesh, S.; Buekenhoudt, A.; Van der Bruggen, B. Shortcut applications of the Hansen Solubility Parameter for Organic Solvent Nanofiltration. J. Membr. Sci. 2018, 546, 120–127. [Google Scholar] [CrossRef]
- Wagner, J.P.; Schreiner, P.R. London dispersion in molecular chemistry--reconsidering steric effects. Angew. Chem. Int. Ed. Engl. 2015, 54, 12274–12296. [Google Scholar] [CrossRef]
- Duereh, A.; Sato, Y.; Smith, R.L.; Inomata, H. Methodology for Replacing Dipolar Aprotic Solvents Used in API Processing with Safe Hydrogen-Bond Donor and Acceptor Solvent-Pair Mixtures. Org. Process. Res. Dev. 2017, 21, 114–124. [Google Scholar] [CrossRef] [Green Version]
- Gauthier, A.M.; Fung, M.; Panko, J.; Kingsbury, T.; Perez, A.L.; Hitchcock, K.; Ferracini, T.; Sahmel, J.; Banducci, A.; Jacobsen, M.; et al. Chemical assessment state of the science: Evaluation of 32 decision-support tools used to screen and prioritize chemicals. Integr. Environ. Assess. Manag. 2015, 11, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Alder, C.M.; Hayler, J.D.; Henderson, R.K.; Redman, A.M.; Shukla, L.; Shuster, L.E.; Sneddon, H.F. Updating and further expanding GSK’s solvent sustainability guide. Green Chem. 2016, 18, 3879–3890. [Google Scholar] [CrossRef]
- Horrocks, H. Conformal coating removal techniques. Circuit World 1998, 24, 13–19. [Google Scholar] [CrossRef]
- Clarke, C.J.; Tu, W.C.; Levers, O.; Brohl, A.; Hallett, J.P. Green and Sustainable Solvents in Chemical Processes. Chem. Rev. 2018, 118, 747–800. [Google Scholar] [CrossRef] [PubMed]
- Jeong, K.M.; Lee, M.S.; Nam, M.W.; Zhao, J.; Jin, Y.; Lee, D.K.; Kwon, S.W.; Jeong, J.H.; Lee, J. Tailoring and recycling of deep eutectic solvents as sustainable and efficient extraction media. J. Chromatogr. A 2015, 1424, 10–17. [Google Scholar] [CrossRef]
- Voros, V.; Drioli, E.; Fonte, C.; Szekely, G. Process Intensification via Continuous and Simultaneous Isolation of Antioxidants: An Upcycling Approach for Olive Leaf Waste. ACS Sustain. Chem. Eng. 2019, 7, 18444–18452. [Google Scholar] [CrossRef]



| Solvent | Purity | HSP | |||
|---|---|---|---|---|---|
| δD | δP | δH | Score a | ||
| Toluene | 99% | 18 | 1.4 | 2 | 1 |
| Dimethyl Carbonate | 98% | 15.5 | 8.6 | 9.7 | 1 |
| p-Xylene | 98% | 17.8 | 1 | 3.1 | 1 |
| Benzyl Alcohol | 99% | 18.4 | 6.3 | 13.7 | 2 |
| Methyl Acetate | 99% | 15.5 | 7.2 | 7.6 | 1 |
| Ethylene Glycol | 99% | 17 | 11 | 26 | 0 |
| Undecane | 99% | 16 | 0 | 0 | 0 |
| Ethyl Acetate | ≥99.5% | 15.8 | 5.3 | 7.2 | 1 |
| Methanol | ≥99.8% | 14.7 | 12.3 | 22.3 | 0 |
| Ethanol | ≥99.5% | 15.8 | 8.8 | 19.4 | 0 |
| 1,3-Dioxolane | 99% | 18.1 | 6.6 | 9.3 | 1 |
| Diethyl Carbonate | 99% | 15.1 | 6.3 | 3.5 | 1 |
| 1-Propanol | 99.5% | 16 | 6.8 | 17.4 | 2 |
| 2-Propanol | 99% | 15.8 | 6.1 | 16.4 | 2 |
| Propylene Carbonate | 99.5% | 20 | 18 | 4.1 | 0 |
| Thiophene | 99% | 18.9 | 2.4 | 7.8 | 1 |
| Propylene Glycol Monomethyl Ether | 99.5% | 15.6 | 6.3 | 11.6 | 2 |
| Dimethyl Sulfoxide (DMSO) | ≥99.7% | 18.4 | 16.4 | 10.2 | 0 |
| Acetone | 99% | 15.5 | 10.4 | 7 | 1 |
| 1-Butanol | 99% | 16 | 5.7 | 15.8 | 2 |
| Dimethyl Glutarate | 98% | 16.1 | 7.7 | 8.3 | 2 |
| Anisole | 99% | 17.8 | 4.4 | 6.9 | 1 |
| Ethylene Glycol Butyl Ether Acetate | 98% | 15.3 | 7.5 | 6.8 | 2 |
| Ethyl Lactate | 99% | 16 | 7.6 | 12.5 | 2 |
| Diethyl Ether | 99% | 14.5 | 2.9 | 4.6 | 1 |
| Butyl Benzoate | 98% | 18.3 | 5.6 | 5.5 | 1 |
| Hexane | 99% | 14.9 | 0 | 0 | 0 |
| Tetrahydrofuran (THF) | 99% | 16.8 | 5.7 | 8 | 1 |
| n-Butyl Acetate | 99.5% | 15.8 | 3.7 | 6.3 | 1 |
| Chlorobenzene | 99% | 19 | 4.3 | 2 | 1 |
| Diethylene Glycol Monobutyl Ether | 97% | 16 | 7 | 10.6 | 1 |
| o-Dichlorobenzene | 99% | 19.2 | 6.3 | 3.3 | 1 |
| Methyl Ethyl Ketone (MEK) | 99.5% | 16 | 9 | 5.1 | 1 |
| Cyclohexanone | 99% | 17.8 | 8.4 | 5.1 | 1 |
| Acetophenone | 99% | 18.8 | 9 | 4 | 2 |
| 1-Bromonaphthalene | 97% | 20.6 | 3.1 | 4.1 | 2 |
| 1-Chlorobutane | 99% | 16.2 | 5.5 | 2 | 1 |
| Formic Acid | 99% | 14.6 | 10 | 14 | 0 |
| Tetrahydronaphthalene | 97% | 19.6 | 2 | 2.9 | 1 |
| Solvent A | Solvent B | Vol. % of A | Vol. % B | δD | δP | δH | Distance(MPa1/2) | RED | Dwell Time Needed to Remove > 90% of the Conformal Coating |
|---|---|---|---|---|---|---|---|---|---|
| Butyl Benzoate | Ethyl Acetate | 36 | 64 | 15.8 | 5.3 | 7.2 | 1.9 | 0.28 | 2 min |
| Anisole | Acetone | 83 | 17 | 17.4 | 5.4 | 6.9 | 2.8 | 0.41 | 2–3 min |
| Acetone | Butyl Benzoate | 14 | 86 | 17.9 | 6.3 | 5.7 | 3.6 | 0.53 | 3 min |
| Butyl Benzoate | DMSO | 78 | 22 | 18.3 | 8.0 | 6.5 | 5.4 | 0.80 | 3–5 min |
| Acetone | 1-propanol | 87 | 13 | 15.6 | 9.9 | 7.1 | 6.3 | 0.93 | 3 min |
| DMSO | Methyl Acetate | 33 | 67 | 16.5 | 10.2 | 8.5 | 6.9 | 1.01 | 3 min |
| DMSO | Isopentyl Alcohol | 12 | 88 | 16.1 | 6.5 | 12.9 | 8.1 | 1.19 | 8 min |
| 2-butanol | DMSO | 81 | 19 | 16.3 | 7.7 | 13.7 | 9.2 | 1.35 | 6–7 min |
| DMSO | 2- propanol | 33 | 67 | 16.7 | 9.5 | 14.4 | 10.6 | 1.55 | 6–8 min |
| DMSO | 1-propanol | 40 | 60 | 17 | 10.6 | 14.5 | 11.4 | 1.67 | 8 min |
| DMSO | Ethanol | 80 | 20 | 17.9 | 14.9 | 12.0 | 13.0 | 1.91 | >20 min |
| Premium Stripper | 16.9 | 7.5 | 7.8 | 4.9 | 0.72 | 2 min | |||
| Strip X | 16.5 | 7.1 | 10.1 | 4.0 | 0.59 | 2 min | |||
| DCM | 17 | 7.3 | 7.1 | 3.8 | 0.56 | 2 min | |||
| Human Health Group I | Human Health Group II and II | Environmental Toxicity and Fate | Physical Hazards |
|---|---|---|---|
| Carcinogenicity, | Acute Toxicity | Acute Aquatic Toxicity | Reactivity |
| Mutagenicity, and Genotoxicity | Systemic Toxicity and Organ Effects | Chronic Aquatic Toxicity | Flammability |
| Reproductive Toxicity | Neurotoxicity | Other Ecotoxicity Studies when Available | / |
| Developmental Toxicity | Skin Sensitization Respiratory Sensitization | Persistence | / |
| Endocrine Activity | Skin IrritationEye Irritation | Bioaccumulation | / |
| Solvent A | Solvent B | Ratio A (Vol. %) | Ratio B (Vol.%) | δD | δP | δH | Distance (MPa1/2) | RED | GSK (Solvent A) | GSK (Solvent B) |
|---|---|---|---|---|---|---|---|---|---|---|
| Butyl Diglycol Acetate | Methyl Cyclohexane | 69 | 31 | 16 | 2.8 | 6 | 1.781 | 0.262 | N/A | 8 |
| Butyl Diglycol Acetate | Cyclohexane | 70 | 30 | 16.2 | 2.9 | 5.8 | 1.448 | 0.213 | N/A | 7 |
| Diacetone Alcohol | Methyl Cyclohexane | 46 | 54 | 15.9 | 3.8 | 5.5 | 1.288 | 0.189 * | N/A | 8 |
| Diethylene Glycol Monobutyl Ether | Methyl Cyclohexane | 49 | 51 | 16 | 3.4 | 5.7 | 1.308 | 0.192 | 7 | 8 |
| Butyl Diglycol Acetate | p-Cymene | 56 | 44 | 16.6 | 3.3 | 5.6 | 0.894 | 0.131 | N/A | N/A |
| Ethyl Acetate | Methyl Cyclohexane | 72 | 28 | 15.9 | 3.8 | 5.5 | 1.370 | 0.201 | 8 | 8 |
| Dibasic Esters (Dbe) | Methyl Cyclohexane | 60 | 40 | 16.1 | 3.9 | 5.4 | 0.840 | 0.124 * | N/A | 8 |
| Methyl Cyclohexane | Propylene Glycol Monomethyl Ether | 54 | 46 | 15.8 | 2.9 | 5.9 | 1.945 | 0.286 * | 8 | N/A |
| Conformal Coating Types | Characteristics |
|---|---|
| Acrylic | Ease of rework Moisture resistant Fast drying |
| Polyurethane | Good dielectric properties Moisture resistant Abrasion resistant Good solvent resistance |
| Silicones | Wide use temperature range Flexible Moisture resistant |
| Epoxy | Good dielectric properties Abrasion resistant High use temperature Excellent solvent resistance |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, T.; Reimonn, G.; Morose, G.; Yu, E.; Chen, W.-T. Removing Acrylic Conformal Coating with Safer Solvents for Re-Manufacturing Electronics. Polymers 2021, 13, 937. https://doi.org/10.3390/polym13060937
Lu T, Reimonn G, Morose G, Yu E, Chen W-T. Removing Acrylic Conformal Coating with Safer Solvents for Re-Manufacturing Electronics. Polymers. 2021; 13(6):937. https://doi.org/10.3390/polym13060937
Chicago/Turabian StyleLu, Taofeng, Gregory Reimonn, Gregory Morose, Evan Yu, and Wan-Ting Chen. 2021. "Removing Acrylic Conformal Coating with Safer Solvents for Re-Manufacturing Electronics" Polymers 13, no. 6: 937. https://doi.org/10.3390/polym13060937
APA StyleLu, T., Reimonn, G., Morose, G., Yu, E., & Chen, W.-T. (2021). Removing Acrylic Conformal Coating with Safer Solvents for Re-Manufacturing Electronics. Polymers, 13(6), 937. https://doi.org/10.3390/polym13060937







