Semi-Crystalline Hydrophobic Polyamidoamines: A New Family of Technological Materials?
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
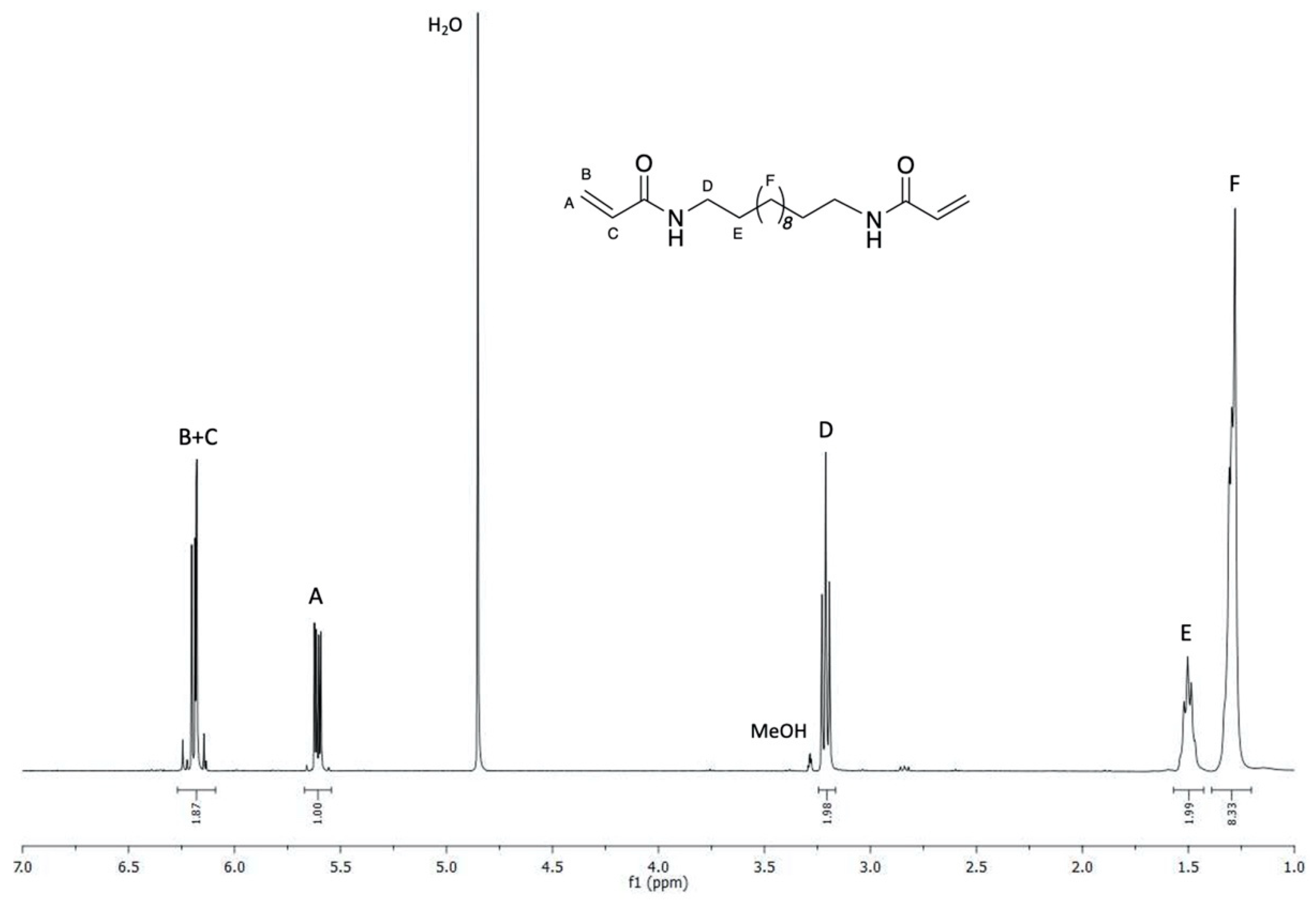
3.1. Synthesis of Hydrophobic Bis-Acrylamides
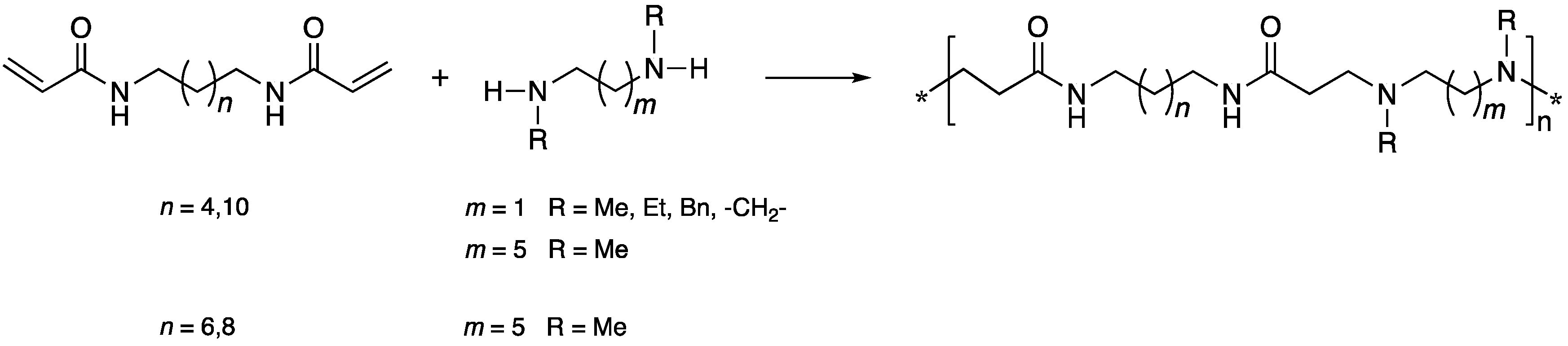
3.2. Synthesis of H-PAAs by the Solution Process
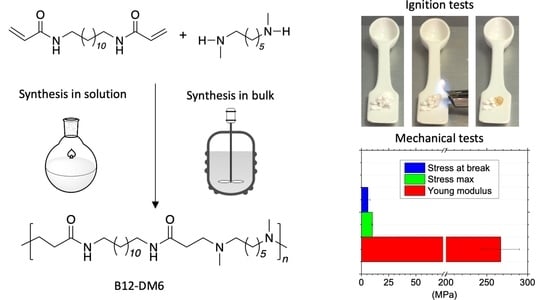
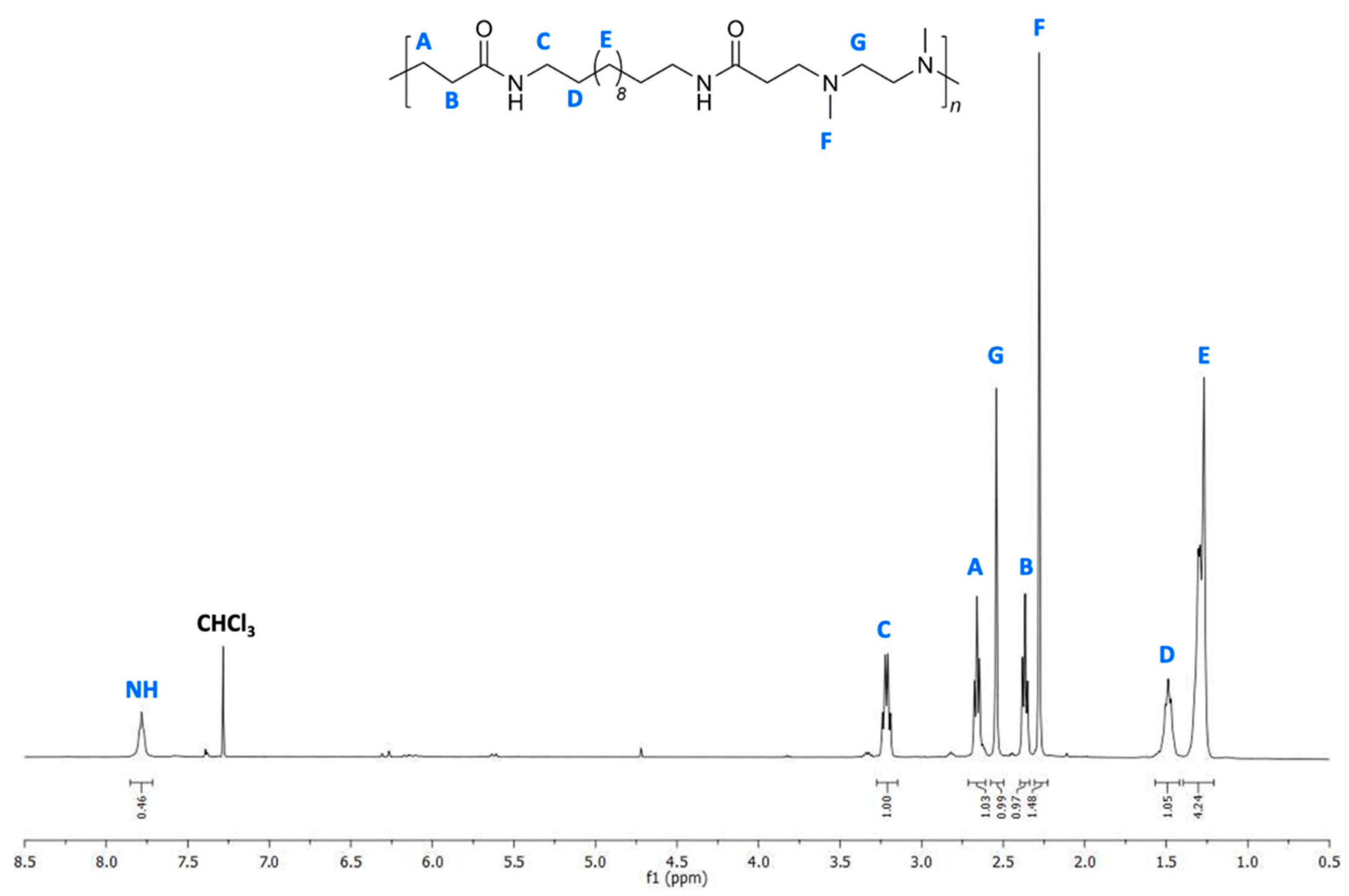
3.3. Synthesis of H-PAAs by the Bulk Process
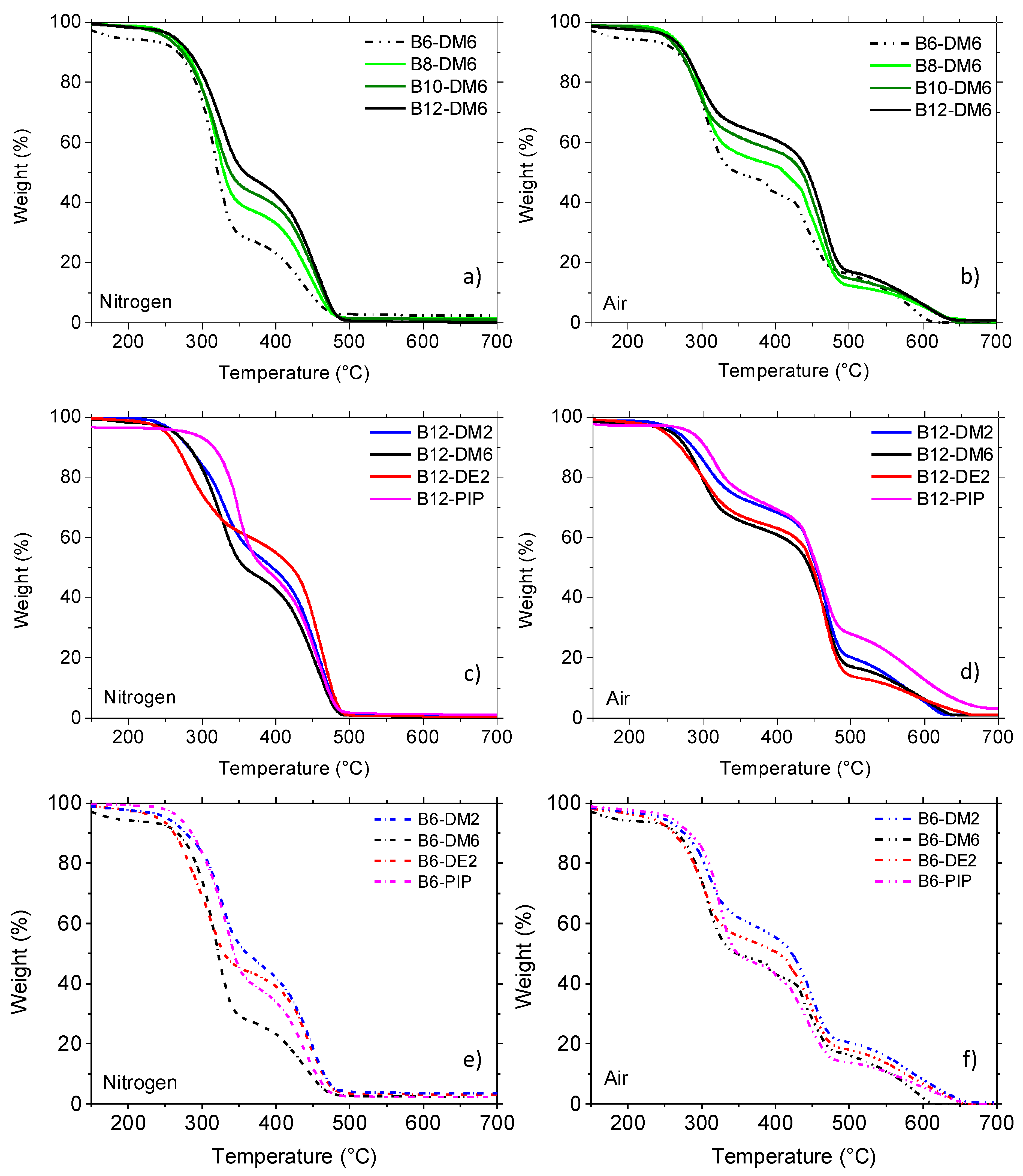
3.4. Thermal Stability of H-PAAs
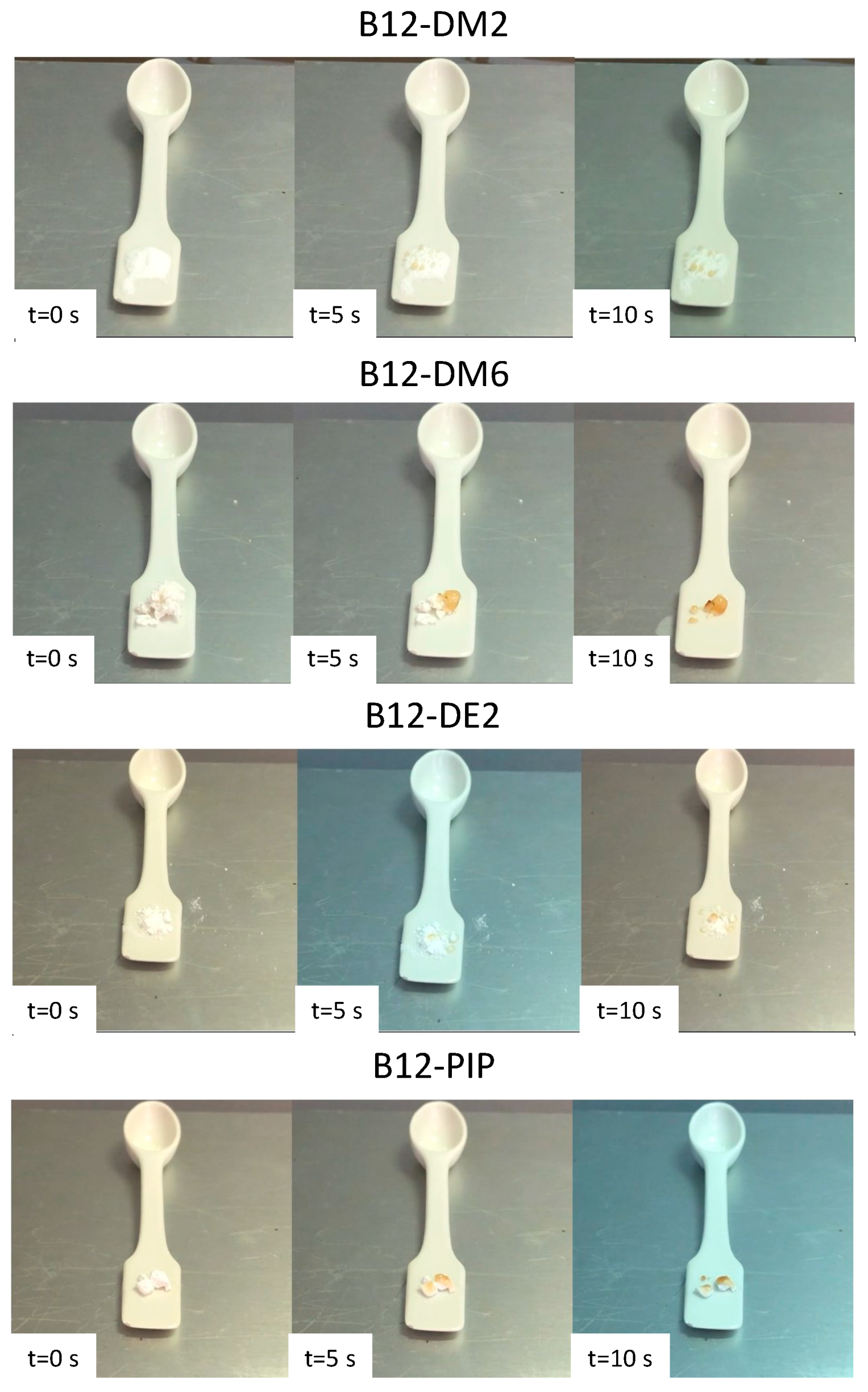
3.5. Preliminary Evaluation of the Effect of Water Contact on the Macroscopic Properties of H-PAA Films
3.6. Ignitability Tests
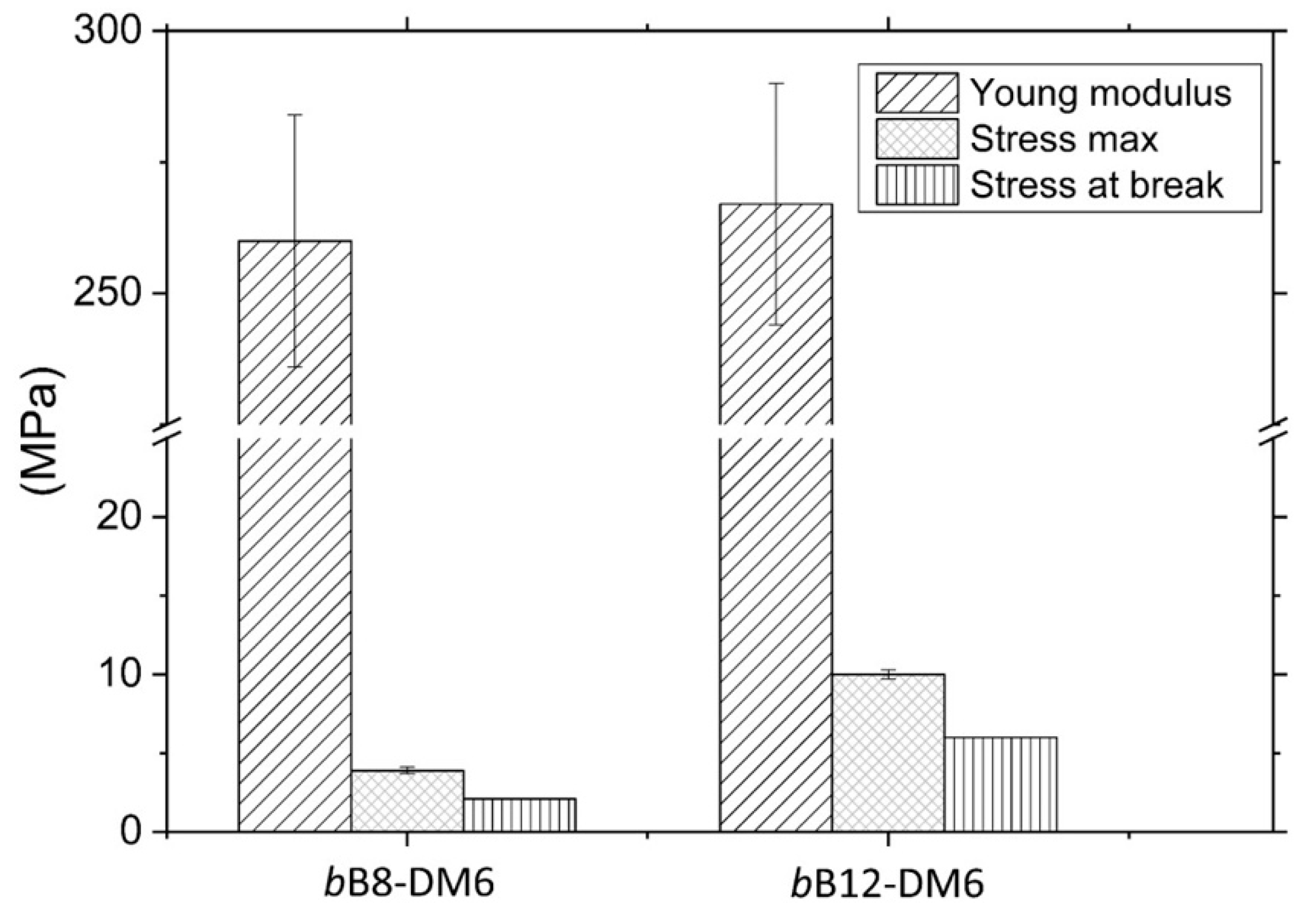
3.7. Preliminary Evaluation of Tensile Properties of bB12-DM6 and bB8-DM6
3.8. Wettability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferruti, P. Polyamidoamines: Past, Present and Perspectives. J. Polym. Sci. Polym. Chem. 2013, 51, 2319–2353. [Google Scholar] [CrossRef]
- Ranucci, E.; Manfredi, A. Polyamidoamines: Versatile bioactive polymers with potential for biotechnological applications. Chem. Afr. 2019, 2, 167–193. [Google Scholar] [CrossRef]
- Mather, B.D.; Visvanathan, K.; Millerb, K.M.; Long, T.E. Michael addition reactions in macromolecular design for emerging technologies. Prog. Polym. Sci. 2006, 31, 487–531. [Google Scholar] [CrossRef]
- Ferruti, P.; Marchisio, M.A.; Duncan, R. Poly(amido-amine)s: Biomedical Applications. Macromol. Rapid Commun. 2002, 23, 332–355. [Google Scholar] [CrossRef]
- Ferruti, P.; Ranucci, E.; Bignotti, F.; Sartore, L.; Bianciardi, P.; Marchisio, M.A. Degradation Behaviour of Ionic Stepwise Polyaddition Polymers of Medical Interest. J. Biomater. Sci. Polym. Ed. 1995, 6, 833–844. [Google Scholar] [CrossRef]
- Arioli, M.; Manfredi, A.; Alongi, J.; Ferruti, P.; Ranucci, E. Highlight on the Mechanism of Linear Polyamidoamine Degradation in Water. Polymers 2020, 12, 1376. [Google Scholar] [CrossRef] [PubMed]
- Barbucci, R.; Casolaro, M.; Nocentini, M.; Corezzi, S.; Ferruti, P.; Barone, V. Acid-Base and Metal Ion Complex Formation Properties of Polymers Containing Amino Acid Residues. Macromolecules 1986, 19, 37–42. [Google Scholar] [CrossRef]
- Sartore, L.; Penco, M.; Della Sciucca, S.; Borsarini, G.; Ferrari, V. New carbon black composite vapor detectors based on multifunctional polymers. Sensors Actuat. B Chem. 2005, 111, 160–165. [Google Scholar] [CrossRef]
- Sartore, L.; Barbaglio, M.; Borgese, L.; Bontempi, E. Polymer-grafted QCM chemical sensor and application to heavy metal ions real time detection. Sensors Actuat. B Chem. 2011, 155, 538–544. [Google Scholar] [CrossRef]
- Sartore, L.; Barbaglio, M.; Penco, M.; Bergese, R.; Bontempi, E.; Colombi, P.; Depero, L.E. Polymer-coated quartz crystal microbalance chemical sensor for heavy cations in water. J. Nanosci. Nanotechnol. 2009, 9, 1164–1168. [Google Scholar] [CrossRef] [PubMed]
- Barbucci, R.; Magnani, A. Physiochemical characterization and coating of polyurethane with a new heparin-adsorbing material. Biomaterials 1989, 10, 429–432. [Google Scholar] [CrossRef]
- Barbucci, R.; Casolaro, M.; Magnani, A.; Roncolini, C. FTi.r. and potentiometric study of basic polymer behaviour in the free form, in different bulks, in both solid state and aqueous solution. Polymer 1991, 32, 897–903. [Google Scholar] [CrossRef]
- Barbucci, R.; Albanese, A.; Magnani, A.; Tempesti, F. Coating of commercially available materials with a new heparinizable material. J. Biomed. Mater. Res. 1991, 25, 1259–1274. [Google Scholar] [CrossRef]
- Caldwell, G.; Neuse, E.; Stephanou, A. Synthesis of water-soluble polyamidoamines for biomedical applications. II. Polymers possessing intrachain-type secondary amino groups suitable for side-chain attachment. J. Appl. Polym. Sci. 1993, 50, 393–401. [Google Scholar] [CrossRef]
- Caldwell, G.; Neuse, E.W.; van Rensburg, C.E.J. Cytotoxicity of Selected Water-Soluble Polymer–cis-Diaminedichloroplatinum (II) Conjugates Against the Human HeLa Cancer Cell Line. J. Inorg. Organomet. Polym. 1997, P7, 217–231. [Google Scholar] [CrossRef]
- Bencini, M.; Ranucci, E.; Ferruti, P.; Trotta, F.; Donalisio, M.; Cornaglia, M.; Lembo, D.; Cavalli, R. Preparation and in vitro evaluation of the antiviral activity of the Acyclovir complex of a β-cyclodextrin/poly(amidoamine) copolymer. J. Control. Release 2008, 126, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Mauro, N.; Ferruti, P.; Ranucci, E.; Manfredi, A.; Berzi, A.; Clerici, M.; Cagno, V.; Lembo, D.; Palmioli, A.; Sattin, S. Linear biocompatible glyco-polyamidoamines as dual action mode virus infection inhibitors with potential as broad-spectrum microbicides for sexually transmitted diseases. Sci. Rep. 2016, 6, 33393. [Google Scholar] [CrossRef]
- Donalisio, M.; Quaranta, P.; Chiuppesi, F.; Pistello, M.; Cagno, V.; Cavalli, R.; Volante, M.; Bugatti, A.; Rusnati, M.; Ranucci, E.; et al. The AGMA1 poly(amidoamine) inhibits the infectivity of herpes simplex virus in cell lines, in human cervicovaginal histocultures, and in vaginally infected mice. Biomaterials 2016, 85, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Elzes, M.R.; Akeroyd, N.; Engbersen, J.F.J.; Paulusse, J.M.J. Disulfide-functional poly(amido amine)s with tunable degradability for gene delivery. J. Control. Release 2016, 244, 357–365. [Google Scholar] [CrossRef]
- Lim, H.; Noh, J.; Kim, Y.; Kim, H.; Kim, J.; Khang, G.; Lee, D. Acid-Degradable Cationic Poly (ketal amidoamine) for Enhanced RNAInterference In Vitro and In Vivo. Biomacromolecules 2013, 14, 240–247. [Google Scholar] [CrossRef]
- Cavalli, R.; Primo, L.; Sessa, R.; Chiaverina, G.; di Blasio, L.; Alongi, J.; Manfredi, A.; Ranucci, E.; Ferruti, P. The AGMA1 polyamidoamine mediates the efficient delivery of siRNA. J. Drug Target. 2017, 25, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, H.; Xing, H.; Lang, L.; Cheng, L.; Yang, T.; Yang, L.; Ding, P. Bioreducible poly (amido amine) copolymers derived from histamine and agmatine for highly efficient gene delivery. Polym. Int. 2019, 68, 447–455. [Google Scholar] [CrossRef]
- Ranucci, E.; Bignotti, F.; Paderno, P.L.; Ferruti, P. Modification of albumins by grafting poly (amido amine) chains. Polymer 1995, 36, 2989–2994. [Google Scholar] [CrossRef]
- Magnaghi, V.; Conte, V.; Procacci, P.; Pivato, G.; Cortese, P.; Cavalli, E.; Pajardi, G.; Ranucci, E.; Fenili, F.; Manfredi, A.; et al. Biological performance of a novel biodegradable polyamidoamine hydrogel as guide for peripheral nerve regeneration. J. Biomed. Mater. Res. Part A 2011, 98 A, 19–30. [Google Scholar] [CrossRef]
- Dos Reis, G.; Fenili, F.; Gianfelice, A.; Bongiorno, G.; Marchesi, D.; Scopelliti, P.E.; Borgonovo, A.; Podestà, A.; Indrieri, M.; Ranucci, E.; et al. Direct microfabrication of topographical and chemical cues for the guided growth of neural cell networks on polyamidoamine hydrogels. Macromol. Biosci. 2010, 10, 842–852. [Google Scholar] [CrossRef] [PubMed]
- Martello, F.; Tocchio, A.; Tamplenizza, M.; Gerges, I.; Pistis, V.; Recenti, R.; Bortolin, M.; Del Fabbro, M.; Argentiere, S.; Milani, P.; et al. Poly (amido-amine)-based hydrogels with tailored mechanical properties and degradation rates for tissue engineering. Acta Biomater. 2014, 10, 1206–1215. [Google Scholar] [CrossRef] [PubMed]
- Mauro, N.; Chiellini, F.; Bartoli, C.; Gazzarri, M.; Laus, M.; Antonioli, D.; Griffiths, P.; Manfredi, A.; Ranucci, E.; Ferruti, P. RGD-mimic polyamidoamine–montmorillonite composites with tunable stiffness as scaffolds for bone tissue-engineering applications. J. Tissue Eng. Regen. Med. 2017, 11, 2164–2175. [Google Scholar] [CrossRef]
- Gualandi, C.; Bloise, N.; Mauro, N.; Ferruti, P.; Manfredi, A.; Sampaolesi, M.; Liguori, A.; Laurita, R.; Gherardi, M.; Colombo, V.; et al. Poly-l-Lactic Acid Nanofiber–Polyamidoamine Hydrogel Composites: Preparation, Properties, and Preliminary Evaluation as Scaffolds for Human Pluripotent Stem Cell Culturing. Macromol. Biosci. 2016, 1533–1544. [Google Scholar] [CrossRef] [PubMed]
- Manfredi, A.; Carosio, F.; Ferruti, P.; Ranucci, E.; Alongi, J. Linear polyamidoamines as novel biocompatible phosphorus-free surface-confined intumescent flame retardants for cotton fabrics. Polym. Degrad. Stabil. 2018, 151, 52–64. [Google Scholar] [CrossRef]
- Schotten, C. Ueber die Oxydation des Piperidins. Ber. Dtsch. Chem. Ges. 1884, 17, 2544. [Google Scholar] [CrossRef]
- Baumann, E. Ueber eine einfache Methode der Darstellung von Benzoësäureäthern. Ber. Dtsch. Chem. Ges. 1886, 19, 3218. [Google Scholar] [CrossRef]
- Vogel, A.I. Practical Organic Chemistry, 3rd ed.; Longman Group Ltd.: London, UK, 1957; pp. 582–584. [Google Scholar]
- Fulmer, G.R.; Miller, A.J.M.; Sherden, N.H.; Gottlieb, H.E.; Nudelman, A.; Stoltz, B.M.; Bercaw, J.E.; Goldberg, K.I. NMR Chemical Shifts of Trace Impurities: Common Laboratory Solvents, Organics, and Gases in Deuterated Solvents Relevant to the Organometallic Chemist. Organometallics 2010, 29, 2176–2179. [Google Scholar] [CrossRef]
- Babij, N.R.; McCusker, E.O.; Whiteker, G.T.; Canturk, B.; Choy, N.; Creemer, L.C.; De Amicis, C.V.; Hewlett, N.M.; Johnson, P.L.; Knobelsdorf, J.A.; et al. NMR Chemical Shifts of Trace Impurities: Industrially Preferred Solvents Used in Process and Green Chemistry. Org. Process. Res. Dev. 2016, 20, 661–667. [Google Scholar] [CrossRef]
- Aboulkas, A.; El Harfi, K.; El Bouadili, A. Thermal degradation behaviors of polyethylene and polypropylene. Part I: Pyrolysis kinetics and mechanisms. Energy Convers. Manag. 2010, 51, 1363–1369. [Google Scholar] [CrossRef]
- Bolbukh, Y.; Kuzema, P.; Tertykh, V.; Laguta, I. Thermal degradation of polyethylene containing antioxidant and hydrophilic/hydrophobic silica. J. Therm. Anal. Calorim. 2008, 94, 727–736. [Google Scholar] [CrossRef]
- Matzen, M.; Kandola, B.; Huth, C.; Schartel, B. Influence of Flame Retardants on the Melt Dripping Behaviour of Thermoplastic Polymers. Materials 2015, 8, 5621–5646. [Google Scholar] [CrossRef] [PubMed]
- Gorrasi, G.; Milone, C.; Piperopoulos, E.; Pantani, R. Preparation, processing and analysis of physical properties of calciumferrite-CNTs/PET nano-composite. Compos. Part B 2015, 81, 44–52. [Google Scholar] [CrossRef]
- Holland, B.J.; Hay, J.N. The thermal degradation of PET and analogous polyesters measured by thermal analysis-Fourier transform infrared spectroscopy. Polymer 2002, 43, 1835–1847. [Google Scholar] [CrossRef]
- Zhao, M.; Yi, D.; Camino, G.; Frache, A.; Yang, R. Interdigitated crystalline MMT-MCA in polyamide 6. RSC Adv. 2017, 7, 861–869. [Google Scholar] [CrossRef]
- Fina, A.; Camino, G. Ignition mechanisms in polymers and polymer nanocomposites. Polym. Adv. Technol. 2011, 22, 1147–1155. [Google Scholar] [CrossRef]
- Manfredi, A.; Carosio, F.; Ferruti, P.; Alongi, J.; Ranucci, E. Disulfide-containing polyamidoamines with remarkable flame retardant activity for cotton fabrics. Polym. Degrad. Stab. 2018, 156, 1–13. [Google Scholar] [CrossRef]
- Alongi, J.; Cuttica, F.; Di Blasio, A.; Carosio, F.; Malucelli, G. Intumescent features of nucleic acids and proteins. Termochim. Acta 2014, 591, 31–39. [Google Scholar] [CrossRef]
- Costes, L.; Laoutid, F.; Brohez, S.; Dubois, P. Bio-based flame retardants: When nature meets fire protection. Mater. Sci. Eng. R 2017, 117, 1–25. [Google Scholar] [CrossRef]
- Salmeia, K.A.; Gaan, S.; Malucelli, G. Recent Advances for Flame Retardancy of Textiles Based on Phosphorus Chemistry. Polymers 2016, 8, 319. [Google Scholar] [CrossRef] [PubMed]

 | ||||
|---|---|---|---|---|
| Code | N (b) | Amine (c) (g) | Yield (g) | Yield (%) |
| B12 | 10 | 31.0 | 27.7 | 60.0 |
| B10 | 8 | 26.4 | 24.0 | 57.0 |
| B8 | 6 | 22.1 | 26.5 | 70.0 |
| B6 | 4 | 17.8 | 20.5 | 61.0 |
| Sample Code (d) | Bis-Acrylamide (g) | Bis-Amine (g) | Benzyl Alcohol (mL) | Time (h) | Yield [g, (%)] | |
|---|---|---|---|---|---|---|
| B12-DM2 | 15.4 | 4.5 | 57.0 | 48 | 17.7 (89) | 9700 |
| B12-DM6 | 15.4 | 7.4 | 65.0 | 48 | 21.3 (93) | >10,000 |
| B12-DE2 | 15.4 | 5.9 | 61.0 | 48 | 18.7 (88) | 6000 |
| B12-DB2 | 15.4 | 12.4 | 83.0 | 96 | 19.5 (70) | <3000 |
| B12-PIP | 15.4 | 4.4 | 59.0 | 48 | 19.6 (99) | 6400 |
| B10-DM6 | 14.0 | 7.4 | 61.0 | 48 | 20.4 (95) | 6000 |
| B8-DM6 | 12.6 | 7.4 | 60.0 | 48 | 18.5 (92) | 6400 |
| B6-DM2 | 11.2 | 4.5 | 46.0 | 48 | 13.3 (85) | >10,000 |
| B6-DM6 | 11.2 | 7.4 | 55.0 | 48 | 15.5 (83) | >10,000 |
| B6-DE2 | 11.2 | 5.9 | 51.0 | 48 | 11.3 (66) | 6000 |
| B6-DB2 | 11.2 | 12.4 | 69.0 | 96 | n.d. (e) | <3000 |
| B6-PIP | 11.2 | 4.4 | 45.0 | 48 | (98) | >10,000 |
| bB12-DM6 (f) | 15.4 | 7.4 | - | 3 | (~100) | 7500 |
| bB8-DM6 (f) | 12.6 | 7.4 | - | 3 | (~100) | 6400 |
| Polymer Code | Structure of the Repeat Unit |
|---|---|
| B12-DM2 |  |
| B12-DE2 |  |
| B12-DB2 | 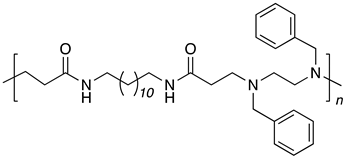 |
| B12-DM6 | 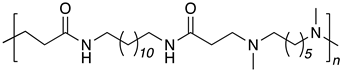 |
| B12-PIP | 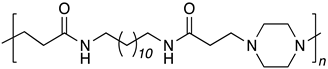 |
| B10-DM6 | 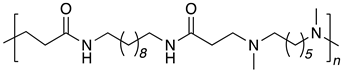 |
| B8-DM6 | 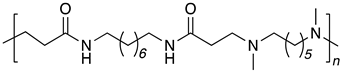 |
| B6-DM2 |  |
| B6-DE2 | 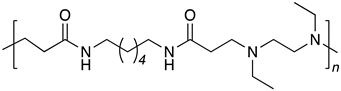 |
| B6-DB2 |  |
| B6-DM6 | 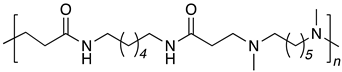 |
| B6-PIP | 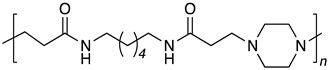 |
| B12-DM2 | B12-DM6 | B12-DE2 | B12-DB2 | B12-PIP | B10-DM6 | B8-DM6 | B6-DM2 | B6-DM6 | B6-DE2 | B6-DB2 | B6-PIP | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Methanol | i | i | sh | sh | i | sh | sh | sh | sh | sh | sh | i |
| Ethanol | sh | sh | sh | sh | i | sh | sh | sh | sh | sh | sh | i |
| Isopropanol | sh | sh | sh | sh | i | sh | sh | sh | sh | sh | sh | i |
| Butanol | sh | sh | sh | sh | sh | sh | sh | sh | sh | sh | sh | i |
| Benzyl alcohol | sh | s | s | s | sh | sh | sh | sh | s | S | s | sh |
| Acetone | i | i | i | i | i | i | i | i | i | i | i | i |
| Dimethyl sulfoxide | i | i | i | i | i | i | i | i | i | i | i | i |
| Diisopropyl ether | i | i | i | i | i | i | i | i | i | i | i | i |
| Diethyl ether | i | i | i | i | i | i | i | i | i | i | i | i |
| Chloroform | s | s | s | s | i | s | s | s | s | s | s | i |
| Dichloromethane | i | s | s | s | i | s | s | s | s | s | s | i |
| Acetic acid/ water 1:2 | i | i | i | i | s | i | i | i | i | i | i | s |
| Water | i | i | i | i | i | i | i | i | i | i | i | i |
| Polymer | Nitrogen | Air | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tonset10% (a) (°C) | Tmax1 (b) (°C) | Tmax2 (°C) | RMF400 (c) (%) | Tonset10% (°C) | Tmax1 (°C) | Tmax2 (°C) | Tmax3 (°C) | RMF400 (%) | RMF550 (d) (%) | |
| B12-DM2 | 281 | 330 | 460 | 49 | 285 | 305 | 462 | 610 | 68 | 14 |
| B12-DE2 | 265 | 280 | 462 | 55 | 268 | 290 | 464 | 575 | 63 | 11 |
| B12-DM6 | 270 | 323 | 459 | 43 | 269 | 299 | 465 | 610 | 61 | 13 |
| B12-PIP | 315 | 347 | 458 | 46 | 297 | 315 | 460 | 585 | 69 | 22 |
| B10-DM6 | 273 | 320 | 456 | 39 | 270 | 292 | 465 | 620 | 57 | 12 |
| B8-DM6 | 278 | 320 | 452 | 33 | 278 | 300 | 442/460 | 610 | 52 | 11 |
| B6-DM2 | 278 | 325 | 445 | 42 | 275 | 310 | 448 | 581 | 55 | 16 |
| B6-DE2 | 265 | 304 | 445 | 39 | 268 | 302 | 447 | 580 | 50 | 12 |
| B6-DM6 | 266 | 316 | 440 | 23 | 266 | 305 | 450 | 587 | 43 | 10 |
| B6-PIP | 286 | 330 | 438 | 34 | 282 | 324 | 446 | 575 | 43 | 11 |
| bB12-DM6 (e) | 271 | 318 | 457 | 41 | 281 | 300 | 464 | 617 | 61 | 13 |
| bB8-DM6 (e) | 279 | 322 | 450 | 34 | 277 | 300 | 441/462 | 612 | 53 | 10 |
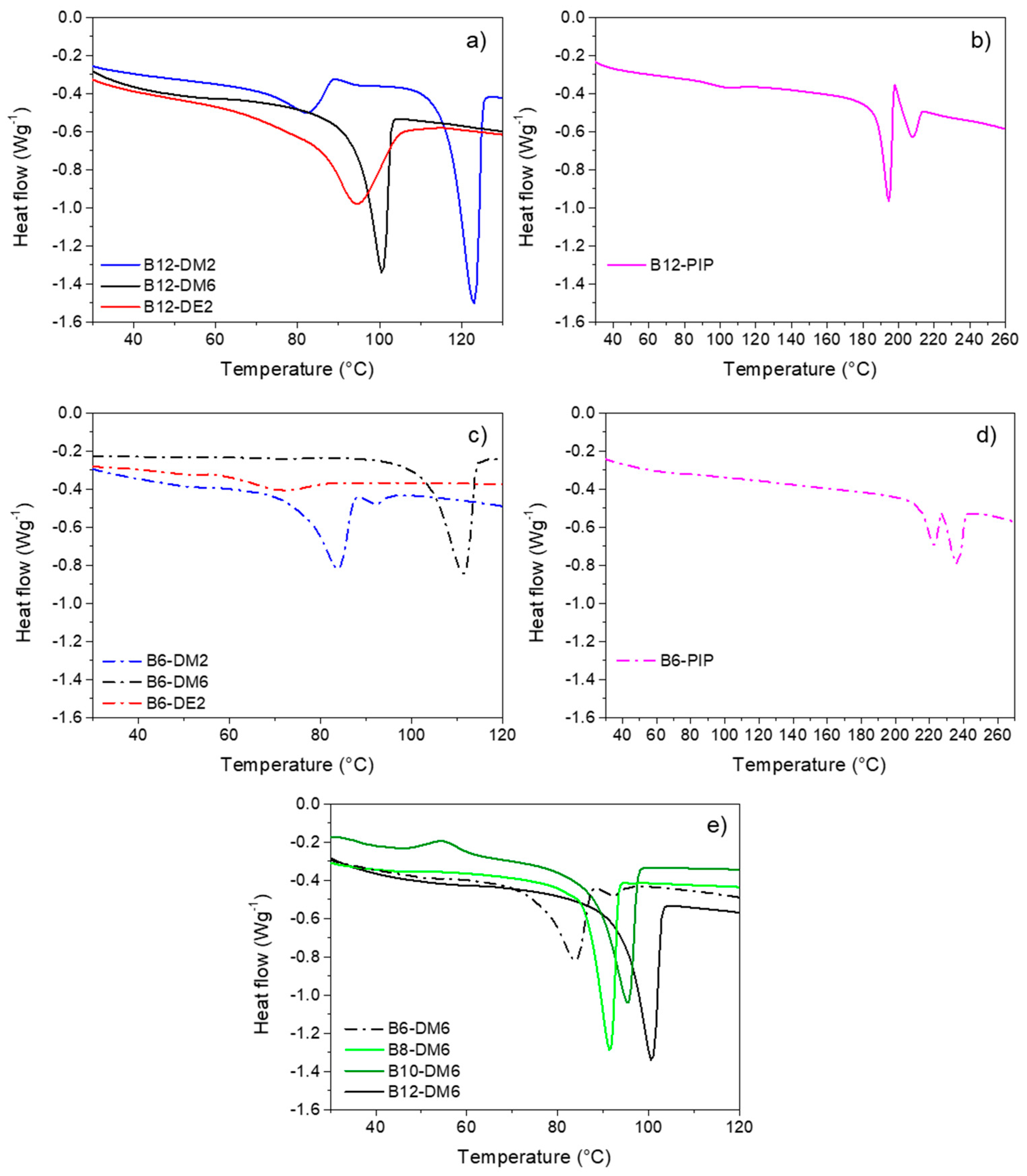
| Polymer | 1st Heating Cycle | Cooling Cycle | 2nd Heating Cycle | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tonset,m1 (a) (°C) | Tm1 (b) (°C) | ΔHm1 (c) (Jg−1) | Tonset,cr (d) (°C) | Tcr (e) (°C) | ΔHcr (f) (Jg−1) | Tonset,m2 (g) (°C) | Tm2 (h) (°C) | ΔHm2 (i) (Jg−1) | Tg (l) (°C) | |
| B12-DM2 | 70 | 83 | −23.4 | 97 | 91 | 49.3 | 116 | 123 | −76.6 | −10 |
| B12-DM6 | - | - | - | 71 | 61 | 58.6 | 95 | 100 | −63.3 | −19 |
| B12-DE2 | - | - | - | 80 | 74 | 82.3 | 83 | 95 | −95.5 | n.d. (m) |
| B12-PIP | 188 | 195 | −51.3 | 181 | 179 | 48.5 | 198 | 196/216 | −18.3 | +32 |
| B10-DM6 | - | - | - | 53 | 44 | 3.2 | 88 | 95 | −62.0 | −19 |
| B8-DM6 | - | - | 53 | 46 | 54.4 | 86 | 90 | −57.1 | −20 | |
| B6-DM2 | - | - | - | 78 | 72 | 53.6 | 103 | 111 | −54.5 | −13 |
| B6-DM6 | 76 | 84 | −37.1 | 55 | 50 | 48.1 | 89 | 84 | −51.1 | −23 |
| B6-DE2 | - | - | - | - | - | - | - | - | - | - |
| B6-PIP | 216 | 222 | −13.2 | 205 | 200 | 53.4 | 228 | 223/236 | −24.2 | +55 |
| bB12-DM6 | - | - | - | 68 | 62 | 34.6 | 98 | 105 | −72.2 | −10 |
| bB8-DM6 | - | - | - | 63 | 57 | 53.0 | 93 | 100 | −50.8 | −16 |
| Polymer | Apparent Flexibility | Resistance to Handling | Resistance after Contact with Water |
|---|---|---|---|
| B12-DM2 | + | + | - |
| B12-DM6 | + | + | + |
| B12-DE2 | + | + | - |
| B12-PIP | - | - | - |
| B10-DM6 | + | + | + |
| B8-DM6 | + | + | + |
| B6-DM2 | + | + | - |
| B6- DM6 | + | + | + |
| B6-DE2 | + | + | - |
| B6-PIP | - | - | + |
| Sample | Thickness (mm) | Young Modulus (MPa) | Stress Max (a) (MPa) | Stress at Break (MPa) | Strain at Break (%) |
|---|---|---|---|---|---|
| bB8-DM6 | 0.10 ± 0.02 | 260 ± 24 | 3.9 ± 0.2 | 2.1 ± 0.5 | 1.7 ± 0.2 |
| bB12-DM6 | 0.12 ± 0.02 | 267 ± 23 | 10.0 ± 0.3 | 5.9 ± 1.9 | 6.9 ± 0.3 |
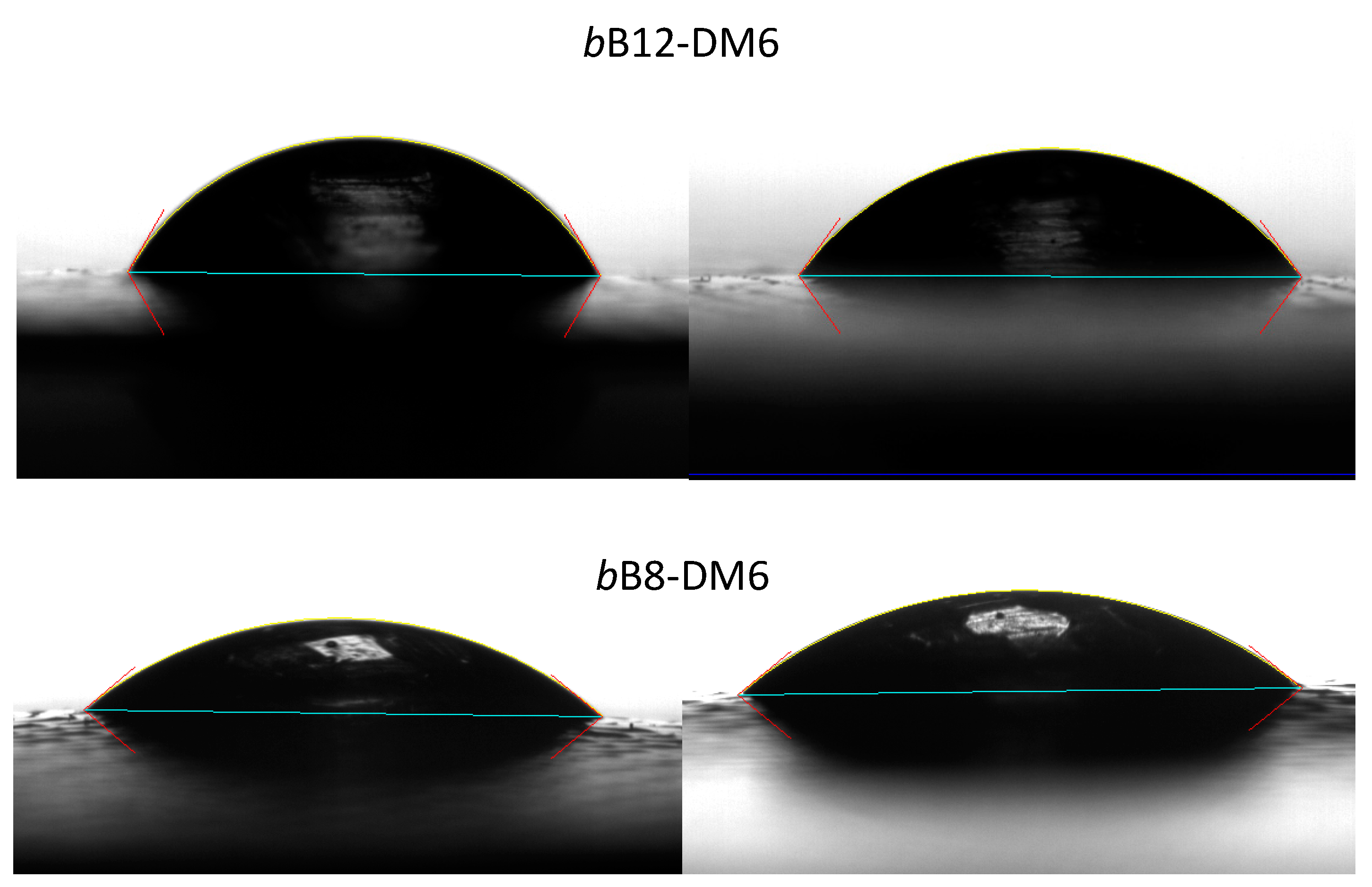
| Polymer Sample | Place | Contact Angle (deg) | Wetting Tension (dy/cm) | Base (mm) | Base Area (mm2) | Sessile Volume (μL) |
|---|---|---|---|---|---|---|
| bB8-DM6 | Border | 39.9 | 56.0 | 7.6 | 44.8 | 32.0 |
| bB8-DM6 | Center | 40.3 | 55.7 | 7.3 | 40.9 | 28.4 |
| bB12-DM6 | Border | 60.5 | 35.9 | 6.1 | 28.8 | 28.3 |
| bB12-DM6 | Center | 54.2 | 42.7 | 6.7 | 33.9 | 31.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcioni, M.; Alongi, J.; Ranucci, E.; Malinconico, M.; Laurienzo, P.; Ferruti, P.; Manfredi, A. Semi-Crystalline Hydrophobic Polyamidoamines: A New Family of Technological Materials? Polymers 2021, 13, 1018. https://doi.org/10.3390/polym13071018
Marcioni M, Alongi J, Ranucci E, Malinconico M, Laurienzo P, Ferruti P, Manfredi A. Semi-Crystalline Hydrophobic Polyamidoamines: A New Family of Technological Materials? Polymers. 2021; 13(7):1018. https://doi.org/10.3390/polym13071018
Chicago/Turabian StyleMarcioni, Massimo, Jenny Alongi, Elisabetta Ranucci, Mario Malinconico, Paola Laurienzo, Paolo Ferruti, and Amedea Manfredi. 2021. "Semi-Crystalline Hydrophobic Polyamidoamines: A New Family of Technological Materials?" Polymers 13, no. 7: 1018. https://doi.org/10.3390/polym13071018
APA StyleMarcioni, M., Alongi, J., Ranucci, E., Malinconico, M., Laurienzo, P., Ferruti, P., & Manfredi, A. (2021). Semi-Crystalline Hydrophobic Polyamidoamines: A New Family of Technological Materials? Polymers, 13(7), 1018. https://doi.org/10.3390/polym13071018