Poly(Ethylene Glycol)-Poly(l-Alanine)/Hyaluronic Acid Complex as a 3D Platform for Understanding Cancer Cell Migration in the Tumor Microenvironment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the mPEG-PA, HA and mPEG-PA/HA Complex Aqueous Solutions
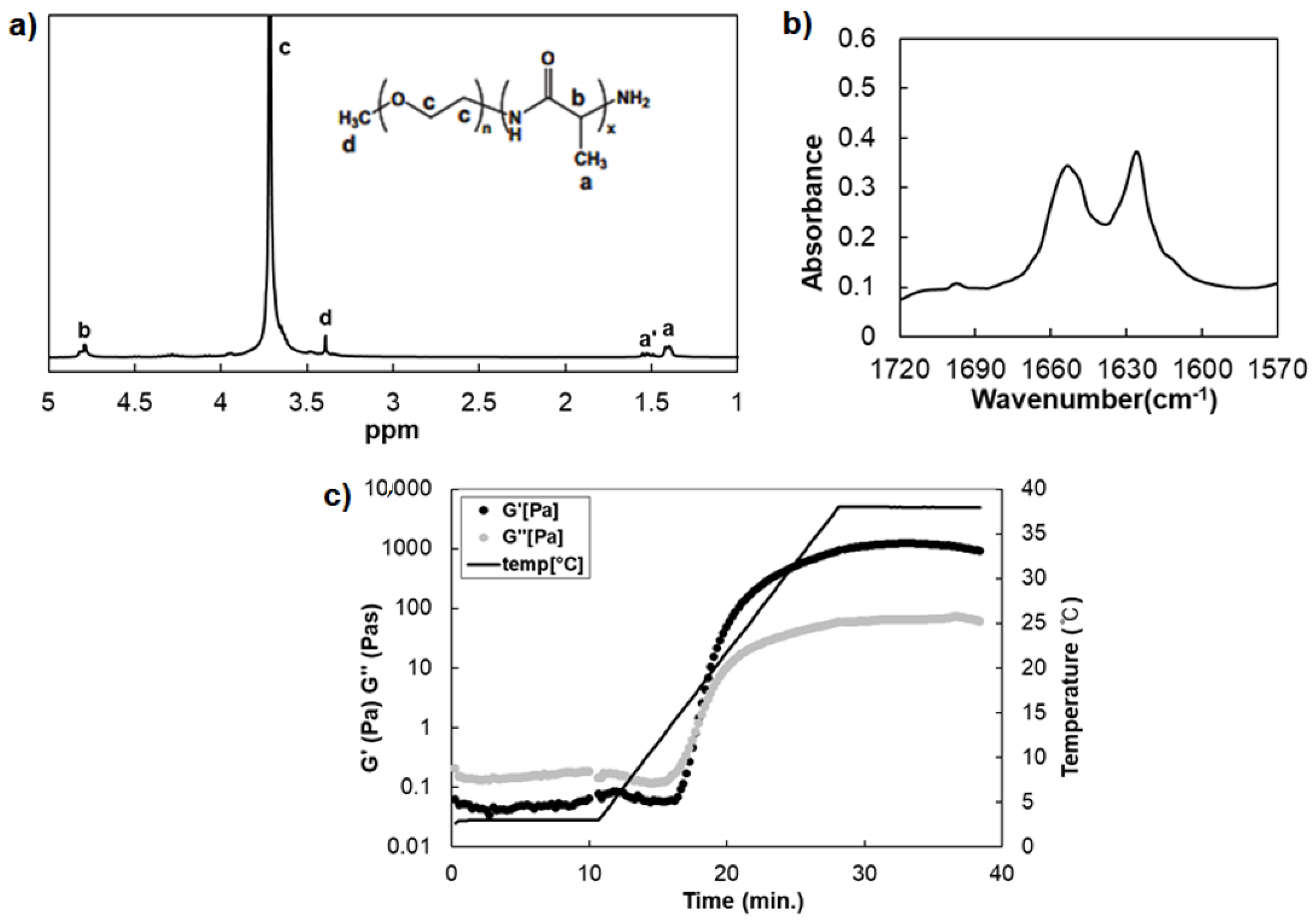
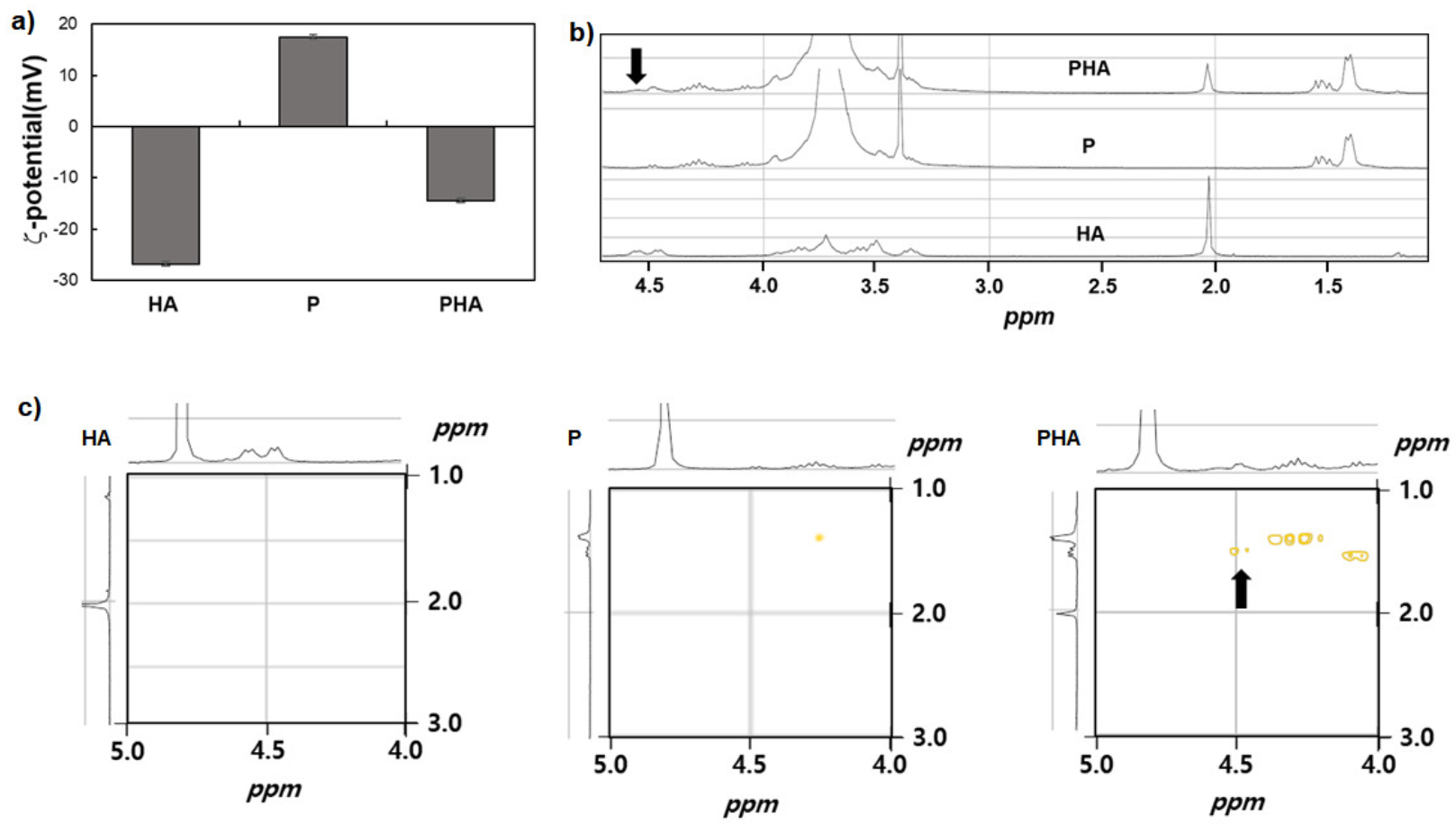
2.3. H-NMR and 2-Dimesional NMR Spectroscopy
2.4. Fourier Transform Infrared Spectroscopy (FTIR)
2.5. Dynamic Mechanical Analysis
2.6. Dynamic Light Scattering(DLS) for Zeta-Potential
2.7. Circular Dichroism (CD) Spectroscopy
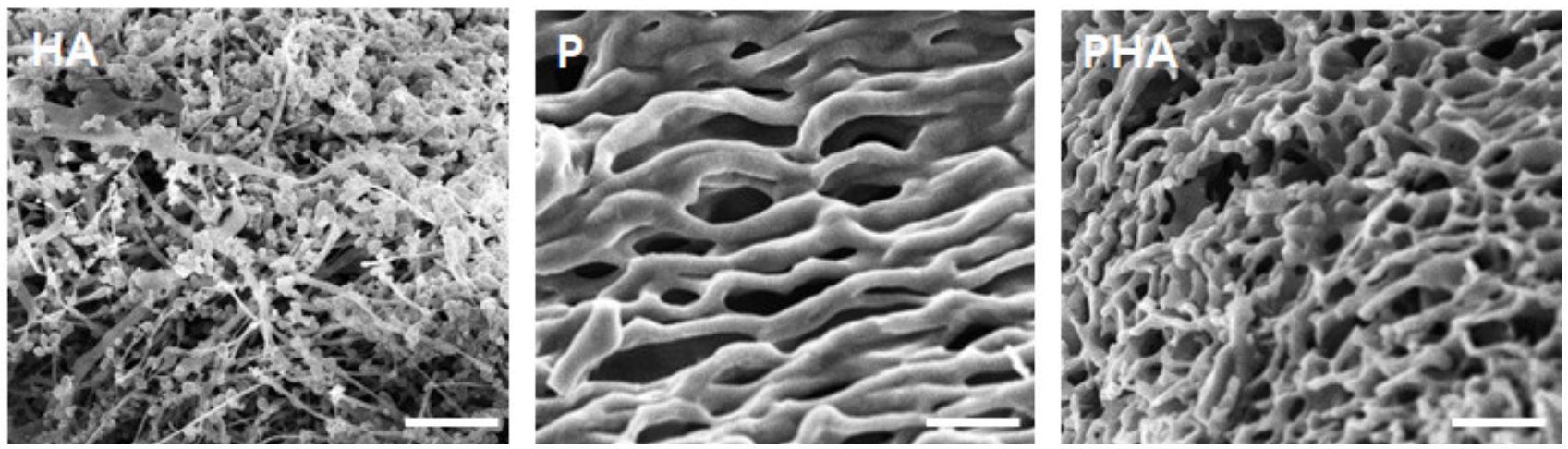
2.8. Scanning Electron Microscopy (SEM)
2.9. Cell Culture
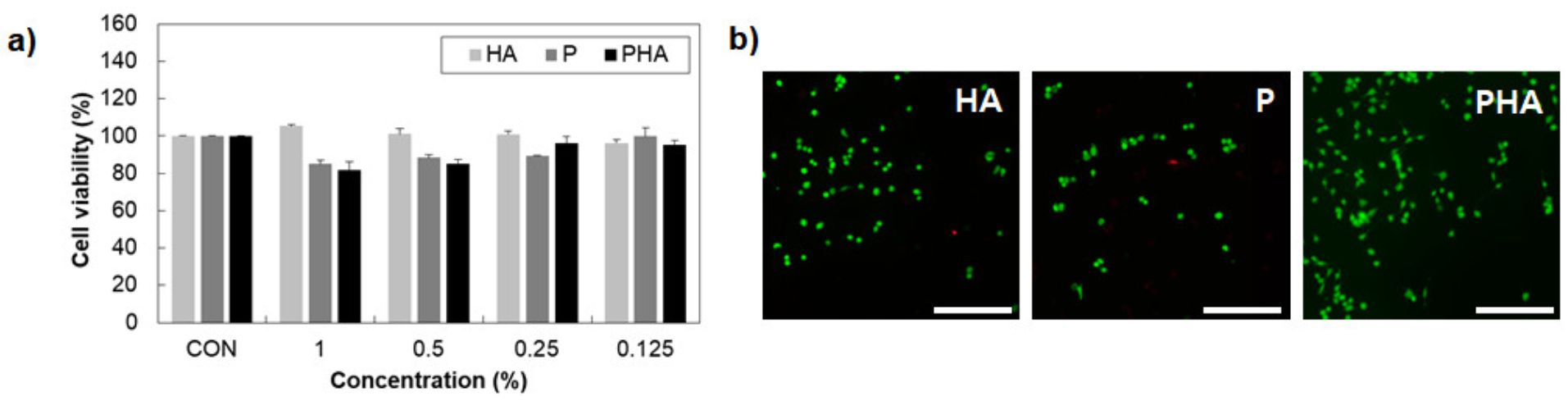
2.10. MTT Assay
2.11. Live/Dead Assay
2.12. D Cell Culture
3. Results and Discussion
3.1. Preparation of Thermosensitive Poly(l-Alanine) Hydrogel
3.2. Characterization of PHA Complex
3.3. Fabrication of Tumor Microenvironment Mimicking the 3D Matrix
3.4. Cell Viability
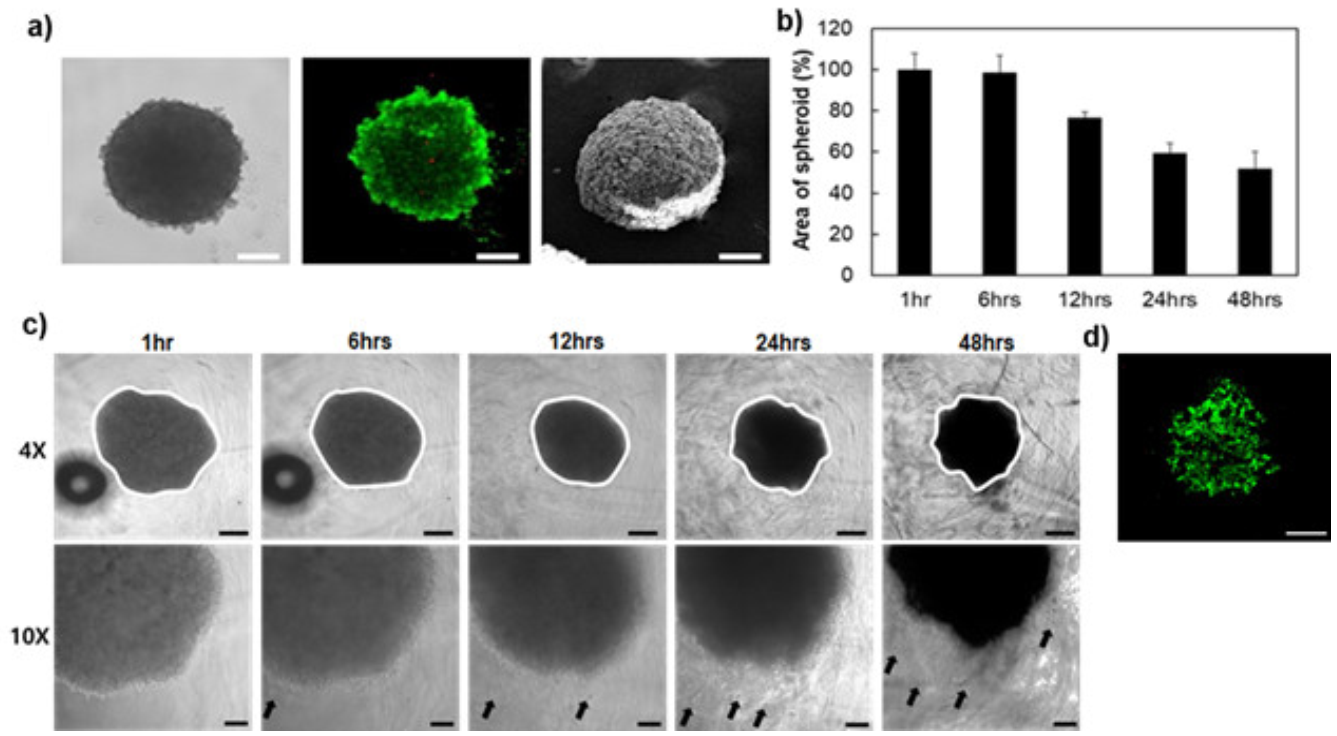
3.5. Cell Behavior in 3D Matrix
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y. Molecular principles of metastasis: A hallmark of cancer revisited. Signal Transduct. Target. Ther. 2020, 5, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Jahanban-Esfahlan, R.; Seidi, K.; Banimohamad-Shotorbani, B.; Jahanban-Esfahlan, A.; Yousefi, B. Combination of nanotechnology with vascular targeting agents for effective cancer therapy. J. Cell. Physiol. 2018, 233, 2982–2992. [Google Scholar] [CrossRef]
- Swartz, M.A.; Iida, N.; Roberts, E.W.; Sangaletti, S.; Wong, M.H.; Yull, F.E.; Coussens, L.M.; DeClerck, Y.A. Tumor Microenvironment Complexity: Emerging Roles in Cancer Therapy. Cancer Res. 2012, 72, 2473–2480. [Google Scholar] [CrossRef] [Green Version]
- Nunes, A.S.; Barros, A.S.; Costa, E.C.; Moreira, A.F.; Correia, I.J. 3D tumor spheroids as in vitro models to mimic in vivo human solid tumors resistance to therapeutic drugs. Biotechnol. Bioeng. 2019, 116, 206–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barsky, S.H.; Rao, C.; Grotendorst, G.R.; Liotta, L.A. Increased content of Type V Collagen in desmoplasia of human breast carcinoma. Am. J. Pathol. 1982, 108, 276. [Google Scholar]
- Provenzano, P.P.; Inman, D.R.; Eliceiri, K.W.; Knittel, J.G.; Yan, L.; Rueden, C.T.; White, J.G.; Keely, P.J. Collagen density promotes mammary tumor initiation and progression. BMC Med. 2008, 6, 11. [Google Scholar] [CrossRef] [Green Version]
- Levental, K.R.; Yu, H.; Kass, L.; Lakins, J.N.; Egeblad, M.; Erler, J.T.; Fong, S.F.; Csiszar, K.; Giaccia, A.; Weninger, W. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 2009, 139, 891–906. [Google Scholar] [CrossRef] [Green Version]
- Riching, K.M.; Cox, B.L.; Salick, M.R.; Pehlke, C.; Riching, A.S.; Ponik, S.M.; Bass, B.R.; Crone, W.C.; Jiang, Y.; Weaver, A.M. 3D collagen alignment limits protrusions to enhance breast cancer cell persistence. Biophys. J. 2014, 107, 2546–2558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, M.H.; Choi, B.G.; Jeong, B. Complexation-Induced Biomimetic Long Range Fibrous Orientation in a Rigid-Flexible Block Copolymer Thermogel. Adv. Funct. Mater. 2012, 22, 5118–5125. [Google Scholar] [CrossRef]
- Bhattacharya, D.S.; Svechkarev, D.; Souchek, J.J.; Hill, T.K.; Taylor, M.A.; Natarajan, A.; Mohs, A.M. Impact of structurally modifying hyaluronic acid on CD44 interaction. J. Mater. Chem. B 2017, 5, 8183–8192. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, S.; Karnad, A.; Freeman, J.W. The biology and role of CD44 in cancer progression: Therapeutic implications. J. Hematol. Oncol. 2018, 11, 64. [Google Scholar] [CrossRef] [Green Version]
- Burkoth, T.S.; Benzinger, T.L.; Jones, D.N.; Hallenga, K.; Meredith, S.C.; Lynn, D.G. C-terminal PEG blocks the irreversible step in β-amyloid (10-35) fibrillogenesis. J. Am. Chem. Soc. 1998, 120, 7655–7656. [Google Scholar] [CrossRef]
- Klok, H.A. Protein-inspired materials: Synthetic concepts and potential applications. Angew. Chem. Int. Ed. 2002, 41, 1509–1513. [Google Scholar] [CrossRef]
- Choi, Y.Y.; Joo, M.K.; Sohn, Y.S.; Jeong, B. Significance of secondary structure in nanostructure formation and thermosensitivity of polypeptide block copolymers. Soft Matter 2008, 4, 2383–2387. [Google Scholar] [CrossRef]
- Hamley, I.W.; Krysmann, M.J.; Castelletto, V.; Noirez, L. Multiple lyotropic polymorphism of a poly (ethylene glycol)-peptide conjugate in aqueous solution. Adv. Mater. 2008, 20, 4394–4397. [Google Scholar] [CrossRef]
- Choi, Y.Y.; Jang, J.H.; Park, M.H.; Choi, B.G.; Chi, B.; Jeong, B. Block length affects secondary structure, nanoassembly and thermosensitivity of poly (ethylene glycol)-poly (L-alanine) block copolymers. J. Mater. Chem. 2010, 20, 3416–3421. [Google Scholar] [CrossRef]
- Moon, H.J.; Park, M.H.; Joo, M.K.; Jeong, B. Temperature-responsive compounds as in situ gelling biomedical materials. Chem. Soc. Rev. 2012, 41, 4860–4883. [Google Scholar] [CrossRef]
- Yu, L.; Ding, J. Injectable hydrogels as unique biomedical materials. Chem. Soc. Rev. 2008, 37, 1473–1481. [Google Scholar] [CrossRef]
- Shinde, U.P.; Yeon, B.; Jeong, B. Recent progress of in situ formed gels for biomedical applications. Prog. Polym. Sci. 2013, 38, 672–701. [Google Scholar]
- Kamatar, A.; Gunay, G.; Acar, H. Natural and Synthetic Biomaterials for Engineering Multicellular Tumor Spheroids. Polymers 2020, 12, 2506. [Google Scholar] [CrossRef]
- Park, M.H.; Moon, H.J.; Park, J.H.; Shinde, U.P.; Ko, D.Y.; Jeong, B. PEG-Poly(l-alanine) Thermogel As a 3D Scaffold of Bone-Marrow-Derived Mesenchymal Stem Cells. Macromol. Biosci. 2015, 15, 464–472. [Google Scholar] [CrossRef]
- Kim, E.H.; Joo, M.K.; Bahk, K.H.; Park, M.H.; Chi, B.; Lee, Y.M.; Jeong, B. Reverse thermal gelation of PAF-PLX-PAF block copolymer aqueous solution. Biomacromolecules 2009, 10, 2476–2481. [Google Scholar] [CrossRef]
- Han, C.D.; Jhon, M.S. Correlations of the first normal stress difference with shear stress and of the storage modulus with loss modulus for homopolymers. J. Appl. Polym. Sci. 1986, 32, 3809–3840. [Google Scholar] [CrossRef]
- Saxena, V.; Wetlaufer, D. A new basis for interpreting the circular dichroic spectra of proteins. Proc. Natl. Acad. Sci. USA 1971, 68, 969–972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tifany, M.L.; Krimm, S. Effwct of temperature on the circular dichroism spectra of polypeptides in the extended state. Biopolym. Orig. Res. Biomol. 1972, 11, 2309–2316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staskus, P.W.; Johnson, W.C., Jr. Conformational transition of hyaluronic acid in aqueous-organic solvent monitored by vacuum ultraviolet circular dichroism. Biochemistry 1988, 27, 1522–1527. [Google Scholar] [CrossRef]
- Chen, H.; Qin, J.; Hu, Y. Efficient degradation of high-molecular-weight hyaluronic acid by a combination of ultrasound, hydrogen peroxide, and copper ion. Molecules 2019, 24, 617. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Zhao, X.; Zhao, L.; Li, Q.; D’Ortenzio, M.; Nguyen, B.; Xu, X.; Wen, Y. Development of a hybrid gelatin hydrogel platform for tissue engineering and protein delivery applications. J. Mater. Chem. B 2015, 3, 6368–6376. [Google Scholar] [CrossRef]
- Poltavets, V.; Kochetkova, M.; Pitson, S.M.; Samuel, M.S. The Role of the Extracellular Matrix and Its Molecular and Cellular Regulators in Cancer Cell Plasticity. Front. Oncol. 2018, 8, 431. [Google Scholar] [CrossRef] [Green Version]
- Sutherland, R.M. Cell and environment interactions in tumor microregions: The multicell spheroid model. Science 1988, 240, 177–184. [Google Scholar] [CrossRef]
- Vaira, V.; Fedele, G.; Pyne, S.; Fasoli, E.; Zadra, G.; Bailey, D.; Snyder, E.; Faversani, A.; Coggi, G.; Flavin, R. Preclinical model of organotypic culture for pharmacodynamic profiling of human tumors. Proc. Natl. Acad. Sci. USA 2010, 107, 8352–8356. [Google Scholar] [CrossRef] [Green Version]
- Kyle, A.H.; Huxham, L.A.; Chiam, A.S.J.; Sim, D.H.; Minchinton, A.I. Direct Assessment of Drug Penetration into Tissue Using a Novel Application of Three-Dimensional Cell Culture. Cancer Res. 2004, 64, 6304–6309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischbach, C.; Chen, R.; Matsumoto, T.; Schmelzle, T.; Brugge, J.S.; Polverini, P.J.; Mooney, D.J. Engineering tumors with 3D scaffolds. Nat. Methods 2007, 4, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Ho, W.J.; Wu, B.M. The Role of the 3D Environment in Hypoxia-induced Drug and Apoptosis Resistance. Anticancer Res. 2011, 31, 3237–3245. [Google Scholar]
- Riffle, S.; Hegde, R.S. Modeling tumor cell adaptations to hypoxia in multicellular tumor spheroids. J. Exp. Clin. Cancer Res. 2017, 36, 102. [Google Scholar] [CrossRef]
- Brüningk, S.C.; Rivens, I.; Box, C.; Oelfke, U.; Ter Haar, G. 3D tumour spheroids for the prediction of the effects of radiation and hyperthermia treatments. Sci. Rep. 2020, 10, 1653. [Google Scholar] [CrossRef] [PubMed]
- Karnoub, A.E.; Dash, A.B.; Vo, A.P.; Sullivan, A.; Brooks, M.W.; Bell, G.W.; Richardson, A.L.; Polyak, K.; Tubo, R.; Weinberg, R.A. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 2007, 449, 557–563. [Google Scholar] [CrossRef]
- Martin, F.; Dwyer, R.M.; Kelly, J.; Khan, S.; Murphy, J.; Curran, C.; Miller, N.; Hennessy, E.; Dockery, P.; Barry, F. Potential role of mesenchymal stem cells (MSCs) in the breast tumour microenvironment: Stimulation of epithelial to mesenchymal transition (EMT). Breast Cancer Res. Treat. 2010, 124, 317–326. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sim, J.; Lee, H.J.; Jeong, B.; Park, M.H. Poly(Ethylene Glycol)-Poly(l-Alanine)/Hyaluronic Acid Complex as a 3D Platform for Understanding Cancer Cell Migration in the Tumor Microenvironment. Polymers 2021, 13, 1042. https://doi.org/10.3390/polym13071042
Sim J, Lee HJ, Jeong B, Park MH. Poly(Ethylene Glycol)-Poly(l-Alanine)/Hyaluronic Acid Complex as a 3D Platform for Understanding Cancer Cell Migration in the Tumor Microenvironment. Polymers. 2021; 13(7):1042. https://doi.org/10.3390/polym13071042
Chicago/Turabian StyleSim, Jooyoung, Hyun Jung Lee, Byeongmoon Jeong, and Min Hee Park. 2021. "Poly(Ethylene Glycol)-Poly(l-Alanine)/Hyaluronic Acid Complex as a 3D Platform for Understanding Cancer Cell Migration in the Tumor Microenvironment" Polymers 13, no. 7: 1042. https://doi.org/10.3390/polym13071042





