A Review of Polysiloxanes in Terms of Their Application in Explosives
Abstract
:1. Introduction
2. Physicochemical Properties of Polysiloxanes Depending on the Functionalization of the Silicon–Oxygen Backbone
3. Implementation of Polysiloxanes in PBX Explosives—Current State and Perspectives
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maranda, A. Przemysłowe Materiały Wybuchowe (Industrial Explosives); Wojskowa Akademia Techniczna: Warsaw, Poland, 2010. [Google Scholar]
- Kaye, S.M. Encyclopedia of Explosives and Related Items; U.S. Army Research and Development Command: Wharton, NJ, USA, 1978; Volume 8. [Google Scholar]
- Cudziło, S.; Maranda, A.; Nowaczewski, J.; Trębiński, R.; Trzciński, W.A. Wojskowe Materiały Wybuchowe (Military Explosives); Wydawnictwo Wydziału Metalurgii i Inżynierii Materiałowej: Czestochowa, Poland, 2000. [Google Scholar]
- Dobratz, B.M. The Insensitive High Explosive Triaminotrinitrobenzene (TATB): Development and Characterization—1888 to 1994; Los Alamos National Laboratory: Los Alamos, NM, USA, 1995.
- Chyłek, Z.; Szala, M. Przegląd potencjalnych wysokoenergetycznych komponentów do wytwarzania nowoczesnych plastycznych materiałów wybuchowych (Review of potential high-energy components for the production of modern plastic explosives). High-Energetic Mater. 2015, 7, 144–155. [Google Scholar]
- Smith, M.W.; Cliff, M.D. NTO-Based Explosive Formulations: A Technology Review; DSTO Aeronautical and Maritime Research Laboratory: Salisbury, UK, 1999. [Google Scholar]
- Cudziło, S.; Trzciński, W.A. Topliwe kruszące materiały wybuchowe (Castable High Explosives). Biul. WAT 2014, 63, 43–55. [Google Scholar] [CrossRef]
- Akhavan, J. The Chemistry of Explosives; Royal Society of Chemistry: Cambridge, UK, 2011. [Google Scholar]
- Zeman, S.; Jungova, M. Technology of Explosives, A Textbook; University of Pardubice: Pardubice, Czech Republic, 2019. [Google Scholar]
- Chyłek, Z.; Jurkiewicz, R. Investigation of the Properties of Polymer Bonded Explosives Based on 1,1-Diamino-2,2-dinitroethene (FOX-7) and 1,3,5,7-Tetranitro-1,3,5,7-tetraazacyclooctane (HMX). Cent. Eur. J. Energ. Mater. 2016, 13, 859–870. [Google Scholar] [CrossRef]
- Dvornic, P.R. Thermal Properties of Polysiloxanes. In Silicon-Containing Polymers; Jones, R.G., Ando, W., Chojnowski, J., Eds.; Springer: Dordrecht, The Netherlands, 2000. [Google Scholar]
- Mazurek, M. Polimery krzemoorganiczne (Organosilicon polymers). In Chemia Polimerów (Polymer Chemistry); Florjańczyk, Z., Penczka, S., Eds.; Oficyna Wydawnicza Politechniki Warszawskiej: Warsaw, Poland, 2002. [Google Scholar]
- Chandrasekhar, V. Inorganic and Organomatallic Polymers; Springer: Berlin, Germany, 2005. [Google Scholar]
- Moretto, H.; Schulze, M.; Wagner, G. Silicones. In Ullmann’s Encyclopedia of Industrial Chemistry; Gehartz, W., Schulz, G., Kellersohn, T., Elvers, B., Hawkins, S., Winter, U., Eds.; Wiley: Weinheim, Germany, 2000. [Google Scholar]
- Boutevin, B.; Guida-Pietrasanta, F.; Ratsimihety, A. Side Group Modified Polysiloxanes. In Silicon-Containing Polymers; Jones, R.G., Ando, W., Chojnowski, J., Eds.; Springer: Dordrecht, The Netherlands, 2000. [Google Scholar]
- Mark, J.E.; Schaefer, D.W.; Lin, G. The Polysiloxanes; Oxford University Press: New York, NY, USA, 2015. [Google Scholar]
- Maciejewski, H.; Szubert, K.; Marciniec, B. Technologie otrzymywania funkcjonalizowanych polisiloksanów (Technologies for obtaining functionalized polysiloxanes). Polimery 2009, 54, 706–711. [Google Scholar]
- Poly(methylphenylsiloxane), Polymer Database. Available online: https://polymerdatabase.com/polymers/Polymethylphenylsiloxane.html (accessed on 19 January 2020).
- Patwardhan, D.V.; Zimmer, H.; Mark, J.E. Synthesis of Some Fluorinated Phenylmethylsiloxane Polymers and Characterization of Their Surface Properties. Polymers 1997, 7, 93–109. [Google Scholar]
- Owen, M.J. Poly[Methyl(3,3,3-Trifluoropropyl)Siloxane]. In Handbook of Fluoropolymer Science and Technology; Smith, D.W., Jr., Iacono, S.T., Iyer, S.S., Eds.; Wiley: Hoboken, NJ, USA, 2014. [Google Scholar]
- Owen, M.J. Siloxane Surface Activity. In Silicone-Based Polymer Science; Zeigler, J.M., Gordon Fearon, F.W., Eds.; American Chemical Society: Washington, DC, USA, 1990. [Google Scholar]
- Maciejewski, H.; Karasiewicz, J.; Marciniec, B. Efektywna synteza fluorofunkcyjnych (poli)siloksanów (Efficient synthesis of fluorofunctional polysiloxanes). Polimery 2012, 57, 449–455. [Google Scholar] [CrossRef]
- Łubkowska, M.; Stańczyk, W. Aminoalkyl functionalized siloxanes. Polimery 2014, 59, 763–768. [Google Scholar] [CrossRef]
- Pozuelo, J.; Baselga, J. Glass transition temperature of low molecular weight poly(3-aminopropyl methyl siloxane). A molecular dynamics study. Polymer 2002, 43, 6049–6055. [Google Scholar] [CrossRef] [Green Version]
- Poly[dimethylsiloxane-co-(3-aminopropyl)methylsiloxane], Sigma Aldrich (Merck). Available online: https://www.sigmaaldrich.com/catalog/product/aldrich/480304?lang=pl®ion=P&cm_sp=Insite-_-prodRecCold_xviews-_-prodRecCold10-1 (accessed on 28 January 2020).
- Xu, Y.; Yin, H.; Zheng, H.; Yuan, S.; Chen, Z. Application performance and surface morphologies of amino polysiloxanes with different amino values and amino types. J. Appl. Polym. Sci. 2011, 119, 2326–2333. [Google Scholar] [CrossRef]
- Yu, T.; Wakuda, K.; Blair, D.L.; Weiss, R.G. Reversibly Cross-Linking Amino-Polysiloxanes by Simple Triatomic Molecules. Facile Methods for Tuning Thermal, Rheological, and Adhesive Properties. J. Phys. Chem. C 2009, 113, 11546–11553. [Google Scholar] [CrossRef]
- Jiang, H.; Zheng, Z.; Song, W.; Li, Z.; Wang, X. Alkoxysilane Functionalized Polyurethane/Polysiloxane Copolymers: Synthesis and the Effect of End-Capping Agent. Polym. Bull. 2007, 59, 53–63. [Google Scholar] [CrossRef]
- Poly(dimethylsiloxane), bis(3-aminopropyl) terminated, Sigma Aldrich (Merck). Available online: https://www.sigmaaldrich.com/catalog/product/aldrich/481696?lang=pl®ion=PL&cm_sp=Insite-_-prodRecCold_xviews-_-prodRecCold10-2 (accessed on 28 January 2020).
- Poly(dimethylsiloxane), diglycidyl ether terminated, Sigma Aldrich (Merck). Available online: https://www.sigmaaldrich.com/catalog/product/aldrich/480282?lang=pl®ion=PL&cm_sp=Insite-_-prodRecCold_xviews-_-prodRecCold10-4 (accessed on 28 January 2020).
- Poly(dimethylsiloxane-co-diphenylsiloxane), divinyl terminated, Sigma Aldrich (Merck). Available online: https://www.sigmaaldrich.com/catalog/product/aldrich/482048?lang=pl®ion=PL&cm_sp=Insite-_-prodRecCold_xviews-_-prodRecCold10-10 (accessed on 28 January 2020).
- Poly(dimethylsiloxane), hydroxy terminated, Sigma Aldrich (Merck). Available online: https://www.sigmaaldrich.com/catalog/product/aldrich/481939?lang=pl®ion=PL&cm_sp=Insite-_-recent_fixed-_-recent5-4 (accessed on 28 January 2020).
- Nikolczuk, K.; Hadzik, J.; Wilk, Z. Nowe ładunki kumulacyjne o właściwościach elastycznych jako przykład zastosowania polimerów silikonowych w technologii materiałów wybuchowych (New shaped charges with elastic properties as an example of the use of silicone polymers in the technology of explosives). Chemik 2013, 67, 33–40. [Google Scholar]
- Golopol, H.; Hetherington, N.; North, K. Aging Effects on the Detonation Velocity of XTX-8003. J. Hazard. Mater. 1980, 4, 45–55. [Google Scholar] [CrossRef]
- Elbeih, A.; Zeman, S.; Jungova, M.; Vavra, P.; Akstein, Z. Effect of Different Polymeric Matrices on Some Properties of Plastic Bonded Explosives. Propellants Explos. Pyrotech. 2012, 37, 676–684. [Google Scholar] [CrossRef]
- Elbeih, A.; Zeman, S.; Jungova, M.; Akstein, Z. Effect of Different Polymeric Matrices on the Sensitivity and Performance of Interesting Cyclic Nitramines. Cent. Eur. J. Energy Mater. 2012, 9, 131–138. [Google Scholar]
- Zeman, S.; Elbeih, A.; Hussein, A.K.; Elshenawy, T.; Pelikan, V.; Yan, Q. Concerning the Shock Sensitivities of Certain Plastic Bonded Explosives Based on Attractive Cyclic Nitramines. Cent. Eur. J. Energetic Mater. 2017, 14, 775–787. [Google Scholar] [CrossRef]
- Daniel, M.A. Polyurethane Binder System for Polymer Bonded Explosives. Report No. DSTO-GD-0492; Defence Science and Technology Organisation: Edinburgh, Australia, 2006. [Google Scholar]
- Riviera, T.; Matuszak, M.L. Surface properties of potential plastic-bonded explosives (PBX). J. Colloid Interface Sci. 1983, 93, 105–108. [Google Scholar] [CrossRef]
- Xiao, J.; Huang, H.; Li, J.; Zhang, H.; Zhu, W.; Xiao, H. A molecular dynamics study of interface interactions and mechanical properties of HMX-based PBXs with PEG and HTPB. J. Mol. Struct. Theochem 2008, 851, 242–248. [Google Scholar] [CrossRef]
- Bower, J.K.; Kolb, J.R.; Pruneda, C.O. Polymeric Coatings Effect on Surface Activity and Mechanical Behavior of High Explosives. Ind. Eng. Chem. Prod. Res. Dev. 1980, 19, 326–329. [Google Scholar] [CrossRef]
- Pang, W.Q.; De Luca, L.T.; Fan, X.Z.; Golotov, O.G.; Zhao, F.Q. Boron-Based Fuel-Rich Propellant: Properties, Combustion and Technology Aspects; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Yadav, A.; Pant, C.S.; Das, S. Research Advances in Bonding Agents for Composite Propellants. Propellants Explos. Pyrotech. 2020, 45, 695–704. [Google Scholar] [CrossRef]
- Kishore, K.; Prema, S.; Iyanar, K.; Pandureng, L.P. Mechanistic studies on the effect of ferrocene bonding agents in composite solid propellants. Fuel 1994, 73, 1583–1593. [Google Scholar] [CrossRef]
- Hill, R.M. Silicone Surfactants. In Silicone Surfactants; Hill, R.M., Ed.; Marcel Dekker: New York, NY, USA, 1999. [Google Scholar]
- Silanes, Siloxanes & Silicates, Wacker. Available online: https://www.wacker.com/cms/en-us/products/product-groups/silanes-siloxanes-silicates/silanes-siloxanes-silicates.html (accessed on 4 February 2020).
- Zeng, C.; Yang, Z.; Zhang, J.; Li, Y.; Lin, C.; He, G.; Zhao, X.; Liu, S.; Gong, F. Enhanced Interfacial and Mechanical Properties of PBX Composites via Surface Modification on Energetic Crystals. Polymers 2019, 11, 1308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heath, R. Isocyanate-Based Polymers: Polyurethanes, Polyureas, Polyisocyanurates, and their Copolymers. In Brydson’s Plastic Materials; Gilbert, M., Ed.; Butter-Heinemann: Oxford, UK, 2017. [Google Scholar]
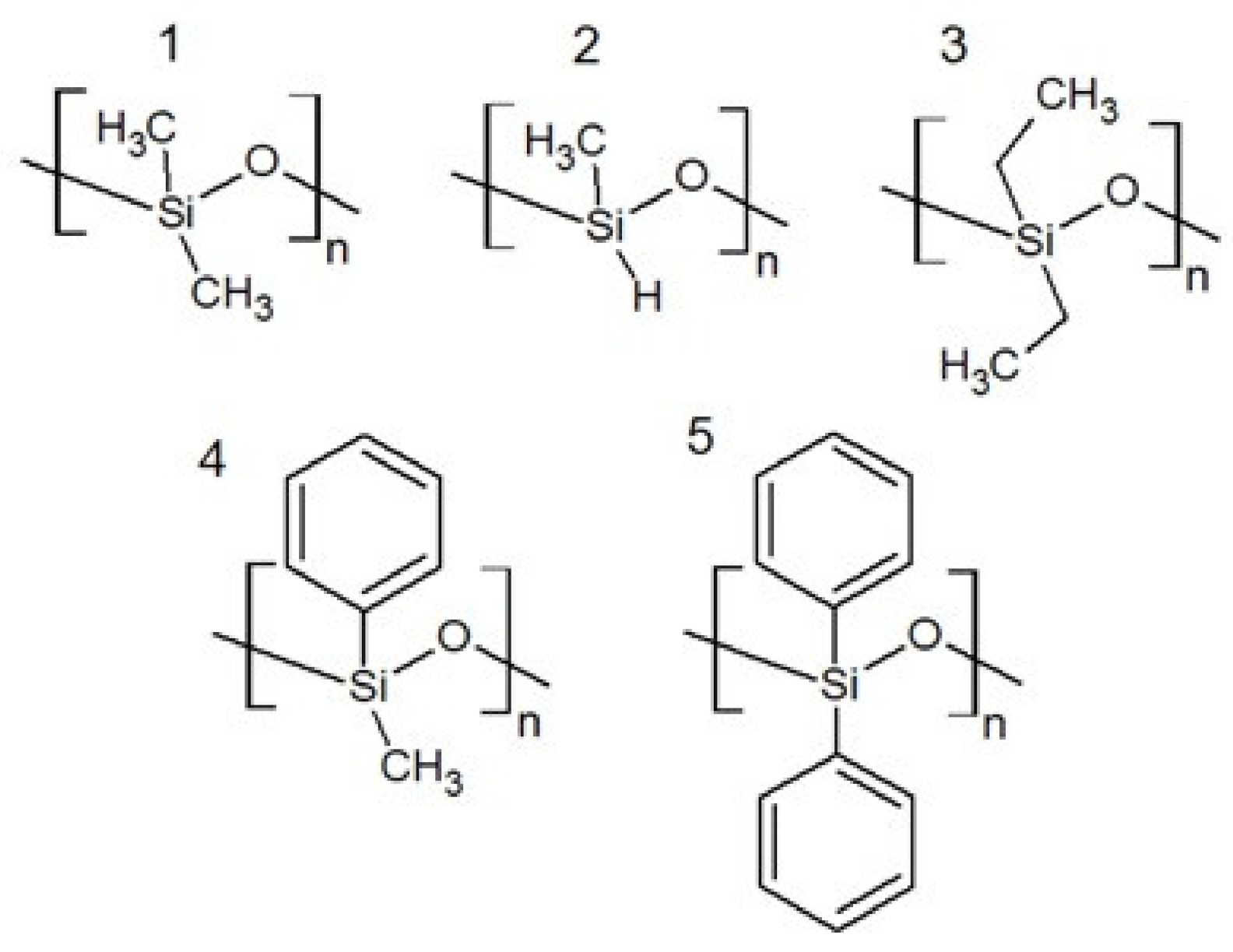

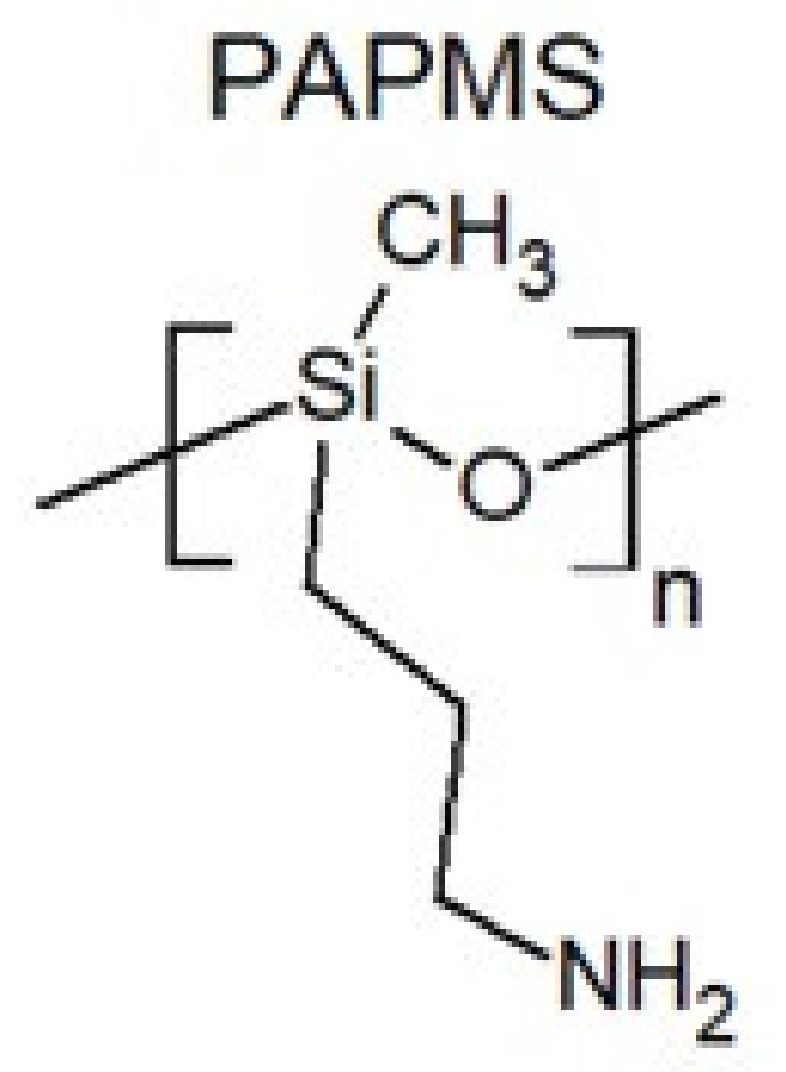
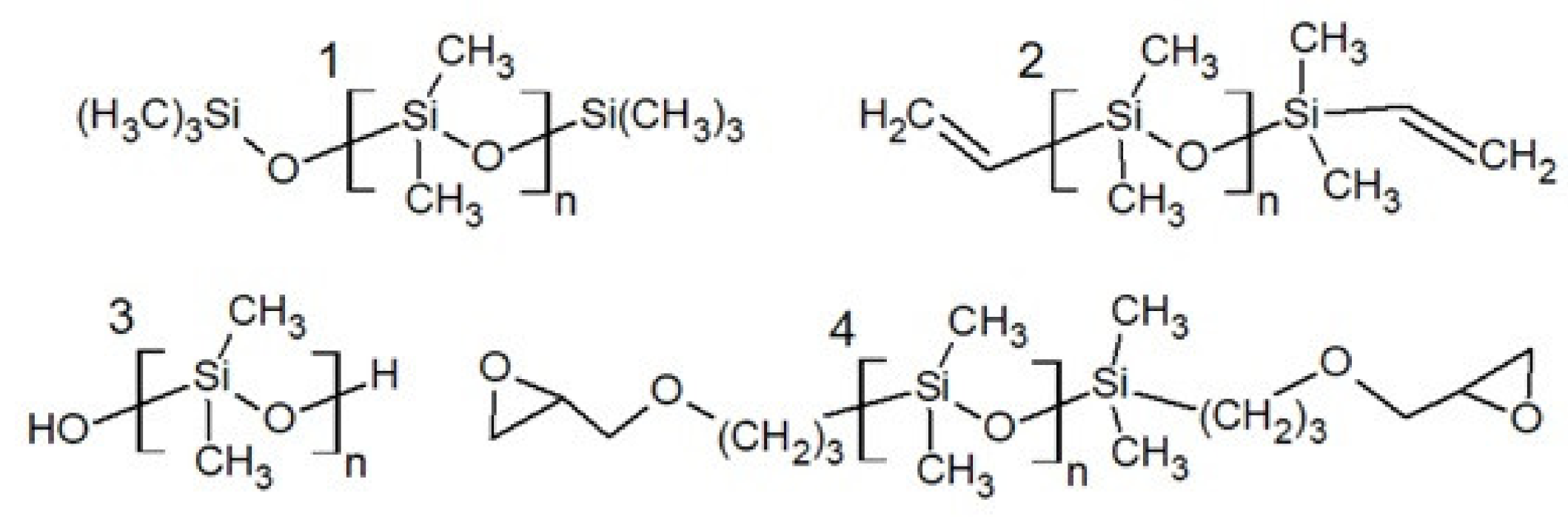


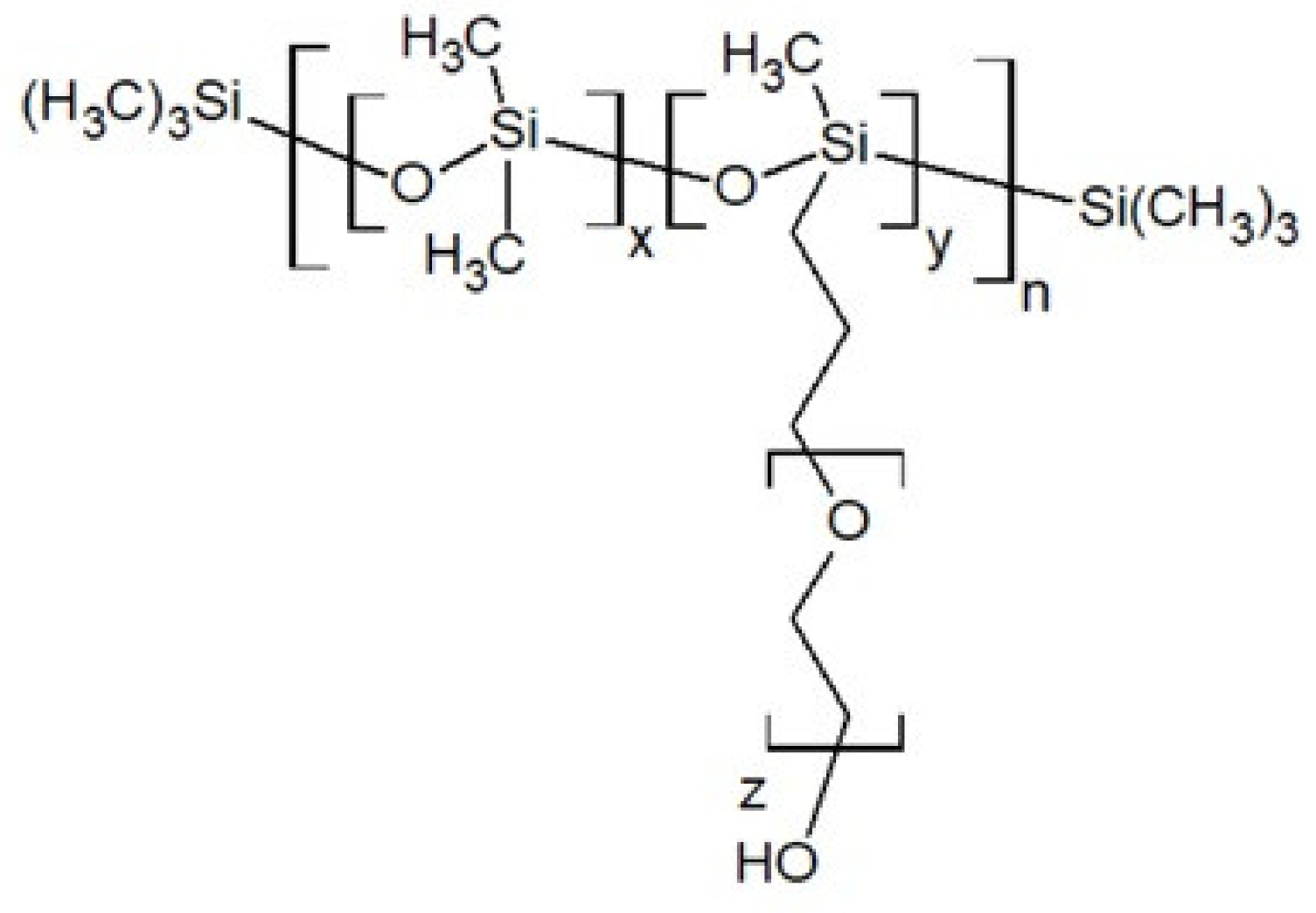

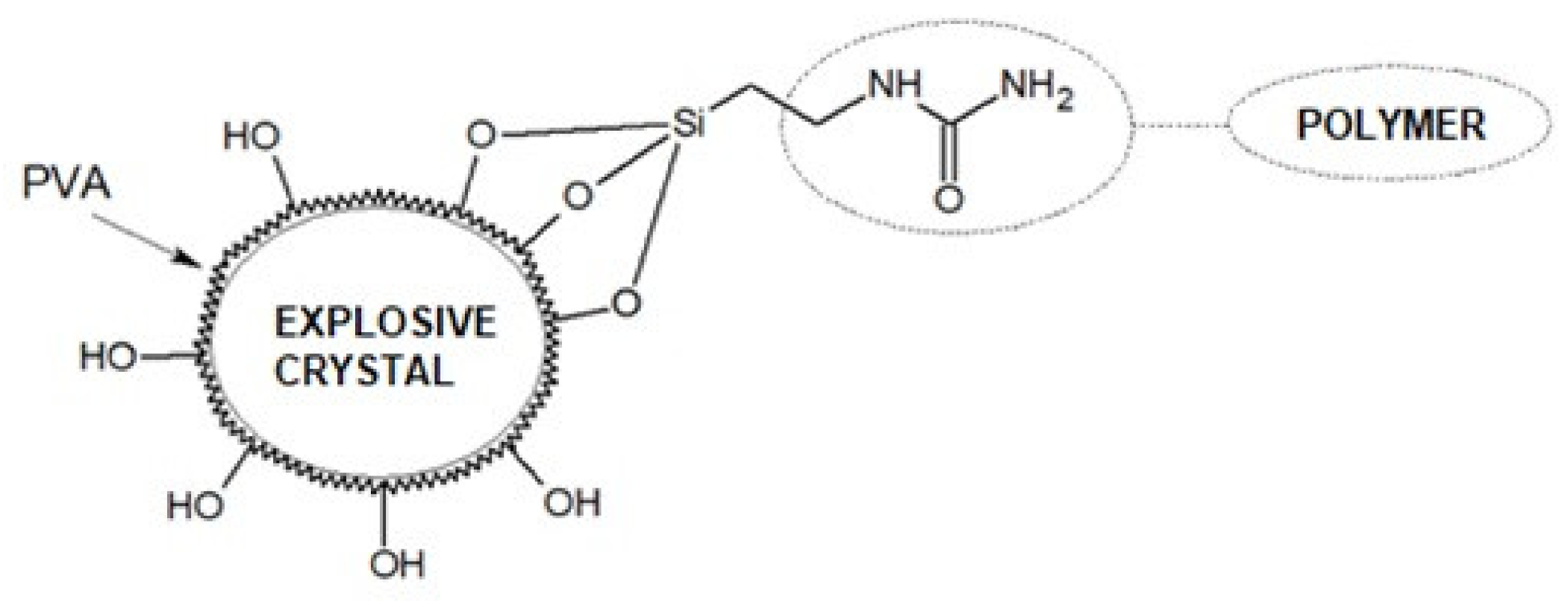
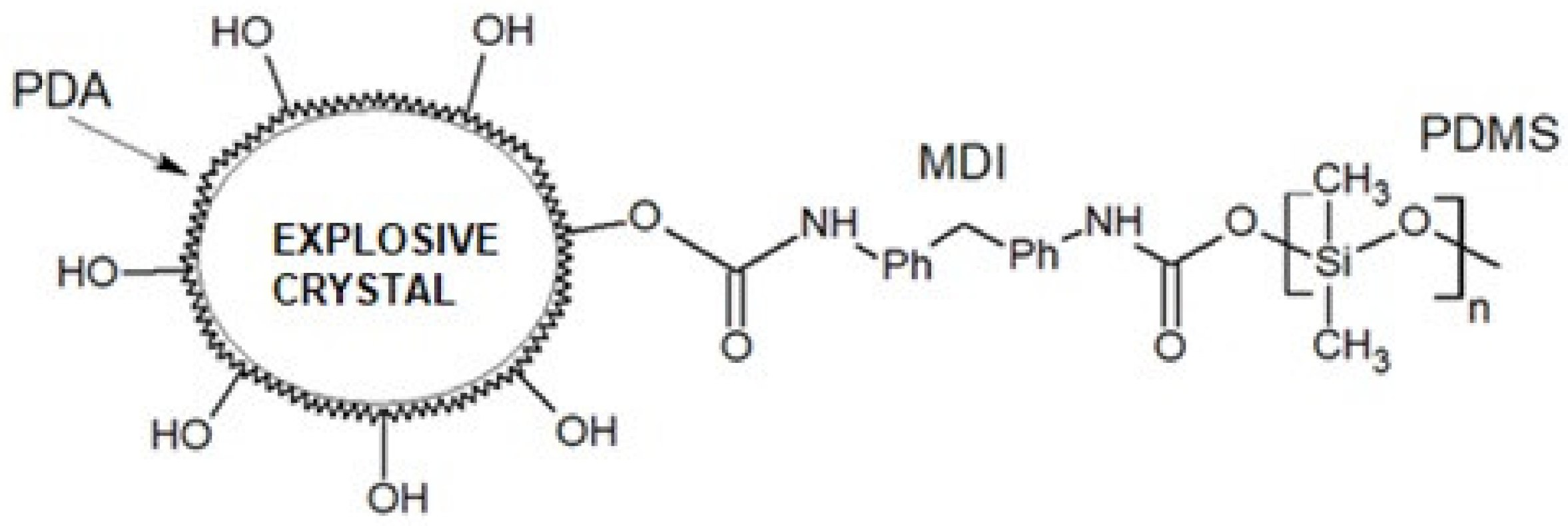
| Average Molecular Mass [g/mol] | Dynamic Viscosity (25 °C) [mPa·s] | Dynamic Viscosity (25 °C) [mPa·s] | Surface Tension [mN/m] |
|---|---|---|---|
| 600 | 3 | 0.90 | 19.3 |
| 1800 | 10 | 0.94 | 19.9 |
| 5800 | 100 | 0.97 | 20.9 |
| 26,000 | 1000 | 0.97 | 21.2 |
| 62,000 | 12,500 | 0.97 | 21.4 |
| 160,000 | 500,000 | 0.97 | 21.5 |
| Polymer | Dynamic Viscosity (25 °C) [mPa·s] | Dynamic Viscosity (25 °C) [mPa·s] | Surface Tension [mN/m] |
|---|---|---|---|
| PDMS | 20.7 [20] | 22.7 [20] | 23.5 [19]; 22.8 [20] |
| PMTFPS | 24.2 [20] | 21.4 [20] | 13.6 [19,20] |
| PMPS | - | - | 33.2 [19] |
| PMFPhS | - | - | 18.7 [19] |
| PMDFPhS | - | - | 19.1 [19] |
| Polymer | Glass Transition Temperature [°C] |
|---|---|
| PDMS | –123 |
| PMHS | –138 |
| PDES | –139 |
| PMPS | –28 |
| PDPS | 40 |
| PMTFPS | –70 |
| PAPMS | –63 |
| Explosive | Loading Density [g/cm3] | Detonation Velocity [m/s] | Impact Sensitivity [J] | Friction Sensitivity [N] |
|---|---|---|---|---|
| RDX | 1.76 | 8750 | 5.58 | 120 |
| HMX | 1.90 | 9100 | 6.37 | 95 |
| BCHMX | 1.79 | 8700 | 2.98 | 88 |
| HNIW | 2.04 | 9800 | 4.2 | 64 |
| RDX/Viton A (91/9) | 1.76 | 8285 | 10.6 | 326 |
| HMX/Viton A (91/9) | 1.84 | 8602 | 10.3 | 304 |
| BCHMX/Viton A (91/9) | 1.81 | 8474 | 5.3 | 283 |
| HNIW/Viton A (91/9) | 1.94 | 9023 | 6.9 | 252 |
| RDX/Viton A (95/5) | 1.76 | 8424 | 9.2 | 271 |
| HMX/Viton A (95/5) | 1.84 | 8730 | 9.2 | 243 |
| BCHMX/Viton A (95/5) | 1.81 | 8612 | 4.8 | 230 |
| HNIW/Viton A (95/5) | 1.95 | 9194 | 6.4 | 186 |
| RDX/PDMS | 1.58 | 7844 | 33.9 | 254 |
| HMX/PDMS | 1.64 | 8083 | 32.2 | 228 |
| BCHMX/PDMS | 1.63 | 7994 | 24.3 | 232 |
| HNIW/PDMS | 1.74 | 8267 | 28.0 | 192 |
| RDX/HTPB | 1.52 | 7526 | 14.6 | >360 |
| HMX/HTPB | 1.57 | 7812 | 15.2 | >360 |
| BCHMX/HTPB | 1.56 | 7746 | 9.6 | 322 |
| HNIW/HTPB | 1.63 | 8167 | 10.8 | 214 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zalewski, K.; Chyłek, Z.; Trzciński, W.A. A Review of Polysiloxanes in Terms of Their Application in Explosives. Polymers 2021, 13, 1080. https://doi.org/10.3390/polym13071080
Zalewski K, Chyłek Z, Trzciński WA. A Review of Polysiloxanes in Terms of Their Application in Explosives. Polymers. 2021; 13(7):1080. https://doi.org/10.3390/polym13071080
Chicago/Turabian StyleZalewski, Karol, Zbigniew Chyłek, and Waldemar A. Trzciński. 2021. "A Review of Polysiloxanes in Terms of Their Application in Explosives" Polymers 13, no. 7: 1080. https://doi.org/10.3390/polym13071080







