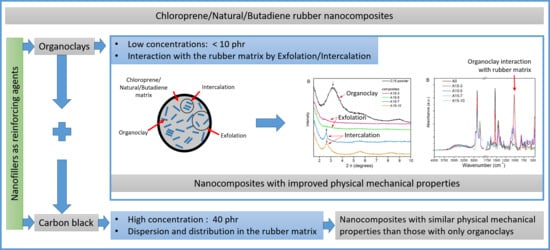
Enhancement of Chloroprene/Natural/Butadiene Rubber Nanocomposite Properties Using Organoclays and Their Combination with Carbon Black as Fillers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Compounding and Vulcanization of Samples
2.3. Characterization Techniques
2.3.1. Physicomechanical Properties
2.3.2. X-Ray Diffraction Analysis
2.3.3. Thermogravimetric Analysis (TGA)
2.3.4. Fourier Transformed Infrared (FT-IR) Analysis
2.3.5. Cure Characteristics
3. Results and Discussion
3.1. Physicomechanical Properties
3.1.1. Tear Properties
3.1.2. Tensile Properties
3.1.3. Abrasion Resistance
3.1.4. Hardness and Density
3.2. X-Ray Diffraction Analysis
3.3. Thermogravimetric Analysis
3.4. FT-IR Analysis
3.5. Cure Characteristics
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kapgate, B.P.; Das, C.; Das, A.; Basu, D.; Wiessner, S.; Reuter, U. Reinforced chloroprene rubber by in situ generated silica particles: Evidence of bound rubber on the silica surface. J. Appl. Polym. Sci. 2016, 43717, 1–10. [Google Scholar] [CrossRef]
- Wu, W.L.; Chen, Z. Modified-diatomite reinforced rubbers. Mater. Lett. 2017, 209, 159–162. [Google Scholar] [CrossRef]
- Garing, C.L.; Pajarito, B.B. Effect of clay loading on the water resistance of ternary-filled natural rubber composites. Mater. Today Proc. 2020, 33, 1959–1962. [Google Scholar] [CrossRef]
- Sethuramalingam, V.C.; Prabagaran, S.; Ganesan, K. Studies on influence of silica filler and rice husk ash on the mechanical properties of vulcanized hybrid rubber composite. Mater. Today Proc. 2020; in press. [Google Scholar] [CrossRef]
- Qiao, J. Elastomeric nano-particle and its applications in polymer modifications. Adv. Ind. Eng. Polym. Res. 2020, 3, 47–59. [Google Scholar] [CrossRef]
- Rajasekar, R.; Pal, K.; Heinrich, G.; Das, A.; Das, C.K. Development of nitrile butadiene rubber-nanoclay composites with epoxidized natural rubber as compatibilizer. Mater. Des. 2009, 30, 3839–3845. [Google Scholar] [CrossRef]
- Zhang, A.; Zhang, Y.; Zhang, Y. Characterization of kaolinite/emulsion-polymerization styrene butadiene rubber (ESBR) nanocomposite prepared by latex blending method: Dynamic mechanic properties and mechanism. Polym. Test. 2020, 89, 1–11. [Google Scholar] [CrossRef]
- Ramesan, M.T.; Alex, R.; Khanh, N.V. Studies on the cure and mechanical properties of blends of natural rubber with dichlorocarbene modified styrene-butadiene rubber and chloroprene rubber. React. Funct. Polym. 2005, 62, 41–50. [Google Scholar] [CrossRef]
- Sirisinha, C.; Baulek-Limcharoen, S.; Thunyarittikorn, J. Relationships among blending conditions, size of dispersed phase, and oil resistance in natural rubber and nitrile rubber blends. J. Appl. Polym. Sci. 2001, 82, 1232–1237. [Google Scholar] [CrossRef]
- Choi, S.S. Improvement of properties of silica-filled natural rubber compounds using polychloroprene. J. Appl. Polym. Sci. 2002, 83, 2609–2616. [Google Scholar] [CrossRef]
- Razavi-Nouri, M.; Sabet, A.; Mohebbi, M. Thermal, tensile and rheological properties of dynamically cross-linked organoclay filled poly(ethylene-co-vinyl acetate)/acrylonitrile-butadiene rubber nanocomposites: Effect of peroxide content. Polymer 2020, 190, 1–10. [Google Scholar] [CrossRef]
- Kanny, K.; Mohan, T.P. Rubber nanocomposites with nanoclay as the filler. In Progress in Rubber Nanocomposites; Sabu, T., Hann, J.M., Eds.; Woodhead Publising: Durban, South Africa, 2017; pp. 153–177. ISBN 978-0081004098. [Google Scholar]
- Ahmed, K. An investigation on chloroprene-compatibilized acrylonitrile butadiene rubber/high density polyethylene blends. J. Adv. Res. 2014, 6, 811–817. [Google Scholar] [CrossRef][Green Version]
- Manohar, N.; Jayaramudu, J.; Suchismita, S.; Rajkumar, K.; Badul Reddy, A.; Sadiku, E.R.; Priti, R.; Maurya, D.J. A unique application of the second order derivative of FTIR–ATR spectra for compositional analyses of natural rubber and polychloroprene rubber and their blends. Polym. Test. 2017, 62, 447–453. [Google Scholar] [CrossRef]
- Sare, I.R.; Mardel, J.I.; Hill, A.J. Wear-resistant metallic and elastomeric materials in the mining and mineral processing industries—An overview. Wear. 2001, 250, 1–10. [Google Scholar] [CrossRef]
- Lai Lai, P.L.; Joon Ching, J.; Nay Ming, H.; Leng Kian, G.; Fook Peng, L.; Yi Yee, L. Effect of graphene oxide particle size on the tensile strength and stability of natural rubber graphene composite. Mater. Sci. Eng. B 2020, 262, 1–10. [Google Scholar] [CrossRef]
- Senthilvel, K.; Arul Jeya Kumar, A.; Seeman, M.; Ashok Kumar, I.; Prabu, B. Studies the effect of halloysite nanotubes on the mechanical and hot air ageing properties of nitrile-polyvinyl chloride rubber nano-composites. Mater. Today Proc. 2020. [Google Scholar] [CrossRef]
- Rooj, S.; Das, A.; Stöckelhuber, K.W.; Wießner, S.; Fischer, D.; Reuter, U.; Heinrich, G. Expanded organoclay assisted dispersion and simultaneous structural alterations of multiwall carbon nanotube (MWCNT) clusters in natural rubber. Compos. Sci. Technol. 2015, 107, 36–43. [Google Scholar] [CrossRef]
- Zongchao, X.; Long, Z.; Shipeng, W.; Li, L. Graphene oxide-supported zinc oxide nanoparticles for chloroprene rubber with improved crosslinking network and mechanical properties. Compos. Part A Appl. Sci. Manuf. 2019, 124, 2–9. [Google Scholar] [CrossRef]
- Lingmin, K.; Yong, Z.; Guangsu, H.; Jinrong, W. Carbon nanodots as dual role of crosslinking and reinforcing chloroprene rubber. Compos. Commun. 2020, 22, 1–8. [Google Scholar]
- Shaojian, H.; Tengfei, H.; Jiaqi, W.; Xiaohui, W.; Yang, X.; Liqun, Z.; Jun, L. A novel method to prepare acrylonitrile-butadiene rubber/clay nanocomposites by compounding with clay gel. Compos. Part. B Eng. 2019, 167, 356–361. [Google Scholar]
- Uddin, F. Clays, nanoclays, and montmorillonite minerals. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2008, 39, 2804–2814. [Google Scholar] [CrossRef]
- Das, A.; Mahaling, R.N.; Stöckelhuber, K.W.; Heinrich, G. Reinforcement and migration of nanoclay in polychloroprene/ethylene-propylene-diene-monomer rubber blends. Compos. Sci. Technol. 2011, 71, 276–281. [Google Scholar] [CrossRef]
- Urrego Yepes, W.; Velásquez Restrepo, S.M.; Giraldo Vásquez, D.H.; Posada Correo, J.C. Revisión- Efecto del sistema de vulcanizacion en la red entrecruzada y en la reacción química de vulcanización del caucho natural. Rev. EIA 2017, 14, 99–115. [Google Scholar] [CrossRef]
- Annadurai, P.; Mukundan, T.; Joseph, R. Influence of carbon black in polychloroprene organoclay nanocomposite with improved mechanical, electrical and morphology characteristics. Plast. Rubber Compos. 2013, 42, 379–384. [Google Scholar] [CrossRef]
- Mathew, G.; Rhee, J.M.; Lee, Y.S.; Park, D.H.; Nah, C. Cure kinetics of ethylene acrylate rubber/clay nanocomposites. J. Ind. Eng. Chem. 2008, 14, 60–65. [Google Scholar] [CrossRef]
- Romli, A.Z.; Mamauod, S.N.L. Physical and Mechanical Properties of ENR Compatibilized NR/NBR Blends Reinforced Nanoclay and Nanosilica. Macromol. Symp. 2017, 371, 27–34. [Google Scholar] [CrossRef]
- Praveen, S.; Chattopadhyay, P.K.; Albert, P.; Dalvi, V.G.; Chakraborty, B.C.; Chattopadhyay, S. Synergistic effect of carbon black and nanoclay fillers in styrene butadiene rubber matrix: Development of dual structure. Compos. Part A Appl. Sci. Manuf. 2009, 40, 309–316. [Google Scholar] [CrossRef]
- Etika, K.C.; Liu, L.; Hess, L.A.; Grunlan, J.C. The influence of synergistic stabilization of carbon black and clay on the electrical and mechanical properties of epoxy composites. Carbon N. Y. 2009, 47, 3128–3136. [Google Scholar] [CrossRef]
- Gent, A.; Pulford, C.T. Mechanism of Rubber Abrasion. J. Appl. Polym. Sci. 1983, 28, 943–960. [Google Scholar] [CrossRef]
- Raji Vijay, V.; Anitha, A.M.; Ravindranatha Menon, A.R. Studies on blends of natural rubber and butadiene rubber containing silica-Organomodified kaolin hybrid filler systems. Polymer 2016, 89, 135–142. [Google Scholar] [CrossRef]
- Sreelekshmi, R.V.; Brahmakumar, M.; Sudha, J.D.; Ravindranatha Menon, A.R. Studies on natural rubber containing kaolin modified with hexamethylenediamine derivative of phosphorylated cashew nut shell liquid prepolymer. Appl. Clay. Sci. 2017, 141, 171–179. [Google Scholar] [CrossRef]
- Tabsan, N.; Wirasate, S.; Suchiva, K. Abrasion behavior of layered silicate reinforced natural rubber. Wear 2010, 269, 394–404. [Google Scholar] [CrossRef]
- Lin, Z.; Joseph, T.G.; Curley, M. Specific energy and the modified rubber wheel abrasion test. Wear 2017, 370–371, 9–16. [Google Scholar] [CrossRef]
- Mohomane, S.M.; Djokovic, V.; Thomas, S.; Luyt, A.S. Polychloroprene nanocomposites filled with different organically modified clays: Morphology, thermal degradation and stress relaxation behaviour. Polym. Test. 2011, 30, 585–593. [Google Scholar] [CrossRef]
- Das, A.; Costa, F.R.; Wagenknecht, U.; Heinrich, G. Nanocomposites based on chloroprene rubber: Effect of chemical nature and organic modification of nanoclay on the vulcanizate properties. Eur. Polym. J. 2008, 44, 3456–3465. [Google Scholar] [CrossRef]
- Boukerrou, A.; Duchet, J.; Fellahi, S.; Djidjelli, H.; Kaci, M.; Sautereau, H. Synthesis and characterization of rubbery epoxy/organoclay hectorite nanocomposites. Express Polym. Lett. 2007, 1, 824–830. [Google Scholar] [CrossRef]
- Belluci, F.; Camino, G.; Frache, A.; Sarra, A. Catalytic charring-volatizatin competition in organoclay nanocomposites. Polym. Degrad. Stab. 2007, 92, 425–436. [Google Scholar] [CrossRef]
- Xie, W.; Gao, Z.; Liu, K.; Pan, W.P.; Vaia, R.; Hunter, D.; Singh, A. Thermal characterization of organically modified montmorillonite. Thermochim. Acta 2001, 367, 339–350. [Google Scholar] [CrossRef]
- Lu, F.J.; Hsu, S.L. Vibrational Spectroscopic Analysis of the Structure of Natural Rubber. Rubber Chem. Technol. 1987, 60, 647–658. [Google Scholar] [CrossRef]
- Nallasamy, P.; Mohan, S. Vibrational Spectra of Cis-1, 4-Polyisoprene. Arab. J. Sci. Eng. 2004, 29, 17–26. [Google Scholar]
- Rolere, S.; Liengprayoon, S.; Vaysse, L.; Sainte-Beuve, J.; Bonfils, F. Investigating natural rubber composition with Fourier Transform Infrared (FT-IR) spectroscopy: A rapid and non-destructive method to determine both protein and lipid contents simultaneously. Polym. Test. 2015, 43, 83–93. [Google Scholar] [CrossRef]
- Barth, A. Infrared spectroscopy of proteins. Biochim. Biophys. Acta Bioenerg. 2007, 1767, 1073–1101. [Google Scholar] [CrossRef] [PubMed]
- Sarma, A.D.; Le, H.H.; Das, A.; Wießner, S.; Stöckelhuber, K.W.; Bhowmick, A.K.; Heinrich, G. Determination of phase specific localization of carbon black in ternary rubber blends: A macroscopic approach by fourier transform infrared spectroscopy (FTIR). Polymer 2018, 150, 64–71. [Google Scholar] [CrossRef]
- Dimitry, O.I.H.; Abdeen, Z.; Ismail, E.A.; Saad, A.L.G. Studies of particle dispersion in elastomeric polyurethane/organically modified montmorillonite nanocomposites. Int. J. Green Nanotechnol. 2011, 3, 197–212. [Google Scholar] [CrossRef]
- Pack, S.; Kashiwagi, T.; Cao, C.; Korach, C.S.; Lewin, M.; Rafailovich, M.H. Role of surface interactions in the synergizing polymer/clay flame retardant properties. Macromolecules 2010, 43, 5338–5351. [Google Scholar] [CrossRef]
- Trofimov, B.A.; Sinegovskaya, L.M.; Gusarova, N.K. Journal of Sulfur Chemistry Vibrations of the S–S bond in elemental sulfur and organic polysulfides: A structural guide. J. Sulfur. Chem. 2009, 30, 518–554. [Google Scholar] [CrossRef]
- Maji, P.K.; Guchhait, P.K.; Bhowmick, A.K. Effect of the microstructure of a hyperbranched polymer and nanoclay loading on the morphology and properties of novel polyurethane nanocomposites. ACS Appl. Mater. Interfaces 2009, 1, 289–300. [Google Scholar] [CrossRef]





| Filler | Intergallery Spacing d(001) (Å) | Organic Modifier | Particle Size (nm) |
|---|---|---|---|
| Organoclay Cloisite 10 A (C10A) | 19.0 | Dimethyl, benzyl, hydrogenated tallow, quaternary ammonium chloride. | - |
| Organoclay Cloisite 15 (C15) | 36.3 | Dimethyl, dehydrogenated tallow, quaternary ammonium chloride. | - |
| Carbon black N330 (CB) | - | - | 29 |
| Composite Code | Chloroprene Rubber (CR) | Natural Rubber (NR) | Butadiene Rubber (BR) | Cloisite® 10 A (C10A) | Cloisite® 15 (C15) | Carbon Black (CB) | Zinc Oxide (ZnO) | S | Others |
|---|---|---|---|---|---|---|---|---|---|
| A0 | 69.6 | 25.3 | 5.1 | 0.0 | 0.0 | 0.0 | 2.8 | 2.2 | 6.8 |
| A10-3 | 69.6 | 25.3 | 5.1 | 3.0 | 0.0 | 0.0 | 2.8 | 2.2 | 6.8 |
| A10-5 | 69.6 | 25.3 | 5.1 | 5.0 | 0.0 | 0.0 | 2.8 | 2.2 | 6.8 |
| A10-7 | 69.6 | 25.3 | 5.1 | 7.0 | 0.0 | 0.0 | 2.8 | 2.2 | 6.8 |
| A10-10 | 69.6 | 25.3 | 5.1 | 10.0 | 0.0 | 0.0 | 2.8 | 2.2 | 6.8 |
| A15-3 | 69.6 | 25.3 | 5.1 | 0.0 | 3.0 | 0.0 | 2.8 | 2.2 | 6.8 |
| A15-5 | 69.6 | 25.3 | 5.1 | 0.0 | 5.0 | 0.0 | 2.8 | 2.2 | 6.8 |
| A15-7 | 69.6 | 25.3 | 5.1 | 0.0 | 7.0 | 0.0 | 2.8 | 2.2 | 6.8 |
| A15-10 | 69.6 | 25.3 | 5.1 | 0.0 | 10.0 | 0.0 | 2.8 | 2.2 | 6.8 |
| B0 | 69.6 | 25.3 | 5.1 | 0.0 | 0.0 | 40.0 * | 2.8 | 2.2 | 6.8 |
| B10-3 | 69.6 | 25.3 | 5.1 | 3.0 | 0.0 | 40.0 * | 2.8 | 2.2 | 6.8 |
| B10-5 | 69.6 | 25.3 | 5.1 | 5.0 | 0.0 | 40.0 * | 2.8 | 2.2 | 6.8 |
| B10-7 | 69.6 | 25.3 | 5.1 | 7.0 | 0.0 | 40.0 * | 2.8 | 2.2 | 6.8 |
| B10-10 | 69.6 | 25.3 | 5.1 | 10.0 | 0.0 | 40.0 * | 2.8 | 2.2 | 6.8 |
| B15-3 | 69.6 | 25.3 | 5.1 | 0.0 | 3.0 | 40.0 * | 2.8 | 2.2 | 6.8 |
| B15-5 | 69.6 | 25.3 | 5.1 | 0.0 | 5.0 | 40.0 * | 2.8 | 2.2 | 6.8 |
| B15-7 | 69.6 | 25.3 | 5.1 | 0.0 | 7.0 | 40.0 * | 2.8 | 2.2 | 6.8 |
| B15-10 | 69.6 | 25.3 | 5.1 | 0.0 | 10.0 | 40.0 * | 2.8 | 2.2 | 6.8 |
| Composite Code | Tensile Strength (MPa) | Elongation at Break (%) | M 300 (MPa) | Tear Strength (N mm−1) |
|---|---|---|---|---|
| A0 | 5.36 ± 1.52 | 730.91 ± 95.97 | 1.35 ± 0.03 | 16.29 ± 1.56 |
| A10-3 | 6.85 ± 0.80 | 739.10 ± 41.61 | 1.74 ± 0.04 | 21.08 ± 0.80 |
| A10-5 | 11.85 ± 1.09 | 832.15 ± 29.95 | 2.41 ± 0.36 | 24.05 ± 1.20 |
| A10-7 | 13.65 ± 0.76 | 836.82 ± 11.38 | 3.05 ± 0.05 | 31.51 ± 2.29 |
| A10-10 | 10.14 ± 0.79 | 768.33 ± 23.77 | 2.88 ± 0.07 | 30.76 ± 1.91 |
| A15-3 | 9.95 ± 1.13 | 840.35 ± 37.73 | 1.88 ± 0.03 | 19.09 ± 0.87 |
| A15-5 | 13.42 ± 0.83 | 933.19 ± 45.89 | 2.24 ± 0.20 | 25.13 ± 1.45 |
| A15-7 | 12.40 ± 0.85 | 915.79 ± 13.82 | 2.31 ± 0.13 | 28.82 ± 2.84 |
| A15-10 | 10.01 ± 0.90 | 797.01 ± 41.74 | 2.70 ± 0.07 | 26.58 ± 2.32 |
| B0 | 9.95 ± 0.84 | 477.64 ± 15.41 | 6.71 ± 0.18 | 27.97 ± 0.66 |
| B10-3 | 8.55 ± 0.82 | 338.55 ± 22.87 | 7.49 ± 0.15 | 26.64 ± 2.49 |
| B10-5 | 7.44 ± 1.01 | 519.05 ± 6.81 | 6.54 ± 0.13 | 27.42 ± 2.68 |
| B10-7 | 10.73 ± 0.72 | 399.15 ± 16.38 | 7.74 ± 0.35 | 29.83 ± 0.46 |
| B10-10 | 8.83 ± 1.10 | 370.92 ± 31.69 | 6.86 ± 0.18 | 28.73 ± 2.82 |
| B15-3 | 12.77 ± 0.71 | 466.23 ± 25.73 | 7.19 ± 0.22 | 26.87 ± 2.53 |
| B15-5 | 7.02 ± 0.63 | 455.75 ± 17.58 | 6.35 ± 0.09 | 29.38 ± 2.14 |
| B15-7 | 12.43 ± 0.40 | 470.10 ± 18.88 | 6.97 ± 0.14 | 30.85 ± 2.23 |
| B15-10 | 7.31 ± 0.02 | 310.98 ± 1.47 | 7.11 ± 0.10 | 33.06 ± 2.56 |
| Composite Code | Hardness (Shore A) | Loss of Volume by Abrasion (mm3) | Reduction of Volume Loss (%) | Density (g/cm3) |
|---|---|---|---|---|
| A0 | 42 ± 0 | 449.97 ± 5.88 | - | 1.1356 ± 0.0011 |
| A10-3 | 47 ± 0 | 251.29 ± 13.09 | 44.15 | 1.1479 ± 0.0026 |
| A10-5 | 51 ± 0 | 243.42 ± 9.81 | 45.96 | 1.1497 ± 0.0022 |
| A10-7 | 56 ± 1 | 251.35 ± 7.13 | 44.14 | 1.1460 ± 0.0051 |
| A10-10 | 54 ± 1 | 269.68 ± 1.37 | 40.06 | 1.1607 ± 0.0042 |
| A15-3 | 50 ± 1 | 246.23 ± 5.05 | 45.28 | 1.1444 ± 0.0010 |
| A15-5 | 54 ± 1 | 241.73 ± 7.75 | 46.29 | 1.1479 ± 0.0022 |
| A15-7 | 62 ± 1 | 251.99 ± 15.73 | 43.99 | 1.1518 ± 0.0016 |
| A15-10 | 53 ± 0 | 399.11 ± 26.92 | 11.3 | 1.1587 ± 0.0004 |
| B0 | 59 ± 1 | 288.86 ± 6.69 | - | 1.2016 ± 0.0007 |
| B10-3 | 61 ± 1 | 242.07 ± 2.62 | 16.2 | 1.2101 ± 0.0016 |
| B10-5 | 64 ± 1 | 243.56 ± 11.15 | 15.68 | 1.2121 ± 0.0064 |
| B10-7 | 61 ± 1 | 240.51 ± 8.38 | 16.74 | 1.2164 ± 0.0015 |
| B10-10 | 63 ± 0 | 266.96 ± 3.72 | 7.51 | 1.2197 ± 0.0010 |
| B15-3 | 63 ± 1 | 227.12 ± 2.30 | 21.37 | 1.2040 ± 0.0013 |
| B15-5 | 66 ± 1 | 247.14 ± 12.32 | 14.44 | 1.2088 ± 0.0030 |
| B15-7 | 63 ± 0 | 216.48 ± 3.35 | 25.06 | 1.2186 ± 0.0004 |
| B15-10 | 64 ± 0 | 249.51 ± 3.86 | 13.62 | 1.2124 ± 0.0014 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castaño-Rivera, P.; Calle-Holguín, I.; Castaño, J.; Cabrera-Barjas, G.; Galvez-Garrido, K.; Troncoso-Ortega, E. Enhancement of Chloroprene/Natural/Butadiene Rubber Nanocomposite Properties Using Organoclays and Their Combination with Carbon Black as Fillers. Polymers 2021, 13, 1085. https://doi.org/10.3390/polym13071085
Castaño-Rivera P, Calle-Holguín I, Castaño J, Cabrera-Barjas G, Galvez-Garrido K, Troncoso-Ortega E. Enhancement of Chloroprene/Natural/Butadiene Rubber Nanocomposite Properties Using Organoclays and Their Combination with Carbon Black as Fillers. Polymers. 2021; 13(7):1085. https://doi.org/10.3390/polym13071085
Chicago/Turabian StyleCastaño-Rivera, Patricia, Isabel Calle-Holguín, Johanna Castaño, Gustavo Cabrera-Barjas, Karen Galvez-Garrido, and Eduardo Troncoso-Ortega. 2021. "Enhancement of Chloroprene/Natural/Butadiene Rubber Nanocomposite Properties Using Organoclays and Their Combination with Carbon Black as Fillers" Polymers 13, no. 7: 1085. https://doi.org/10.3390/polym13071085
APA StyleCastaño-Rivera, P., Calle-Holguín, I., Castaño, J., Cabrera-Barjas, G., Galvez-Garrido, K., & Troncoso-Ortega, E. (2021). Enhancement of Chloroprene/Natural/Butadiene Rubber Nanocomposite Properties Using Organoclays and Their Combination with Carbon Black as Fillers. Polymers, 13(7), 1085. https://doi.org/10.3390/polym13071085