Biocompatibility and Pharmacological Effects of Innovative Systems for Prolonged Drug Release Containing Dexketoprofen in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substances
2.2. Procedure of Preparation the DEX-Loaded Liposomes Stabilized with Chitosan
2.3. Analysis of the Prepared Liposomes
2.4. The In Vivo Biocompatibility Evaluation of Chitosan-Coated Liposomes Entrapping DEX
- Group I (DW): distilled water 0.3 mL/100 g body weight;
- Group II (DEX): dexketoprofen 10 mg/kg body weight (kbw);
- Group III (nano-DEX): liposomes containing dexketoprofen 10 mg/kbw.
2.5. The Investigation of the Chitosan-Coated Liposomes Entrapping DEX on the Nociceptive Reactivity in Rats
3. Results
3.1. Characterization of Liposomes
3.2. In Vitro Release of DEX from Vesicles Stabilized with Chitosan
3.3. The In Vivo Biocompatibility Testing
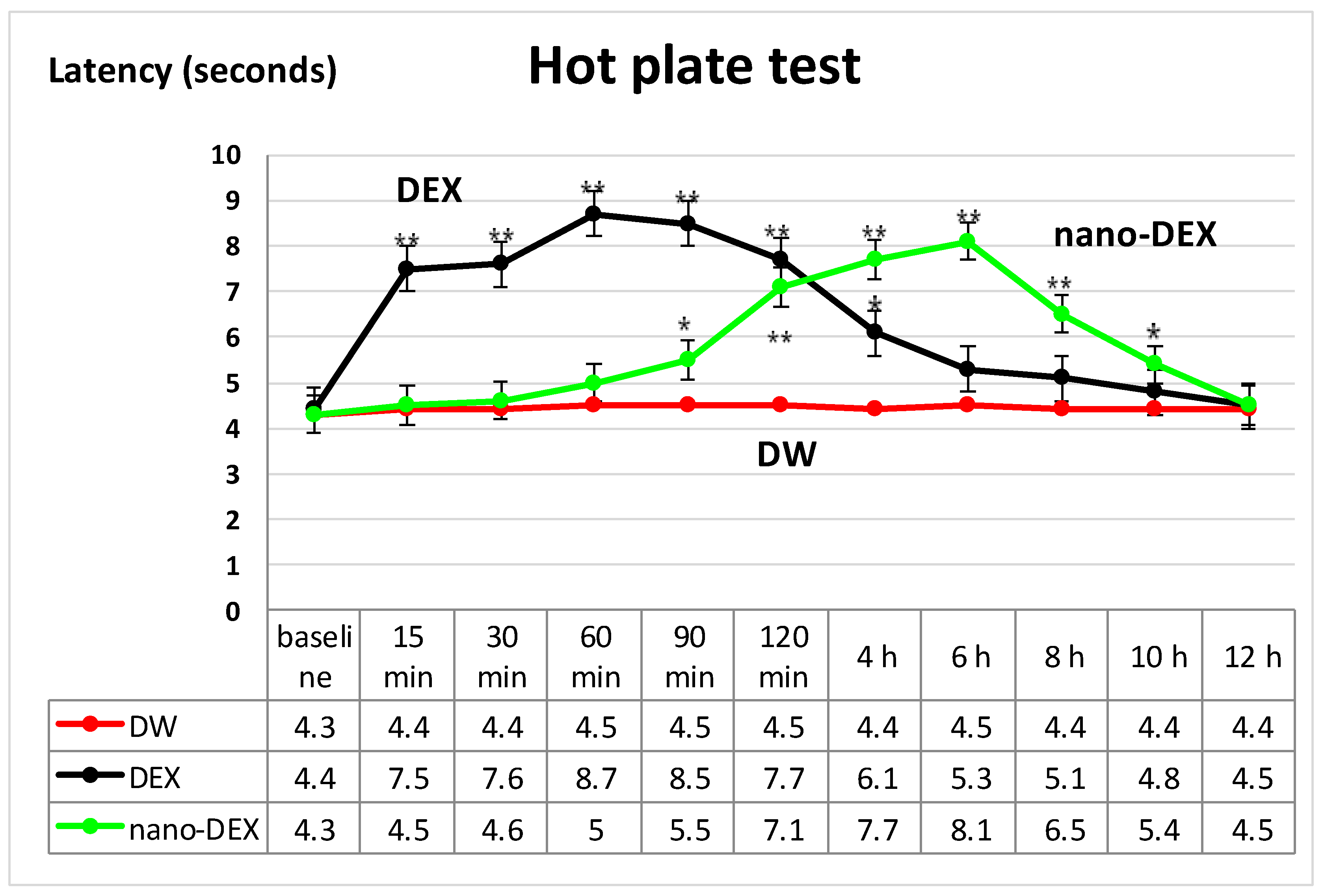
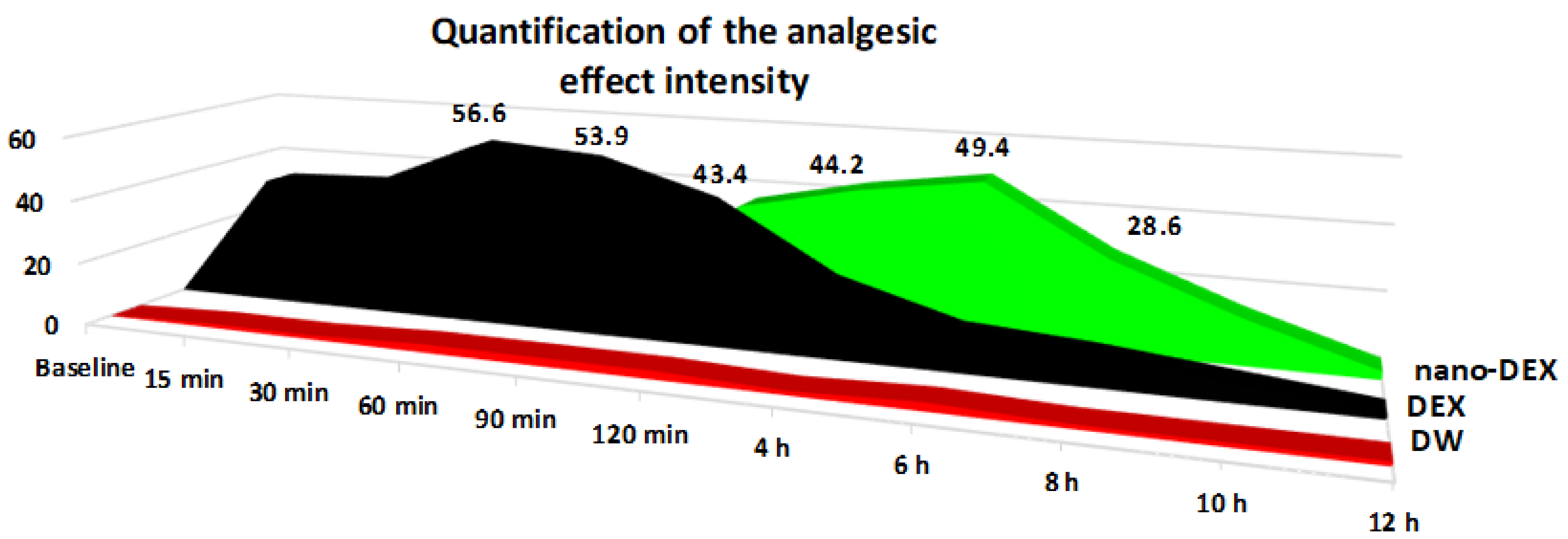
3.4. The Nociceptive Sensitivity Investigation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boisseau, P.; Loubaton, B. Nanomedicine, nanotechnology in medicine. Comptes Rendus Phys. 2011, 12, 620–626. [Google Scholar] [CrossRef] [Green Version]
- Hughes, G.A. Nanostructure-mediated drug delivery. Nanomedicine 2005, 1, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Lillard, J.W., Jr. Nanoparticle-based targeted drug delivery. Exp. Mol. Pathol. 2009, 86, 215–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoicea, N.; Fiorda-Diaz, J.; Joseph, N.; Shabsigh, M.; Arias-Morales, C.; Gonzalez-Zacarias, A.; Mavarez-Martinez, A.; Marjoribanks, S.; Bergese, S. Advanced Analgesic Drug Delivery and Nanobiotechnology. Drugs 2017, 77, 1069–1076. [Google Scholar] [CrossRef]
- Kenyon, N.J.; Bratt, J.M.; Lee, J.; Luo, J.; Franzi, L.M.; Zeki, A.A.; Lam, K.S. Self-assembling nanoparticles containing dexamethasone as a novel therapy in allergic airways inflammation. PLoS ONE 2013, 8, e77730. [Google Scholar] [CrossRef]
- Lu, X.; Howard, M.D.; Mazik, M.; Eldridge, J.; Rinehart, J.J.; Jay, M.; Leggas, M. Nanoparticles containing anti-inflammatory agents as chemotherapy adjuvants: Optimization and in vitro characterization. AAPS J. 2008, 10, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Machelska, H.; Celik, M.Ö. Advances in Achieving Opioid Analgesia Without Side Effects. Front. Pharmacol. 2018, 9, 1388. [Google Scholar] [CrossRef]
- Andreu, V.; Arruebo, M. Current progress and challenges of nanoparticle-based therapeutics in pain management. J. Control. Release 2018, 269, 189–213. [Google Scholar] [CrossRef] [Green Version]
- Al-Lawati, H.; Binkhathlan, Z.; Lavasanifar, A. Nanomedicine for the effective and safe delivery of non-steroidal anti-inflammatory drugs: A review of preclinical research. Eur. J. Pharm. Biopharm. 2019, 142, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, M.J.; Arbós, R.M.; Amaro, S.R. Dexketoprofen trometamol: Clinical evidence supporting its role as a painkiller. Expert Rev. Neurother. 2008, 8, 1625–1640. [Google Scholar]
- Balani, M.; Gawade, P.; Maheshgauri, S.; Ghole, S.; Shinde, V.; Sathe, V.J. Results of two multicentric, comparative, randomized, parallel group clinical trials to evaluate the efficacy and safety of dexketoprofen trometamol in the treatment of dental pain and dysmenorrhoea in Indian patients. Clin. Diag. Res. 2008, 2, 1086–1091. [Google Scholar]
- Sweetman, B.J. Development and use of the quick acting chiral NSAID dexketoprofen trometamol (keral). Acute Pain 2003, 4, 109–115. [Google Scholar] [CrossRef]
- Moore, R.A.; Barden, J. Systematic review of dexketoprofen in acute and chronic pain. BMC Clin. Pharmacol. 2008, 8, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carne, X.; Rios, J.; Torres, F. Postmarketing cohort study to assess the safety profile of oral dexketoprofen trometamol for mild to moderate acute pain treatment in primary care. Methods Find. Exp. Clin. Pharmacol. 2009, 31, 533–540. [Google Scholar] [PubMed]
- Walczak, J.S. Analgesic properties of dexketoprofen trometamol. Pain Manag. 2011, 1, 409–416. [Google Scholar] [CrossRef]
- Barbanoj, M.J.; Antonijoan, R.M.; Gich, I. Clinical pharmacokinetics of dexketoprofen. Clin. Pharmacokinet. 2001, 40, 245–262. [Google Scholar] [CrossRef]
- Barbanoj, M.J. Clinical pharmacokinetics of dexketoprofen trometamol: Recent studies. Methods Find. Exp. Clin. Pharmacol. 2006, 28, 3. [Google Scholar] [PubMed]
- Matsui, H.; Shimokawa, O.; Kaneko, T.; Nagano, Y.; Rai, K.; Hyodo, I. The pathophysiology of non-steroidal anti-inflammatory drug (NSAID)-induced mucosal injuries in stomach and small intestine. J. Clin. Biochem. Nutr. 2011, 48, 107–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, A.; Mubharak, N.M.; Naseem, K.; Tabassum, H.; Rizwan, M.; Najda, A.; Kashif, M.; Bin-Jumah, M.; Hussain, A.; Shaheen, A.; et al. Recent Advancement and development of chitin and chitosan -based nanocomposite for Drug Delivery: Critical approach to clinical research. Arab. J. Chem. 2020, 13, 8935–8964. [Google Scholar] [CrossRef]
- Ali, E.A.; Ismail, M.N.; Elsabee, M. Chitosan based polyelectrolyte complexes development for anionic and cationic dyes adsorption. Egypt J. Chem. 2020, 63, 537–554. [Google Scholar] [CrossRef]
- Quemeneur, F.; Rinaudo, M.; Pépin-Donat, B. Influence of Molecular Weight and pH on Adsorption of Chitosan at the Surface of Large and Giant Vesicles. Biomacromolecules 2008, 9, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Freitas, E.D.; Moura, C.F., Jr.; Kerwald, J.; Beppu, M.M. An Overview of Current Knowledge on the Properties, Synthesis and Applications of Quaternary Chitosan Derivatives. Polymers 2020, 12, 2878. [Google Scholar] [CrossRef]
- Yaneva, Z.; Ivanova, D.; Nikolova, N.; Tzanova, M. The 21st century revival of chitosan in service to bio-organic chemistry. Biotechnol. Biotechnol. Equip. 2020, 34, 221–237. [Google Scholar] [CrossRef]
- Degim, I.T.; Kadioglu, D. Cheap, suitable, predictable and manageable nanoparticles for drug delivery: Quantum dots. Curr. Drug Deliv. 2013, 10, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Sevim, B.B.; Cerci, B.H.; Kucuk, Z.; Bahadori, F.; Kazdal, F.; Eskandari, Z.; Tiris, G.; Demiray, M. Optimization of a new controlled release oral dexketoprofen formulation. J. Nanomater. Mol. Nanotechnol. 2018, 7, 109. [Google Scholar]
- Öztürk, A.A.; Yenilmez, E.; Yazan, Y. Dexketoprofen trometamol-loaded Eudragit® RL 100 nanoparticle formulation, characterization and release kinetics. Acta Pharm. Sci. 2019, 57, 69–84. [Google Scholar]
- Öztürk, A.A.; Kiyan, H.T. Treatment of oxidative stress-induced pain and inflammation with dexketoprofen trometamol loaded different molecular weight chitosan nanoparticles: Formulation, characterization and anti-inflammatory activity by using in vivo HET-CAM assay. Microvasc. Res. 2020, 128, 103961. [Google Scholar] [CrossRef] [PubMed]
- Gârlea, A.; Popa, M.I.; Pohoaţă, V.; Melnig, V. Ibuprofen/ketoprofen entrapment in chitosan based vesicle carrier. Rom. J. Biophys. 2007, 17, 157–168. [Google Scholar]
- Tartau, L.; Cazacu, A.; Melnig, V. Ketoprofen-liposomes formulation for clinical therapy. J. Mater. Sci. Mater. Med. 2012, 23, 2499–2507. [Google Scholar] [CrossRef] [PubMed]
- Vasile, C.; Stoleru, E.; Darie-Nita, R.N.; Dumitriu, R.P.; Pamfil, P.; Tartau, L. Biocompatible materials based on plasticized poly(lactic acid), chitosan and Rosemary ethanolic extract I. Effect of chitosan on the properties of plasticized poly(lactic acid) materials. Polymers 2019, 11, 941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stefan, M.; Melnig, V.; Pricop, D.; Neagu, A.; Mihasan, M.; Tartau, L.; Hritcu, L. Attenuated effects of chitosan-capped gold nanoparticles on LPS-induced toxicity in laboratory rats. Mater. Sci. Eng. C Mater. 2013, 33, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Peniche, C.; Argüelles-Monal, W.; Peniche, H.; Acosta, N. Chitosan: An attractive biocompatible polymer for microencapsulation. Macromol. Biosci. 2003, 3, 511–520. [Google Scholar] [CrossRef]
- Tiyaboonchai, W. Chitosan nanoparticles: A promising system for drug delivery. Naresuan Univ. J. 2003, 11, 51–66. [Google Scholar]
- Li, X.; Wang, L.; Fan, Y.; Feng, Q.; Cui, F.Z. Biocompatibility and toxicity of nanoparticles and nanotubes. J. Nanomater. 2012, 2012, 548389. [Google Scholar]
- Wolf, M.F.; Andwraon, J.M. Practical approach to blood compatibility assessments: General considerations and standards. In Biocompatibility and Performance of Medical Devices; Boutrand, J.-P., Ed.; Woodhead Publishing: Cambridge, UK, 2012; pp. 159–201, 201e–206e. [Google Scholar]
- Bannon, A.W.; Malmberg, A.B. Malmberg, A.B. Models of nociception: Hot-plate, tail-flick, and formalin tests in rodents. Curr. Protoc. Neurosci. 2007, 41, 9.1–9.15. [Google Scholar] [CrossRef] [PubMed]
- Ma, C. Animal models of pain. Int. Anesthesiol. Clin. 2007, 45, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Mogil, J.S. Animal models of pain: Progress and challenges. Nat. Rev. Neurosci. 2009, 10, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Walker, E.A.; Butelman, E.R.; Decosta, B.R. Opioid thermal antinociception in rhesus monkeys: Receptor mechanisms and temperature dependency. J. Pharmacol. Exp. Ther. 1993, 267, 280–286. [Google Scholar]
- European Union. DIRECTIVE 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes; European Union: Brussels, Belgium, 2010. [Google Scholar]
- Zimmerman, M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983, 16, 109–110. [Google Scholar] [CrossRef]
- Ailincai, D.; Tartau-Mititelu, L.; Marin, L. Drug delivery systems based on biocompatible imino-chitosan hydrogels for local anticancer therapy. Drug Deliv. 2018, 25, 1080–1090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.; Dixit, C.K. Methods for characterization of nanoparticles. In Advances in Nanomedicine for the Delivery of Therapeutic Nucleic Acids; Nimesh, S., Chandra, R., Gupta, N., Eds.; Woodhead Publishing: Cambridge, UK, 2017; Chapter 3; pp. 43–58. [Google Scholar]
- Sashmal, S.; Mukherjee, S.; Ray, S.; Thakur, R.S.; Ghosh, L.K.; Gupta, B.K. Design and optimization of NSAID loaded nanoparticles. Pak. J. Pharm. Sci. 2007, 20, 157–162. [Google Scholar]
- Tomić, M.; Micov, A.; Pecikoza, U.; Stepanović-Petrović, R. Clinical Uses of Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) and Potential Benefits of NSAIDs Modified-Release Preparations. In Microsized and Nanosized Carriers for Nonsteroidal Anti-Inflammatory Drugs Formulation Challenges and Potential Benefits; Čalija, B., Ed.; Elsevier Inc.: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2017; pp. 1–29. [Google Scholar]
- Krajišnik, D.; Čalija, B.; Cekić, N. Polymeric Microparticles and Inorganic Micro/Nanoparticulate Drug Carriers: An Overview and Pharmaceutical Application. In Microsized and Nanosized Carriers for Nonsteroidal Anti-Inflammatory Drugs Formulation Challenges and Potential Benefits; Čalija, B., Ed.; Elsevier Inc.: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2017; pp. 31–67. [Google Scholar]
- Moradkhani, M.R.; Karimi, A.; Negahdari, B. Nanotechnology application for pain therapy. Artif. Cells Nanomed. Biotechnol. 2018, 46, 368–373. [Google Scholar] [CrossRef] [Green Version]
- Nita, L.E.; Chiriac, A.P.; Nistor, M.T.; Tartau, L. Indomethacin uptake into poly(2-hydroxyethyl methacrylate-co-3,9-divinyl-2,4,8,10-tetraoxaspiro [5.5]-undecane) network: In vitro and in vivo controlled release study. Int. J. Pharm. 2012, 426, 90–99. [Google Scholar] [CrossRef]
- Sweed, N.M.; Basalious, E.B.; Nour, S.A. Combined site-specific release retardant mini-matrix tablets (C-SSRRMT) for extended oral delivery of dexketoprofen trometamol: In vitro evaluation and single versus multiple doses pharmacokinetic study in human volunteers. Drug Dev. Ind. Pharm. 2019, 45, 1777–1787. [Google Scholar] [CrossRef]
- Ugurlu, T.; Ilhan, E. Development and in vitro evaluation of a novel pulsatile drug delivery system containing dexketoprofen trometamol. J. Pharm. Innov. 2020, 1–13. [Google Scholar] [CrossRef]
- Hossain, M.D.R.; Ahsan, A.; Hossain, M.R.; Dey, R. Formulation & In Vitro Evaluation of Gastroretentive Floating Drug Delivery System of Dexketoprofen. Available online: https://www.researchgate.net/profile/Md_Hossain367/publication/332670659_USTC_RoAR_FDDS_POSTER/links/5cc297c092851c8d220508cf/USTC-RoAR-FDDS-POSTER.pdf (accessed on 8 February 2021).
- Çoban, Ö.; Değim, Z.; Yılmaz, Ş.; Altıntaş, L.; Arsoy, T.; Sözmen, M. Efficacy of targeted liposomes and nanocochleates containing imatinib plus dexketoprofen against fibrosarcoma. Drug Dev. Res. 2019, 80, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Çulcu, Ö.; Tunçel, E.; İlbasmış Tamer, S.; Tırnaksız, F. Characterization of Thermosensitive Gels for the Sustained Delivery of Dexketoprofen Trometamol for Dermal Applications. GUHES 2020, 2, 1–12. [Google Scholar]
- Öztürk, A.A.; Yenilmez, E.; Arslan, R.; Şenel, B.; Yazan, Y. Dexketoprofen trometamol-loaded Kollidon® SR and Eudragit® RS 100 polymeric nanoparticles: Formulation and in vitro-in vivo evaluation. Lat. Am. J. Pharm. 2017, 36, 2153–2165. [Google Scholar]
- Öztürk, A.A.; Banderas, L.M.; Otero, M.D.C.; Yenilmez, E.; Şenel, B.; Yazan, Y. Dexketoprofen trometamol-loaded poly-lactic-co-glycolic acid (PLGA) nanoparticles: Preparation, in vitro characterization and cyctotoxity. Trop. J. Pharm. Res. 2019, 18, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Öztürk, A.A.; Yenilmez, E.; Arslan, R.; Şenel, B.; Yazan, Y. Dexketoprofen trometamol loaded solid lipid nanoparticles (SLNs): Formulation, in vitro and in vivo evaluation. J. Res. Pharm. 2020, 24, 82–89. [Google Scholar] [CrossRef] [Green Version]
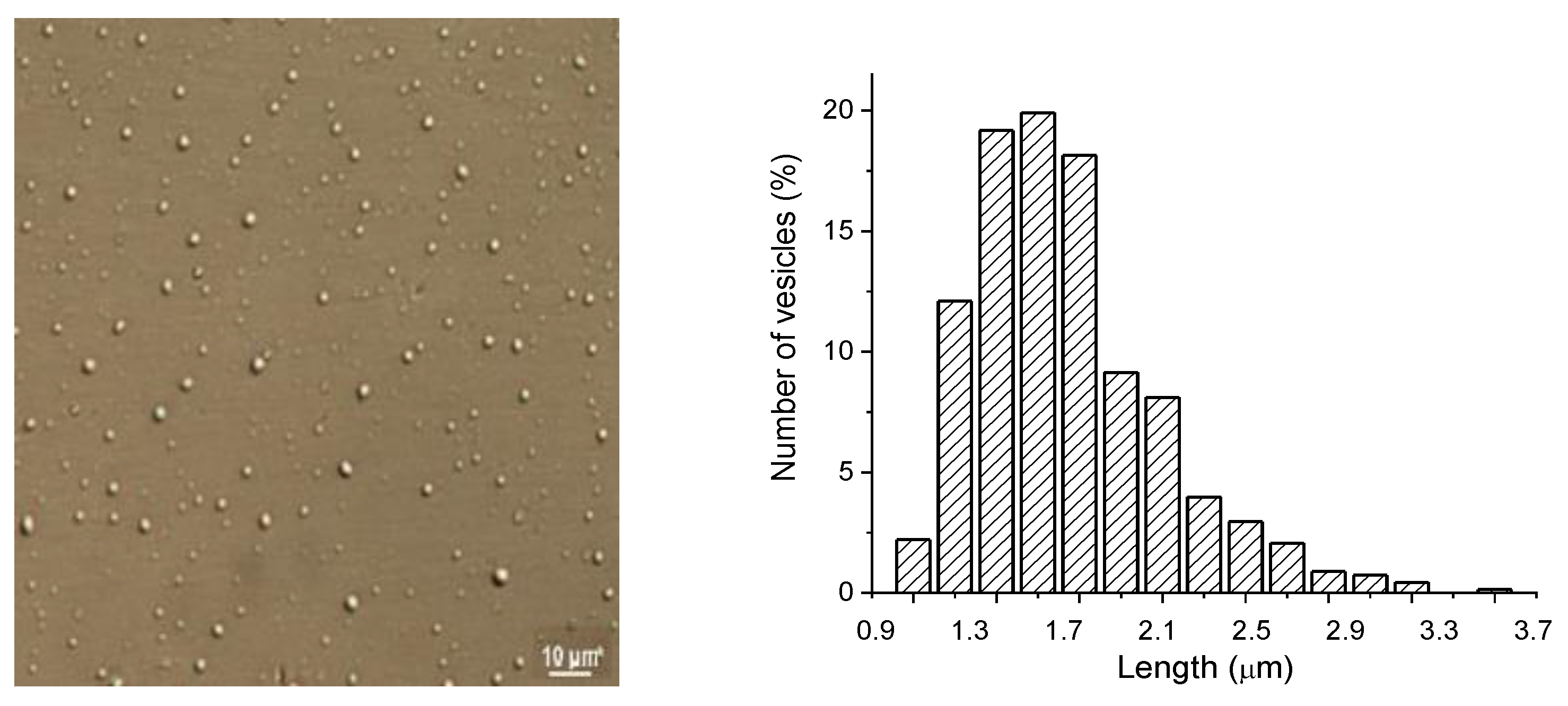
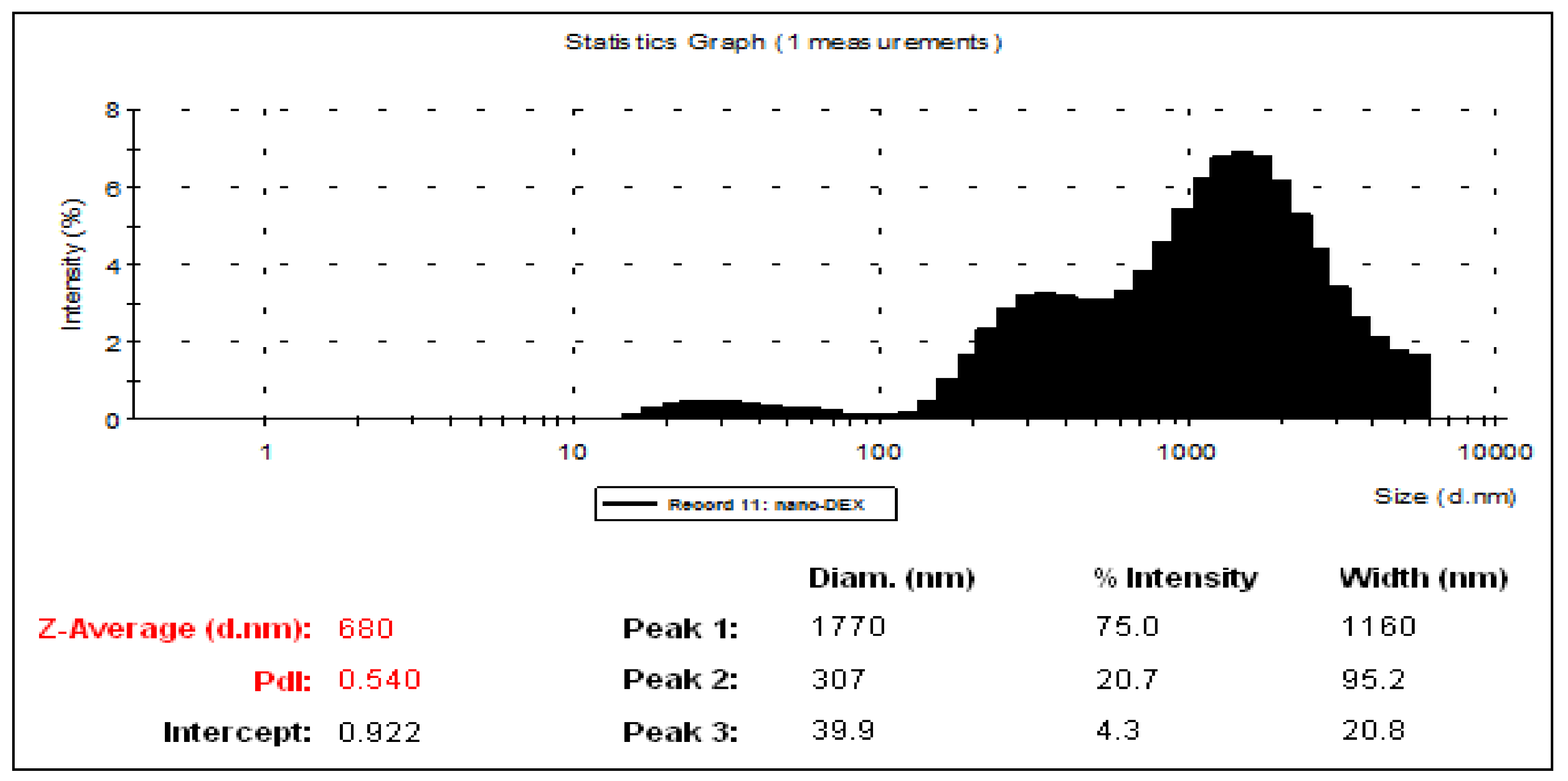

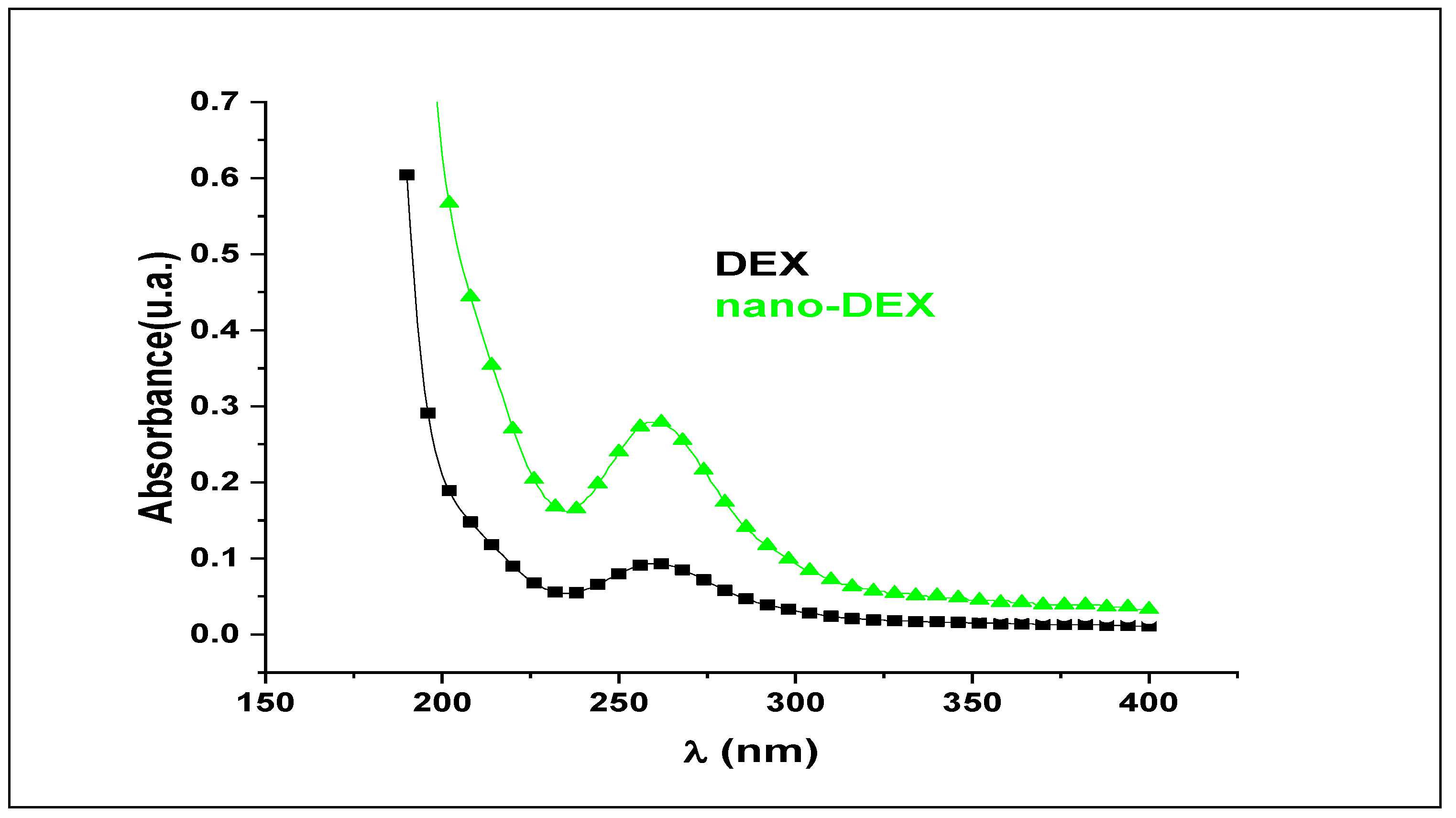
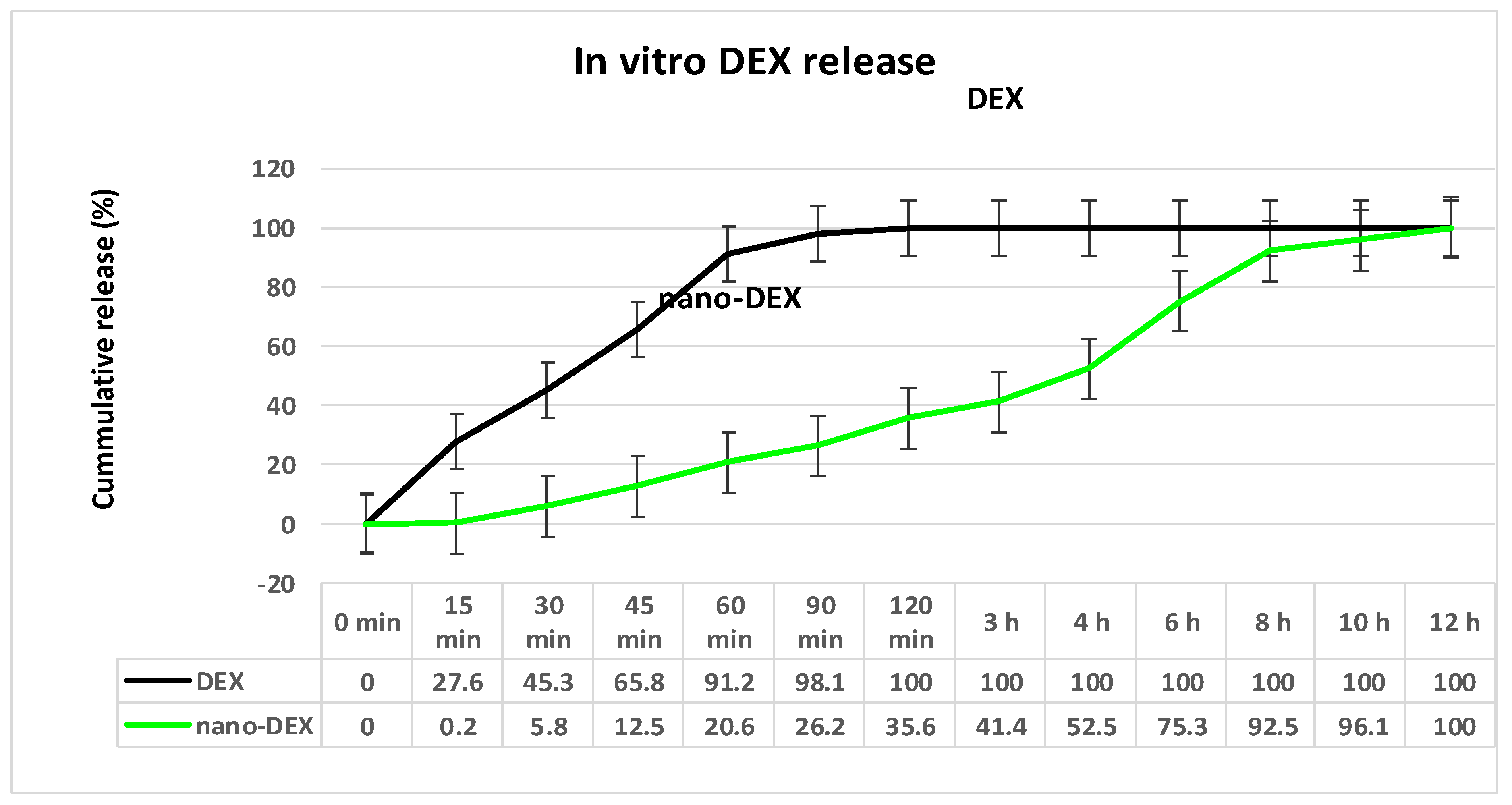
| Liposomes Types | pH | Colloidal Solution Characterization | |||
|---|---|---|---|---|---|
| Polydispersity Index | Z-Average Diameter (nm) | Z Potential (mV) | Stability Criterion | ||
| Liposomes entrapping DEX | 7.12 | 0.472 | 368 | 0.64 | Threshold of agglomeration |
| Chitosan-coated liposomes entrapping DEX | 4 | 0.636 | 1470 | 61.7 | Very good stability |
| Dialyzed chitosan-coated liposomes entrapping DEX | 6.89 | 0.435 | 699 | 3.89 | Threshold of light dispersion |
| Blood Count Formula (% Values) | ||||||
|---|---|---|---|---|---|---|
| PMN | Ly | E | M | B | ||
| DW | 1 day | 27.5 ± 3.3 | 68.1 ± 9.5 | 0.4 ± 0.1 | 3.8 ± 0.05 | 0.2 ± 0.05 |
| 7 days | 28.9 ± 3.5 | 66.7 ± 8.3 | 0.5 ± 0.1 | 3.7 ± 0.2 | 0.2 ± 0.05 | |
| DEX | 1 day | 28.2 ± 3.5 | 67.7 ± 8.7 | 0.4 ± 0.05 | 3.5 ± 0.2 | 0.2 ± 0.1 |
| 7 days | 29.5 ± 4.1 | 66.2 ± 9.1 | 0.5 ± 0.05 | 3.5 ± 0.1 | 0.3 ± 0.05 | |
| nano-DEX | 1 day | 29.3 ± 3.7 | 66.8 ± 8.3 | 0.4 ± 0.05 | 3.3 ± 0.05 | 0.2 ± 0.05 |
| 7 days | 29.7 ± 3.1 | 66.1 ± 8.5 | 0.4 ± 0.1 | 3.6 ± 0.1 | 0.2 ± 0.05 | |
| AST (U/mL) | ALT (U/mL) | LDH (U/L) | ||
|---|---|---|---|---|
| DW | 1 day | 54.5 ± 6.7 | 67.3 ± 8.3 | 336.42 ± 70.17 |
| 7 days | 52.3 ± 5.9 | 68.1 ± 9.3 | 346.29 ± 71.45 | |
| DEX | 1 day | 54.2 ± 6.3 | 66.5 ± 7.5 | 341.33 ± 71.67 |
| 7 days | 56.5 ± 7.1 | 69.3 ± 9.1 | 353.37 ± 69.29 | |
| nano-DEX | 1 day | 53.7 ± 5.5 | 66.1 ± 8.3 | 356.72 ± 69.55 |
| 7 days | 55.3 ± 6.5 | 68.7 ± 8.7 | 360.29 ± 68.13 | |
| Urea (mg/dL) | Creatinine (mg/dL) | ||
|---|---|---|---|
| DW | 1 day | 35.8 ± 6.33 | <0.2 |
| 7 days | 36.7 ± 6.72 | <0.2 | |
| DEX | 1 day | 36.4 ± 6.65 | <0.1 |
| 7 days | 37.6 ± 7.29 | <0.2 | |
| nano-DEX | 1 day | 35.5 ± 6.41 | <0.1 |
| 7 days | 37.9 ± 7.83 | <0.1 | |
| Serum Complement Activity (UCH50) | PC (%) | ||
|---|---|---|---|
| DW | 1 day | 15.9 ± 3.1 | 54.3 ± 8.1 |
| 7 days | 16.5 ± 3.7 | 55.7 ± 8.5 | |
| DEX | 1 day | 16.3 ± 4.1 | 54.5 ± 8.7 |
| 7 days | 16.8 ± 4.5 | 56.1 ± 9.3 | |
| nano-DEX | 1 day | 15.6 ± 3.3 | 53.7 ± 7.7 |
| 7 days | 16.7 ± 3.9 | 55.3 ± 8.5 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mititelu-Tartau, L.; Bogdan, M.; Pricop, D.A.; Buca, B.R.; Hilitanu, L.; Pauna, A.-M.; Dijmarescu, L.A.; Popa, E.G. Biocompatibility and Pharmacological Effects of Innovative Systems for Prolonged Drug Release Containing Dexketoprofen in Rats. Polymers 2021, 13, 1010. https://doi.org/10.3390/polym13071010
Mititelu-Tartau L, Bogdan M, Pricop DA, Buca BR, Hilitanu L, Pauna A-M, Dijmarescu LA, Popa EG. Biocompatibility and Pharmacological Effects of Innovative Systems for Prolonged Drug Release Containing Dexketoprofen in Rats. Polymers. 2021; 13(7):1010. https://doi.org/10.3390/polym13071010
Chicago/Turabian StyleMititelu-Tartau, Liliana, Maria Bogdan, Daniela Angelica Pricop, Beatrice Rozalina Buca, Loredana Hilitanu, Ana-Maria Pauna, Lorena Anda Dijmarescu, and Eliza Gratiela Popa. 2021. "Biocompatibility and Pharmacological Effects of Innovative Systems for Prolonged Drug Release Containing Dexketoprofen in Rats" Polymers 13, no. 7: 1010. https://doi.org/10.3390/polym13071010
APA StyleMititelu-Tartau, L., Bogdan, M., Pricop, D. A., Buca, B. R., Hilitanu, L., Pauna, A.-M., Dijmarescu, L. A., & Popa, E. G. (2021). Biocompatibility and Pharmacological Effects of Innovative Systems for Prolonged Drug Release Containing Dexketoprofen in Rats. Polymers, 13(7), 1010. https://doi.org/10.3390/polym13071010








