The Effect of Poly (Ethylene glycol) Emulation on the Degradation of PLA/Starch Composites
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Film Preparation
2.3. Photodegradation of the Samples
2.4. The Microstructures of the Samples
2.5. Mechanical Performance
2.6. Differential Scanning Calorimetry (DSC) Study
2.7. Fourier Transform Infrared Spectroscopy (FTIR) Study
2.8. Hydrolytic Degradation of the Samples
2.9. Biodegradation of the Samples
3. Results and Discussion
3.1. Mechanical Properties Evaluation
3.2. Morphology and Structural Properties
3.3. Thermal Behavior Assessment
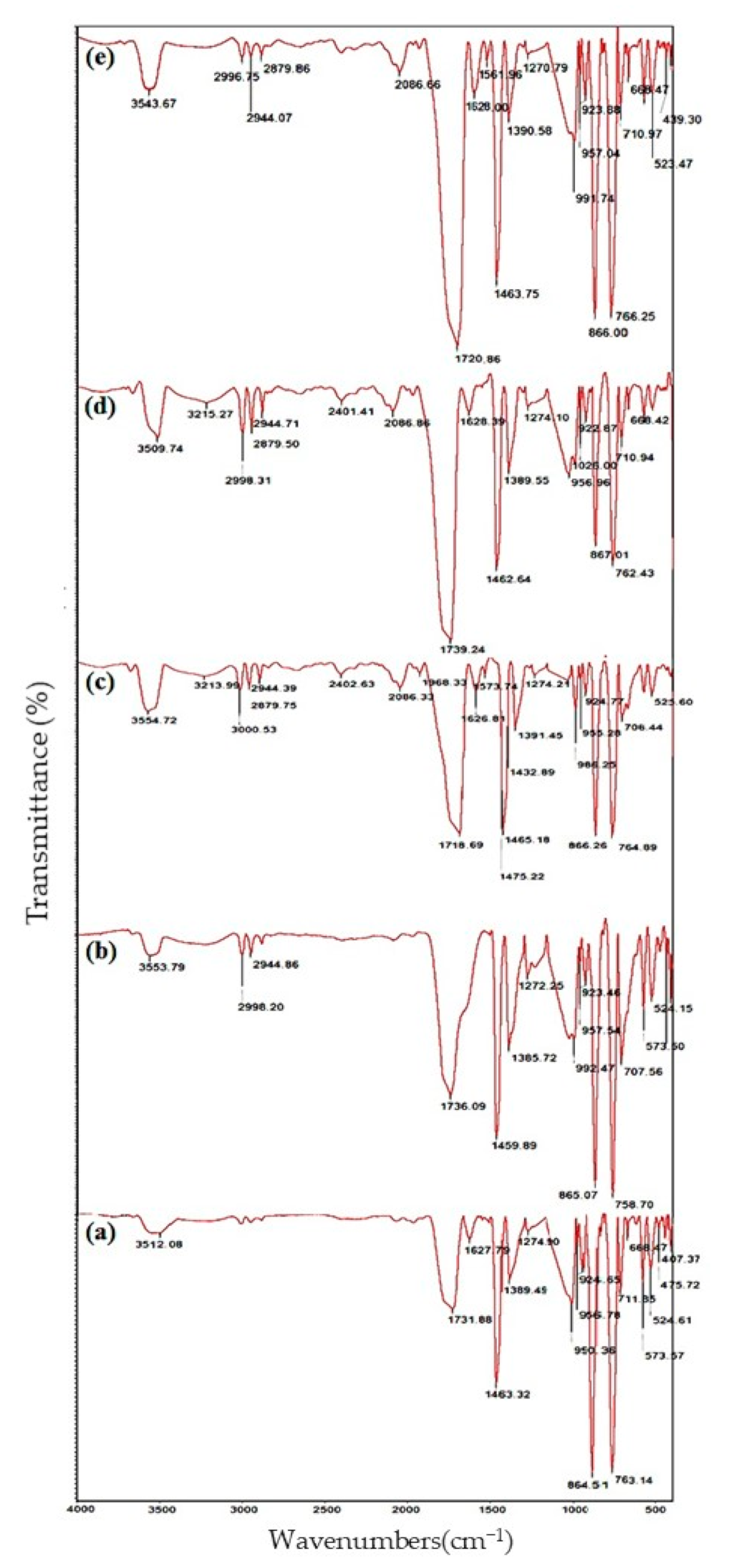
3.4. FTIR
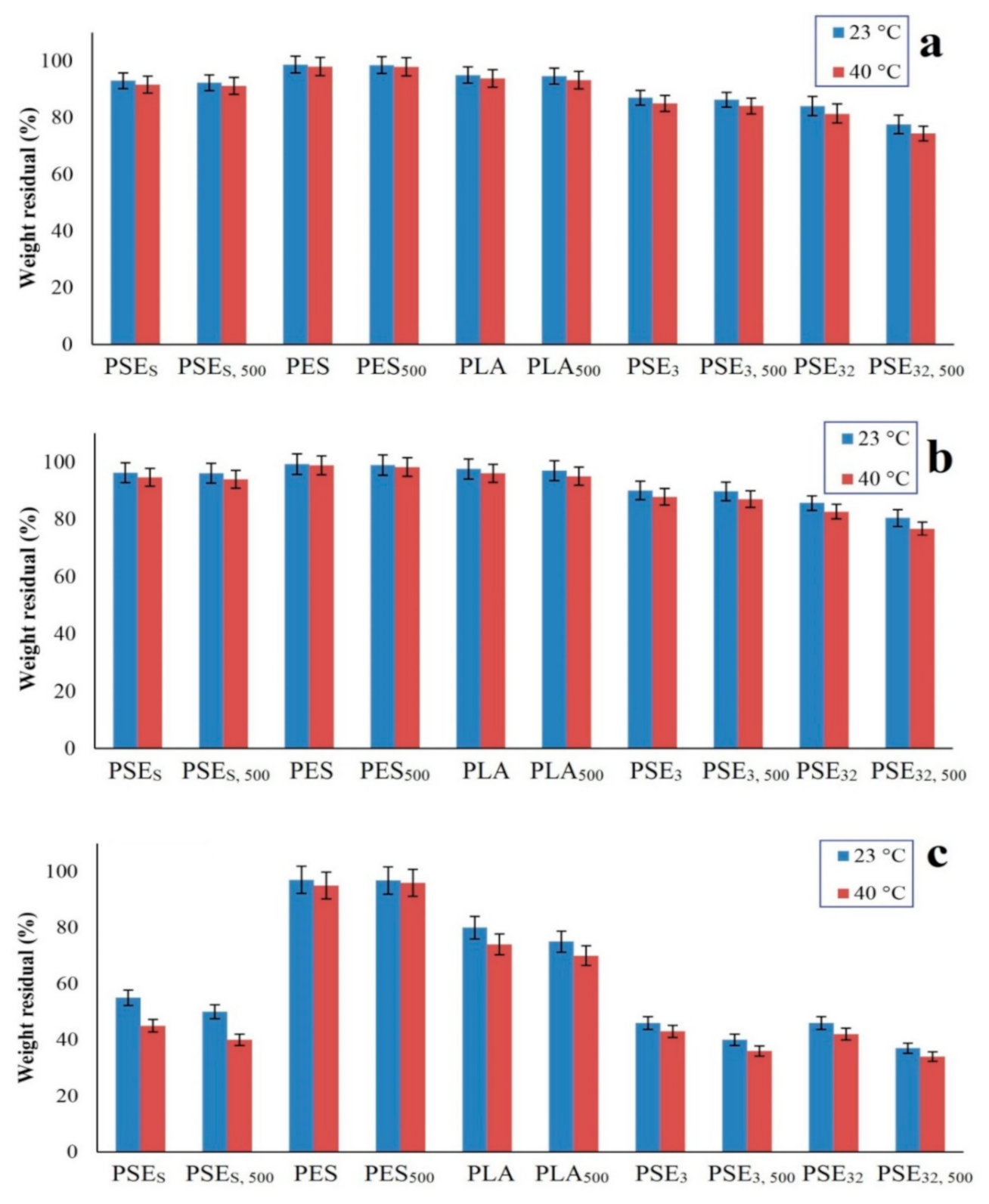
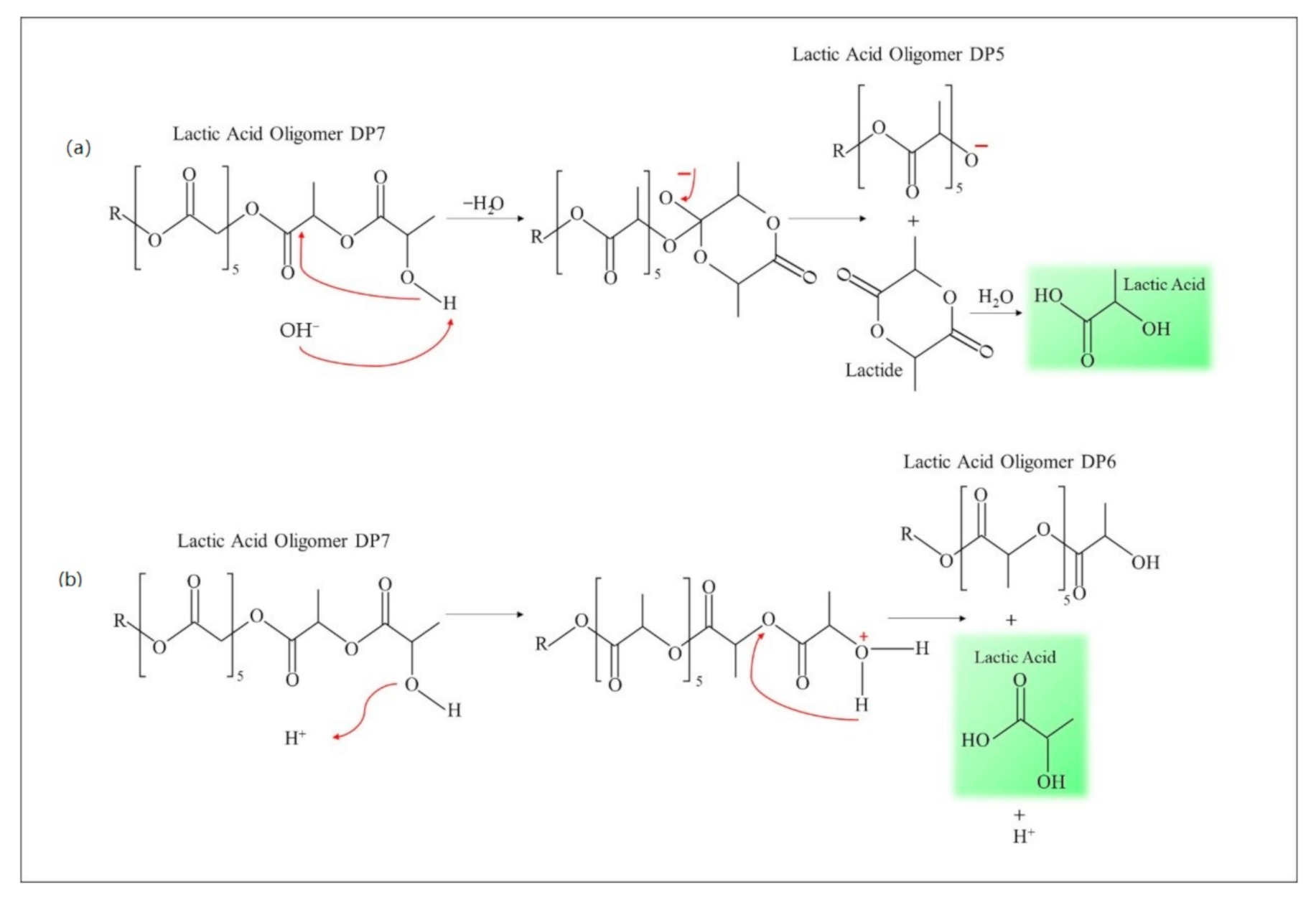
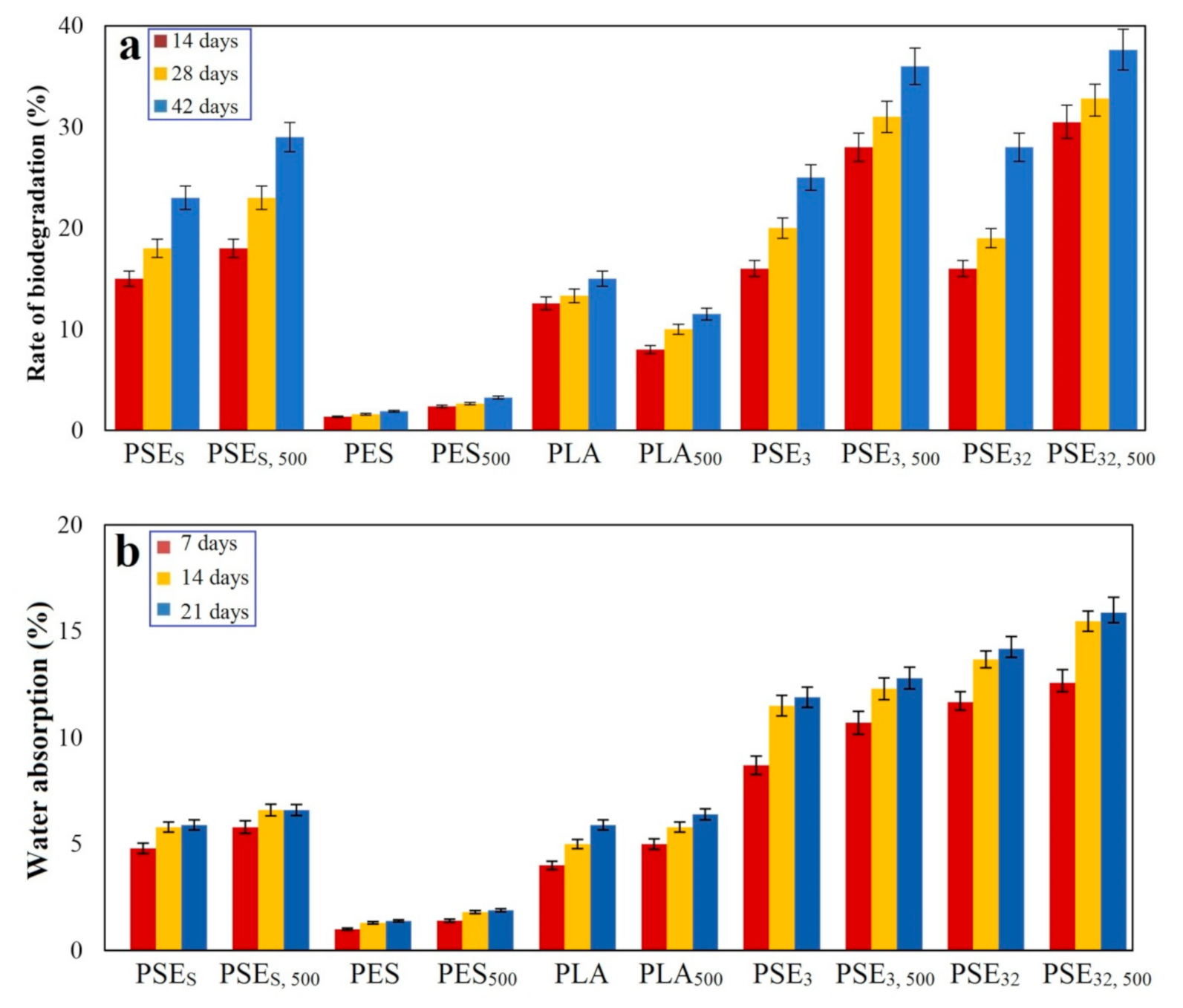
3.5. Hydrolytic Degradation
3.6. Biodegradation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shakiba, M.; Nabavi, S.R.; Emadi, H.; Faraji, M. Development of a superhydrophilic nanofiber membrane for oil/water emulsion separation via modification of polyacrylonitrile/polyaniline composite. Polym. Adv. Technol. 2021, 32, 1301–1316. [Google Scholar] [CrossRef]
- Cinelli, P.; Coltelli, M.B.; Signori, F.; Morganti, P.; Lazzeri, A. Cosmetic packaging to save the environment: Future perspectives. Cosmetics 2019, 6, 26. [Google Scholar] [CrossRef]
- Sarvankar, S.G.; Yewale, S.N. Additive Manufacturing in Automobile Industry. Int. J. Res. Aeronaut. Mech. Eng. 2019, 7, 1–10. [Google Scholar]
- AlSalhi, M.S.; Alam, J.; Dass, L.A.; Raja, M. Recent advances in conjugated polymers for light emitting devices. Int. J. Mol. Sci. 2011, 12, 2036–2054. [Google Scholar] [CrossRef] [PubMed]
- Rezvani Ghomi, E.; Esmaeely Neisiany, R.; Nouri Khorasani, S.; Dinari, M.; Ataei, S.; Koochaki, M.S.; Ramakrishna, S. Development of an epoxy self-healing coating through the incorporation of acrylic acid-co-acrylamide copolymeric gel. Prog. Org. Coat. 2020, 149, 105948. [Google Scholar] [CrossRef]
- Wang, F.; Yang, J.; Cheng, H.; Wu, J.; Liang, X. Study on mechanism of desorption behavior of saturated superabsorbent polymers in concrete. Aci Mater. J. 2015, 112, 463. [Google Scholar] [CrossRef]
- Krzan, A.; Hemjinda, S.; Miertus, S.; Corti, A.; Chiellini, E. Standardization and certification in the area of environmentally degradable plastics. Polym. Degrad. Stab. 2006, 91, 2819–2833. [Google Scholar] [CrossRef]
- Rezvani Ghomi, E.; Khosravi, F.; Tahavori, M.A.; Ramakrishna, S. Circular Economy: A Comparison Between the Case of Singapore and France. Mater. Circ. Econ. 2021, 3, 2. [Google Scholar] [CrossRef]
- Foroughi, F.; Rezvani Ghomi, E.; Morshedi Dehaghi, F.; Borayek, R.; Ramakrishna, S. A Review on the Life Cycle Assessment of Cellulose: From Properties to the Potential of Making It a Low Carbon Material. Materials 2021, 14, 714. [Google Scholar] [CrossRef]
- Göpferich, A. Mechanisms of polymer degradation and erosion. Biomaterials 1996, 17, 103–114. [Google Scholar] [CrossRef]
- Gagliardi, M.; Lenarda, P.; Paggi, M. A reaction-diffusion formulation to simulate EVA polymer degradation in environmental and accelerated ageing conditions. Sol. Energy Mater. Sol. Cells 2017, 164, 93–106. [Google Scholar] [CrossRef]
- Rezvani Ghomi, E.; Khosravi, F.; Mossayebi, Z.; Saedi Ardahaei, A.; Morshedi Dehaghi, F.; Khorasani, M.; Neisiany, R.E.; Das, O.; Marani, A.; Mensah, R.A.; et al. The Flame Retardancy of Polyethylene Composites: From Fundamental Concepts to Nanocomposites. Molecules 2020, 25, 5157. [Google Scholar] [CrossRef]
- Shah, A.A.; Hasan, F.; Hameed, A.; Ahmed, S. Biological degradation of plastics: A comprehensive review. Biotechnol. Adv. 2008, 26, 246–265. [Google Scholar] [CrossRef]
- Hakkarainen, M. Aliphatic polyesters: Abiotic and biotic degradation and degradation products. In Degradable Aliphatic Polyesters; Springer: Berlin, Germany, 2002; pp. 113–138. [Google Scholar]
- Eubeler, J.P.; Zok, S.; Bernhard, M.; Knepper, T.P. Environmental biodegradation of synthetic polymers I. Test methodologies and procedures. Trac Trends Anal. Chem. 2009, 28, 1057–1072. [Google Scholar] [CrossRef]
- Kawai, F. The biochemistry and molecular biology of xenobiotic polymer degradation by microorganisms. Biosci. Biotechnol. Biochem. 2010, 74, 1743–1759. [Google Scholar] [CrossRef]
- Li, K.; Al-Rudainy, B.; Sun, M.; Wallberg, O.; Hulteberg, C.; Tunå, P. Membrane Separation of the Base-Catalyzed Depolymerization of Black Liquor Retentate for Low-Molecular-Mass Compound Production. Membranes 2019, 9, 102. [Google Scholar] [CrossRef]
- Kinane, J.A.; Benakanakere, M.R.; Zhao, J.; Hosur, K.B.; Kinane, D.F. Porphyromonas gingivalis influences actin degradation within epithelial cells during invasion and apoptosis. Cell. Microbiol. 2012, 14, 1085–1096. [Google Scholar] [CrossRef]
- Shakiba, M.; Kakoei, A.; Jafari, I.; Rezvani Ghomi, E.; Kalaee, M.; Zarei, D.; Abdouss, M.; Shafiei-Navid, S.; Khosravi, F.; Ramakrishna, S. Kinetic Modeling and Degradation Study of Liquid Polysulfide Resin-Clay Nanocomposite. Molecules 2021, 26, 635. [Google Scholar] [CrossRef]
- Houchin, M.; Topp, E. Physical properties of PLGA films during polymer degradation. J. Appl. Polym. Sci. 2009, 114, 2848–2854. [Google Scholar] [CrossRef]
- Wei, B.; Qi, H.; Zou, J.; Li, H.; Wang, J.; Xu, B.; Ma, H. Degradation mechanism of amylopectin under ultrasonic irradiation. Food Hydrocoll. 2021, 111, 106371. [Google Scholar] [CrossRef]
- Beltrán-Sanahuja, A.; Casado-Coy, N.; Simó-Cabrera, L.; Sanz-Lázaro, C. Monitoring polymer degradation under different conditions in the marine environment. Environ. Pollut. 2020, 259, 113836. [Google Scholar] [CrossRef] [PubMed]
- Moetazedian, A.; Gleadall, A.; Han, X.; Ekinci, A.; Mele, E.; Silberschmidt, V.V. Mechanical performance of 3D printed polylactide during degradation. Addit. Manuf. 2020, 38, 101764. [Google Scholar]
- Yazdan Mehr, M.; Bahrami, A.; van Driel, W.D.; Fan, X.; Davis, J.L.; Zhang, G. Degradation of optical materials in solid-state lighting systems. Int. Mater. Rev. 2020, 65, 102–128. [Google Scholar] [CrossRef]
- Yuan, J.; Ma, J.; Sun, Y.; Zhou, T.; Zhao, Y.; Yu, F. Microbial degradation and other environmental aspects of microplastics/plastics. Sci. Total Environ. 2020, 715, 136968. [Google Scholar] [CrossRef]
- Lu, T.; Solis-Ramos, E.; Yi, Y.; Kumosa, M. UV degradation model for polymers and polymer matrix composites. Polym. Degrad. Stab. 2018, 154, 203–210. [Google Scholar] [CrossRef]
- Saalah, S.; Saallah, S.; Rajin, M.; Yaser, A.Z. Management of Biodegradable Plastic Waste: A Review. In Advances in Waste Processing Technology; Springer: Berlin, Germany, 2020; pp. 127–143. [Google Scholar]
- Khosravi, F.; Nouri Khorasani, S.; Khalili, S.; Esmaeely Neisiany, R.; Rezvani Ghomi, E.; Ejeian, F.; Das, O.; Nasr-Esfahani, M.H. Development of a Highly Proliferated Bilayer Coating on 316L Stainless Steel Implants. Polymers 2020, 12, 1022. [Google Scholar] [CrossRef] [PubMed]
- Avinc, O.; Khoddami, A. Overview of poly (lactic acid)(PLA) fibre. Fibre Chem. 2009, 41, 391–401. [Google Scholar] [CrossRef]
- Winursito, I. Biodegradabilitas Polikarboksilat Dari Asam Alginat dan Tapioka. J. Litbang Ind. 2013, 3, 39–47. [Google Scholar] [CrossRef]
- Rasal, R.M.; Janorkar, A.V.; Hirt, D.E. Poly (lactic acid) modifications. Prog. Polym. Sci. 2010, 35, 338–356. [Google Scholar] [CrossRef]
- Drieskens, M.; Peeters, R.; Mullens, J.; Franco, D.; Lemstra, P.J.; Hristova-Bogaerds, D.G. Structure versus properties relationship of poly (lactic acid). I. Effect of crystallinity on barrier properties. J. Polym. Sci. Part B: Polym. Phys. 2009, 47, 2247–2258. [Google Scholar] [CrossRef]
- Jeong, J.; Ayyoob, M.; Kim, J.-H.; Nam, S.W.; Kim, Y.J. In situ formation of PLA-grafted alkoxysilanes for toughening a biodegradable PLA stereocomplex thin film. RSC Adv. 2019, 9, 21748–21759. [Google Scholar] [CrossRef]
- Sun, Y.; Lee, D.; Wang, Y.; Li, S.; Ying, J.; Liu, X.; Xu, G.; Gwon, J.; Wu, Q. Thermal decomposition behavior of 3D printing filaments made of wood-filled polylactic acid/starch blend. J. Appl. Polym. Sci. 2021, 138, 49944. [Google Scholar] [CrossRef]
- Ghomi, E.R.; Khosravi, F.; Neisiany, R.E.; Singh, S.; Ramakrishna, S. Future of additive manufacturing in healthcare. Curr. Opin. Biomed. Eng. 2021, 17, 100255. [Google Scholar] [CrossRef]
- Zou, W.; Yu, L.; Liu, X.; Chen, L.; Zhang, X.; Qiao, D.; Zhang, R. Effects of amylose/amylopectin ratio on starch-based superabsorbent polymers. Carbohydr. Polym. 2012, 87, 1583–1588. [Google Scholar] [CrossRef]
- Nafchi, A.M.; Moradpour, M.; Saeidi, M.; Alias, A.K. Thermoplastic starches: Properties, challenges, and prospects. Starch Stärke 2013, 65, 61–72. [Google Scholar] [CrossRef]
- Huneault, M.A.; Li, H. Preparation and properties of extruded thermoplastic starch/polymer blends. J. Appl. Polym. Sci. 2012, 126, E96–E108. [Google Scholar] [CrossRef]
- Rhim, J.-W.; Ng, P.K.W. Natural Biopolymer-Based Nanocomposite Films for Packaging Applications. Crit. Rev. Food Sci. Nutr. 2007, 47, 411–433. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jing, X.; Cheng, H.; Hu, X.; Yang, L.; Huang, Y. Rheology and Crystallization of Long-Chain Branched Poly(l-lactide)s with Controlled Branch Length. Ind. Eng. Chem. Res. 2012, 51, 10731–10741. [Google Scholar] [CrossRef]
- Nozue, Y.; Kawashima, Y.; Seno, S.; Nagamatsu, T.; Hosoda, S.; Berda, E.B.; Rojas, G.; Baughman, T.W.; Wagener, K.B. Unusual Crystallization Behavior of Polyethylene Having Precisely Spaced Branches. Macromology 2011, 44, 4030–4034. [Google Scholar] [CrossRef]
- Esmaeili, M.; Pircheraghi, G.; Bagheri, R.; Altstädt, V. Poly(lactic acid)/coplasticized thermoplastic starch blend: Effect of plasticizer migration on rheological and mechanical properties. Polym. Adv. Technol. 2019, 30, 839–851. [Google Scholar] [CrossRef]
- Yu, Y.; Cheng, Y.; Ren, J.; Cao, E.; Fu, X.; Guo, W. Plasticizing effect of poly(ethylene glycol)s with different molecular weights in poly(lactic acid)/starch blends. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Yue, H.; Zhao, Y.; Ma, X.; Gong, J. Ethylene glycol: Properties, synthesis, and applications. Chem. Soc. Rev. 2012, 41, 4218–4244. [Google Scholar] [CrossRef]
- Jiang, X.; Jiang, T.; Zhang, X.; Dai, H.; Zhang, X. Melt processing of poly(vinyl alcohol) through adding magnesium chloride hexahydrate and ethylene glycol as a complex plasticizer. Polym. Eng. Sci. 2012, 52, 2245–2252. [Google Scholar] [CrossRef]
- Feng, J.; Yang, G.; Zhang, S.; Liu, Q.; Jafari, S.M.; McClements, D.J. Fabrication and characterization of β-cypermethrin-loaded PLA microcapsules prepared by emulsion-solvent evaporation: Loading and release properties. Environ. Sci. Pollut. Res. 2018, 25, 13525–13535. [Google Scholar] [CrossRef]
- Huneault, M.A.; Li, H. Morphology and properties of compatibilized polylactide/thermoplastic starch blends. Polymers 2007, 48, 270–280. [Google Scholar] [CrossRef]
- Walker, A.M.; Tao, Y.; Torkelson, J.M. Polyethylene/starch blends with enhanced oxygen barrier and mechanical properties: Effect of granule morphology damage by solid-state shear pulverization. Polymers 2007, 48, 1066–1074. [Google Scholar] [CrossRef]
- Lambert, S.; Sinclair, C.J.; Bradley, E.L.; Boxall, A.B. Effects of environmental conditions on latex degradation in aquatic systems. Sci. Total. Environ. 2013, 447, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Sheela, T.; Bhajantri, R.; Ravindrachary, V.; Rathod, S.G.; Pujari, P.; Poojary, B.; Somashekar, R. Effect of UV irradiation on optical, mechanical and microstructural properties of PVA/NaAlg blends. Radiat. Phys. Chem. 2014, 103, 45–52. [Google Scholar] [CrossRef]
- Sionkowska, A.; Płanecka, A.; Lewandowska, K.; Michalska, M. The influence of UV-irradiation on thermal and me-chanical properties of chitosan and silk fibroin mixtures. J. Photochem. Photobiol. B Biol. 2014, 140, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Wüst, D.M.; Meyer, D.C.; Favre, P.; Gerber, C. Mechanical and Handling Properties of Braided Polyblend Polyethylene Sutures in Comparison to Braided Polyester and Monofilament Polydioxanone Sutures. Arthrosc. J. Arthrosc. Relat. Surg. 2006, 22, 1146–1153. [Google Scholar] [CrossRef]
- Pukánszky, B.; Tüdős, F. Miscibility and mechanical properties of polymer blends. Makromol. Chemie. Macromol. Symp. 1990, 38, 221–231. [Google Scholar] [CrossRef]
- Sharma, S.; Singh, A.A.; Majumdar, A.; Butola, B.S. Tailoring the mechanical and thermal properties of polylactic ac-id-based bionanocomposite films using halloysite nanotubes and polyethylene glycol by solvent casting process. J. Mater. Sci. 2019, 54, 8971–8983. [Google Scholar] [CrossRef]
- Rogovina, S.Z.; Aleksanyan, K.V.; Loginova, A.A.; Ivanushkina, N.E.; Vladimirov, L.V.; Prut, E.V.; Berlin, A.A. Influence of PEG on Mechanical Properties and Biodegradability of Composites Based on PLA and Starch. Starch Stärke 2018, 70, 1700268. [Google Scholar] [CrossRef]
- Signor, A.W.; VanLandingham, M.R.; Chin, J.W. Effects of ultraviolet radiation exposure on vinyl ester resins: Charac-terization of chemical, physical and mechanical damage. Polym. Degrad. Stab. 2003, 79, 359–368. [Google Scholar] [CrossRef]
- Whiteley, K.S.; Heggs, T.G.; Koch, H.; Mawer, R.L.; Immel, W. Polyolefins. Ullmann’s Encycl. Ind. Chem. 2000. [Google Scholar] [CrossRef]
- Acioli-Moura, R.; Sun, X.S. Thermal degradation and physical aging of poly(lactic acid) and its blends with starch. Polym. Eng. Sci. 2008, 48, 829–836. [Google Scholar] [CrossRef]
- Zhao, C.; Qin, H.; Gong, F.; Feng, M.; Zhang, S.; Yang, M. Mechanical, thermal and flammability properties of polyeth-ylene/clay nanocomposites. Polym. Degrad. Stab. 2005, 87, 183–189. [Google Scholar] [CrossRef]
- Chen, J.; Spear, S.K.; Huddleston, J.G.; Rogers, R.D. Polyethylene glycol and solutions of polyethylene glycol as green reaction media. Green Chem. 2005, 7, 64–82. [Google Scholar]
- Walse, C.; Berg, B.; Sverdrup, H. Review and synthesis of experimental data on organic matter decomposition with respect to the effect of temperature, moisture, and acidity. Environ. Rev. 1998, 6, 25–40. [Google Scholar] [CrossRef]
- Buzarovska, A.; Grozdanov, A. Biodegradable poly(L-lactic acid)/TiO2 nanocomposites: Thermal properties and degra-dation. J. Appl. Polym. Sci. 2012, 123, 2187–2193. [Google Scholar] [CrossRef]
- Jang, W.Y.; Shin, B.Y.; Lee, T.J.; Narayan, R. Thermal properties and morphology of biodegradable PLA/starch com-patibilized blends. J. Ind. Eng. Chem. 2007, 13, 457–464. [Google Scholar]
- Liu, J.; Jiang, H.; Chen, L. Grafting of Glycidyl Methacrylate onto Poly(lactide) and Properties of PLA/Starch Blends Compatibilized by the Grafted Copolymer. J. Polym. Environ. 2012, 20, 810–816. [Google Scholar] [CrossRef]
- Xiong, Z.; Li, C.; Ma, S.; Feng, J.; Yang, Y.; Zhang, R.; Zhu, J. The properties of poly(lactic acid)/starch blends with a functionalized plant oil: Tung oil anhydride. Carbohydr. Polym. 2013, 95, 77–84. [Google Scholar] [CrossRef]
- Pegoretti, A.; Fambri, L.; Migliaresi, C. In vitro degradation of poly (L-lactic acid) fibers produced by melt spinning. J. Appl. Polym. Sci. 1997, 64, 213–223. [Google Scholar] [CrossRef]
- Zhou, Q.; Xanthos, M. Nanoclay and crystallinity effects on the hydrolytic degradation of polylactides. Polym. Degrad. Stab. 2008, 93, 1450–1459. [Google Scholar] [CrossRef]
- Naderi, M.; Aghabararpour, M.; Najafi, M.; Motahari, S. An investigation into resorcinol formaldehyde carbon aero-gel/epoxy coatings: Exploring mechanical properties, ultraviolet stability, and corrosion resistance. Polym. Compos. 2020, 41, 121–133. [Google Scholar] [CrossRef]
- Wang, X.-L.; Yang, K.-K.; Wang, Y.-Z. Properties of Starch Blends with Biodegradable Polymers. J. Macromol. Sci. Part C 2003, 43, 385–409. [Google Scholar] [CrossRef]
- Therias, S.; Rapp, G.; Masson, C.; Gardette, J.-L. Limits of UV-light acceleration on the photooxidation of low-density polyethylene. Polym. Degrad. Stab. 2021, 183, 109443. [Google Scholar] [CrossRef]
- Gharehdashli, A.; Mortazavi, S.; Rashidi, H. Photodegradation of low-density polyethylene with prooxidant and pho-tocatalyst. J. Appl. Polym. Sci. 2020, 137, 48979. [Google Scholar] [CrossRef]
- Rosu, D.; Rosu, L.; Cascaval, C.N. IR-change and yellowing of polyurethane as a result of UV irradiation. Polym. Degrad. Stab. 2009, 94, 591–596. [Google Scholar] [CrossRef]
- Nie, B.; Stutzman, J.; Xie, A. A Vibrational Spectral Maker for Probing the Hydrogen-Bonding Status of Protonated Asp and Glu Residues. Biophys. J. 2005, 88, 2833–2847. [Google Scholar] [CrossRef]
- ElSawy, M.A.; Kim, K.-H.; Park, J.-W.; Deep, A. Hydrolytic degradation of polylactic acid (PLA) and its composites. Renew. Sustain. Energy Rev. 2017, 79, 1346–1352. [Google Scholar] [CrossRef]
- Bos, H.L.; Meesters, K.P.H.; Conijn, S.G.; Corré, W.J.; Patel, M.K. Accounting for the constrained availability of land: A comparison of bio-based ethanol, polyethylene, and PLA with regard to non-renewable energy use and land use. Biofuels, Bioprod. Biorefin. 2012, 6, 146–158. [Google Scholar] [CrossRef]
- Xu, L.; Crawford, K.; Gorman, C.B. Effects of Temperature and pH on the Degradation of Poly(lactic acid) Brushes. Macromol. 2011, 44, 4777–4782. [Google Scholar] [CrossRef]
- Albertsson, A.-C.; Varma, I.K. Aliphatic polyesters: Synthesis, properties and applications. In Degradable Aliphatic Polyesters; Springer: Berlin, Germany, 2002; pp. 1–40. [Google Scholar]
- Liu, H.; Zhang, J. Research progress in toughening modification of poly(lactic acid). J. Polym. Sci. Part B Polym. Phys. 2011, 49, 1051–1083. [Google Scholar] [CrossRef]
- Muroga, S.; Hikima, Y.; Ohshima, M. Visualization of hydrolysis in polylactide using near-infrared hyperspectral imaging and chemometrics. J. Appl. Polym. Sci. 2018, 135, 45898. [Google Scholar] [CrossRef]
- Avérous, L. Biodegradable Multiphase Systems Based on Plasticized Starch: A Review. J. Macromol. Sci. Part C 2004, 44, 231–274. [Google Scholar] [CrossRef]
- Li, G.; Sarazin, P.; Orts, W.J.; Imam, S.H.; Favis, B.D. Biodegradation of Thermoplastic Starch and its Blends with Poly(lactic acid) and Polyethylene: Influence of Morphology. Macromol. Chem. Phys. 2011, 212, 1147–1154. [Google Scholar] [CrossRef]
- Torres, A.V.; Zamudio-Flores, P.B.; Salgado-Delgado, R.; Bello-Pérez, L.A. Biodegradation of low-density polyethylene-banana starch films. J. Appl. Polym. Sci. 2008, 110, 3464–3472. [Google Scholar] [CrossRef]
- Panahi, L.; Gholizadeh, M.; Hajimohammadi, R. Investigating the degradability of polyethylene using starch, oxo-material, and polylactic acid under the different environmental conditions. Asia-Pacific J. Chem. Eng. 2019, 15, 2402. [Google Scholar] [CrossRef]
- Da Silva, A.P.; Pereira, M.D.P.; Passador, F.R.; Montagna, L.S. PLA/Coffee Grounds Composites: A Study of Photodegradation and Biodegradation in Soil. Macromol. Symp. 2020, 394, 2000091. [Google Scholar] [CrossRef]
- Chamas, A.; Moon, H.; Zheng, J.; Qiu, Y.; Tabassum, T.; Jang, J.H.; Abu-Omar, M.M.; Scott, S.L.; Suh, S. Degradation Rates of Plastics in the Environment. ACS Sustain. Chem. Eng. 2020, 8, 3494–3511. [Google Scholar] [CrossRef]



| Sample | PLA (wt.%) | PE (wt.%) | Starch (wt.%) | PEG (wt.%) | PEG Solvent | UV Exposure (h) |
|---|---|---|---|---|---|---|
| PES | – | 70 | 30 | – | – | – |
| PES500 | – | 70 | 30 | – | – | 500 |
| PLA | 100 | – | – | – | – | – |
| PLA500 | 100 | – | – | – | – | 500 |
| PSES | 70 | – | 30 | – | – | – |
| PSES, 500 | 70 | – | 30 | – | – | 500 |
| PSE1 | 70 | – | 27 | 3 | acetone | – |
| PSE2 | 70 | – | 24 | 6 | acetone | – |
| PSE3 | 70 | – | 21 | 9 | acetone | – |
| PSE3, 500 | 70 | – | 21 | 9 | acetone | 500 |
| PSE4 | 70 | – | 18 | 12 | acetone | – |
| PSE5 | 70 | – | 15 | 15 | acetone | – |
| PSE22 | 70 | – | 24 | 6 | ethanol | – |
| PSE32 | 70 | – | 21 | 9 | ethanol | – |
| PSE32,500 | 70 | – | 21 | 9 | ethanol | 500 |
| PSE42 | 70 | – | 18 | 12 | ethanol | – |
| PES52 | 70 | – | 15 | 15 | ethanol | – |
| Sample | Elongation at Break (%) | Tensile Strength (MPa) |
|---|---|---|
| PLA | 9.44 ± 5.23 | 47.04 ± 11.32 |
| PES | 8.14 ± 1.02 | 25.13 ± 6.08 |
| PSES | 5.44 ± 0.70 | 39.91 ± 7.89 |
| PSE2 | 8.66 ± 0.65 | 26.32 ± 6.32 |
| PSE3 | 12.00 ± 0.25 | 23.99 ± 6.92 |
| PSE4 | 8.22 ± 0.23 | 24.05 ± 4.87 |
| PSE5 | 9.09 ± 0.81 | 23.01 ± 5.65 |
| PSE22 | 9.50 ± 1.22 | 13.04 ± 1.12 |
| PSE32 | 14.94 ± 1.85 | 15.50 ± 1.15 |
| PSE42 | 21.11 ± 4.44 | 9.02 ± 0.58 |
| PSE52 | 23.94 ± 6.02 | 8.23 ± 0.51 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Momeni, S.; Rezvani Ghomi, E.; Shakiba, M.; Shafiei-Navid, S.; Abdouss, M.; Bigham, A.; Khosravi, F.; Ahmadi, Z.; Faraji, M.; Abdouss, H.; et al. The Effect of Poly (Ethylene glycol) Emulation on the Degradation of PLA/Starch Composites. Polymers 2021, 13, 1019. https://doi.org/10.3390/polym13071019
Momeni S, Rezvani Ghomi E, Shakiba M, Shafiei-Navid S, Abdouss M, Bigham A, Khosravi F, Ahmadi Z, Faraji M, Abdouss H, et al. The Effect of Poly (Ethylene glycol) Emulation on the Degradation of PLA/Starch Composites. Polymers. 2021; 13(7):1019. https://doi.org/10.3390/polym13071019
Chicago/Turabian StyleMomeni, Sarieh, Erfan Rezvani Ghomi, Mohamadreza Shakiba, Saied Shafiei-Navid, Majid Abdouss, Ashkan Bigham, Fatemeh Khosravi, Zahed Ahmadi, Mehdi Faraji, Hamidreza Abdouss, and et al. 2021. "The Effect of Poly (Ethylene glycol) Emulation on the Degradation of PLA/Starch Composites" Polymers 13, no. 7: 1019. https://doi.org/10.3390/polym13071019
APA StyleMomeni, S., Rezvani Ghomi, E., Shakiba, M., Shafiei-Navid, S., Abdouss, M., Bigham, A., Khosravi, F., Ahmadi, Z., Faraji, M., Abdouss, H., & Ramakrishna, S. (2021). The Effect of Poly (Ethylene glycol) Emulation on the Degradation of PLA/Starch Composites. Polymers, 13(7), 1019. https://doi.org/10.3390/polym13071019









