Chelating Fabrics Prepared by an Organic Solvent-Free Process for Boron Removal from Water
Abstract
:1. Introduction
2. Experimental
2.1. Materials
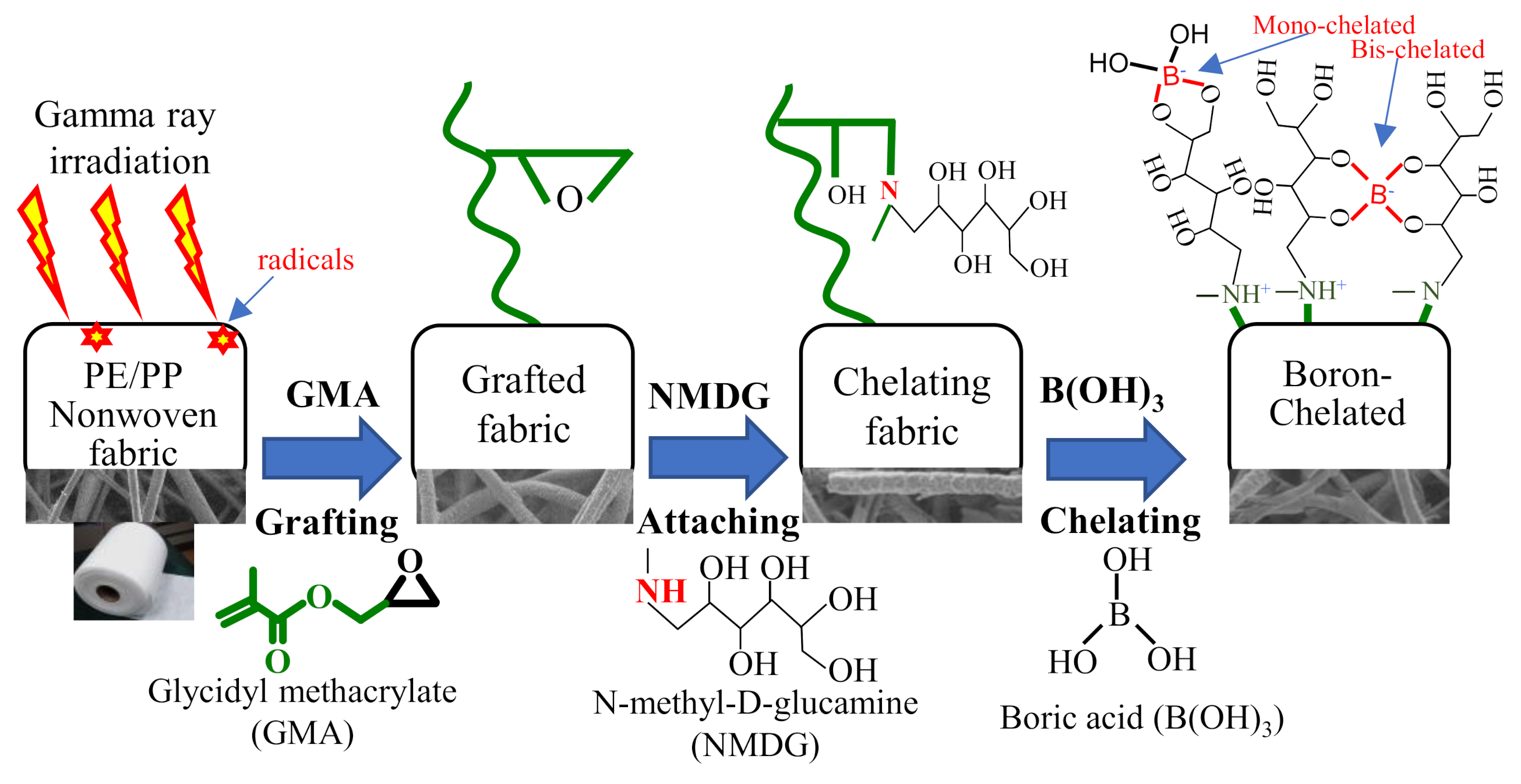
2.2. Preparation of Grafted Fabric
2.3. Preparation of Chelating Fabric
2.4. Characterization
2.5. Evaluation of the Chelating Fabric
3. Results and Discussion
3.1. Preparation of GMA-Grafted Fabric
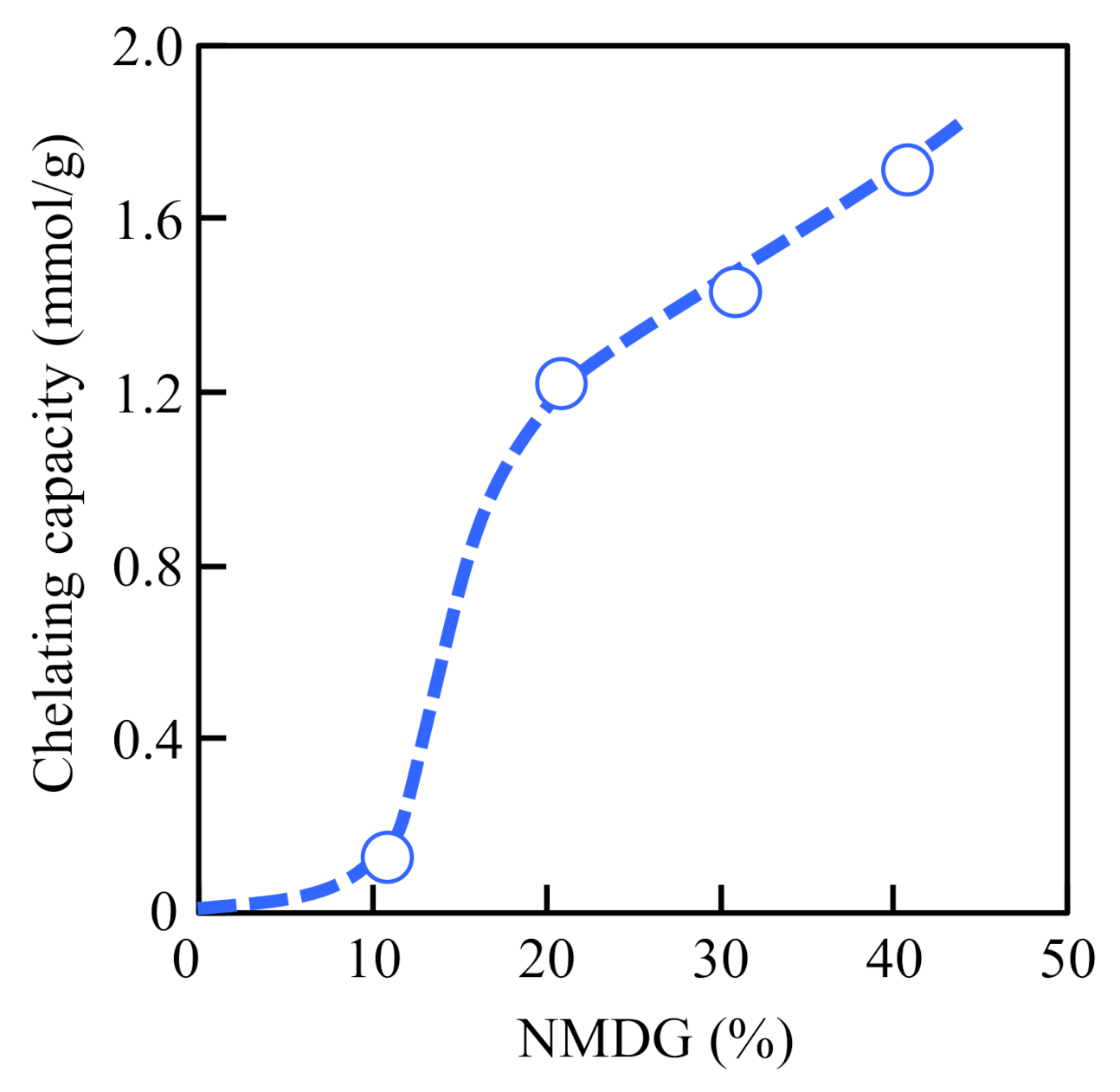
3.2. Preparation of Chelating Fabric
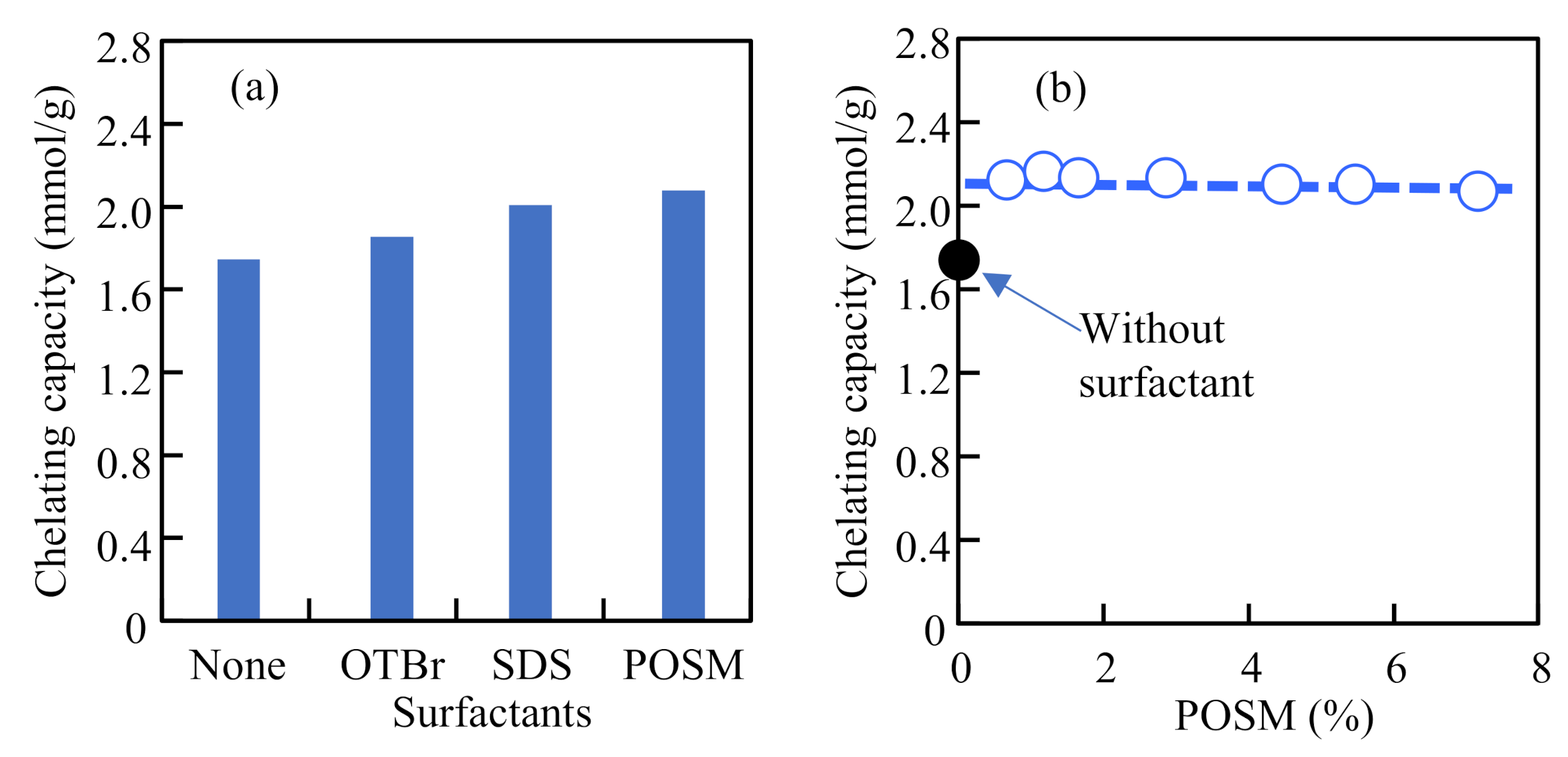
3.3. Enhancing the Attachment Reaction by Surfactants
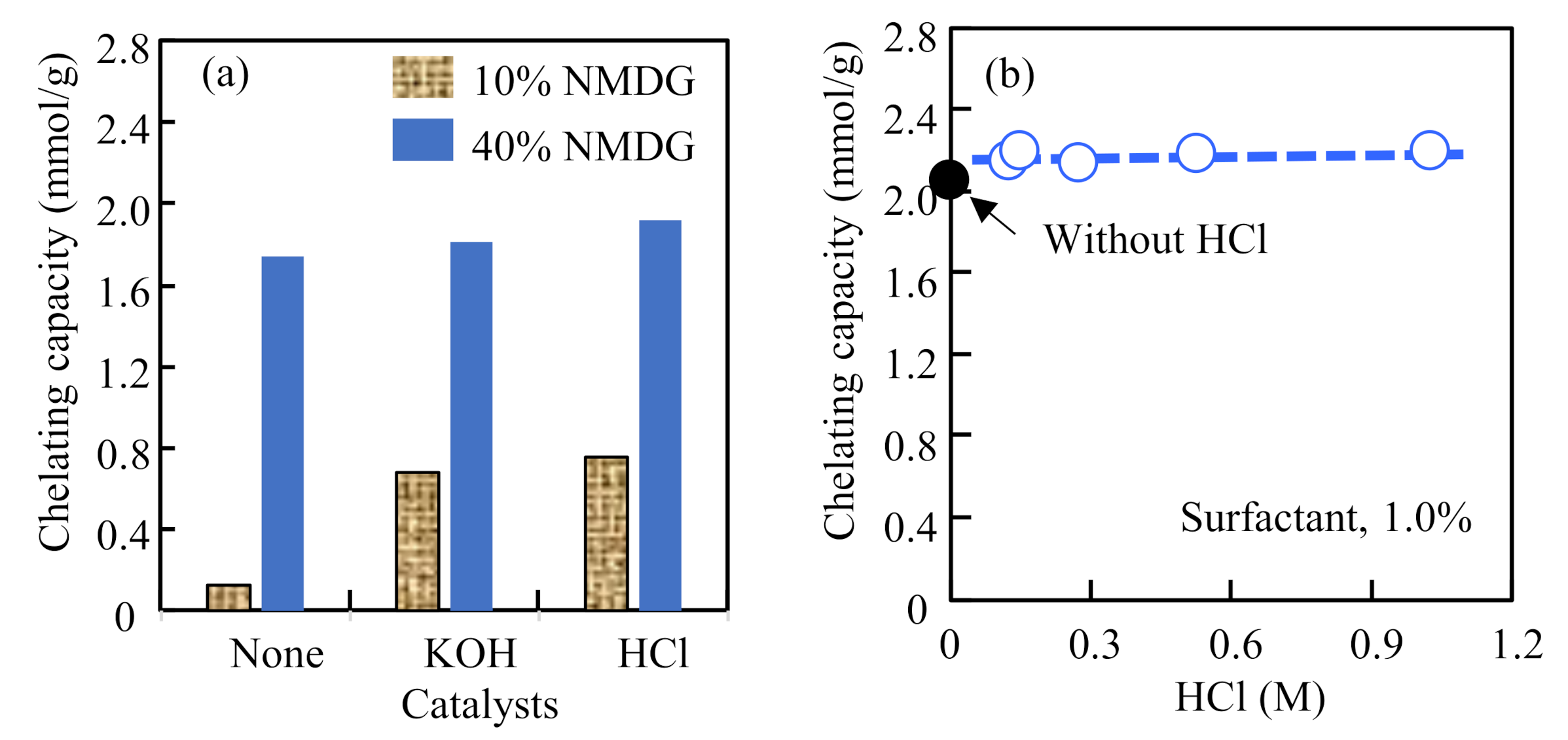
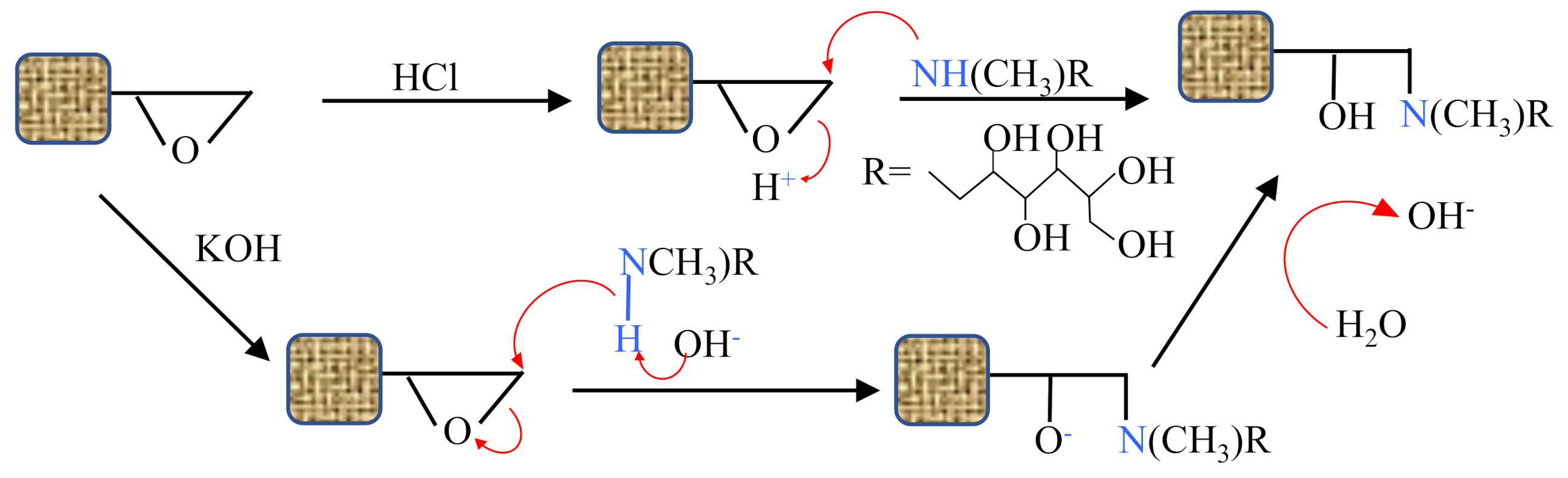
3.4. Accelerating the Attachment Reaction by Base and Acid Catalysts
3.5. Reaction Kinetics
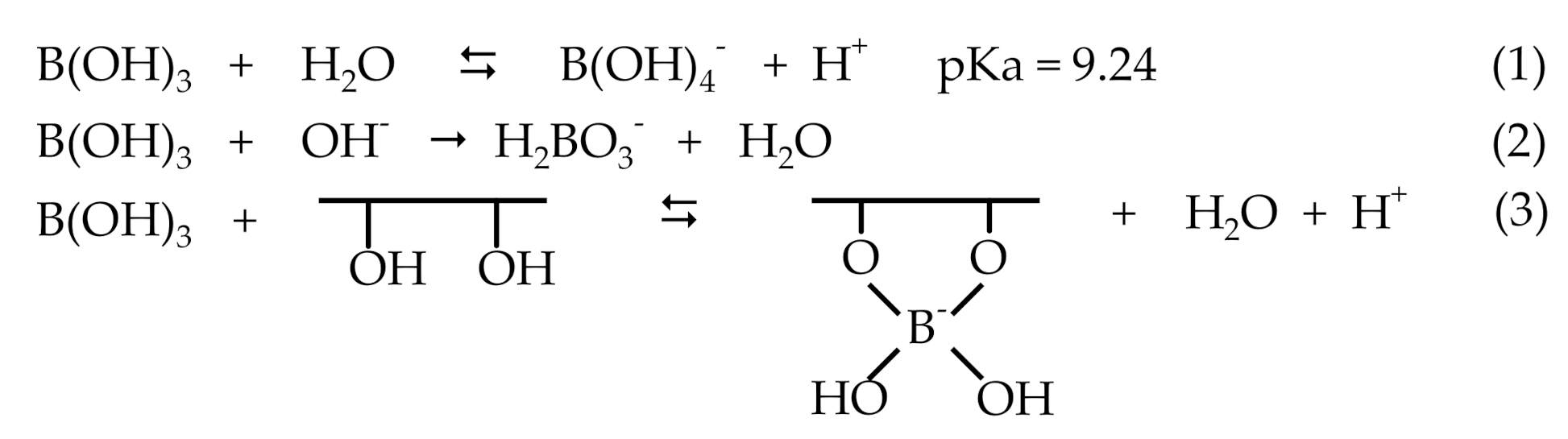
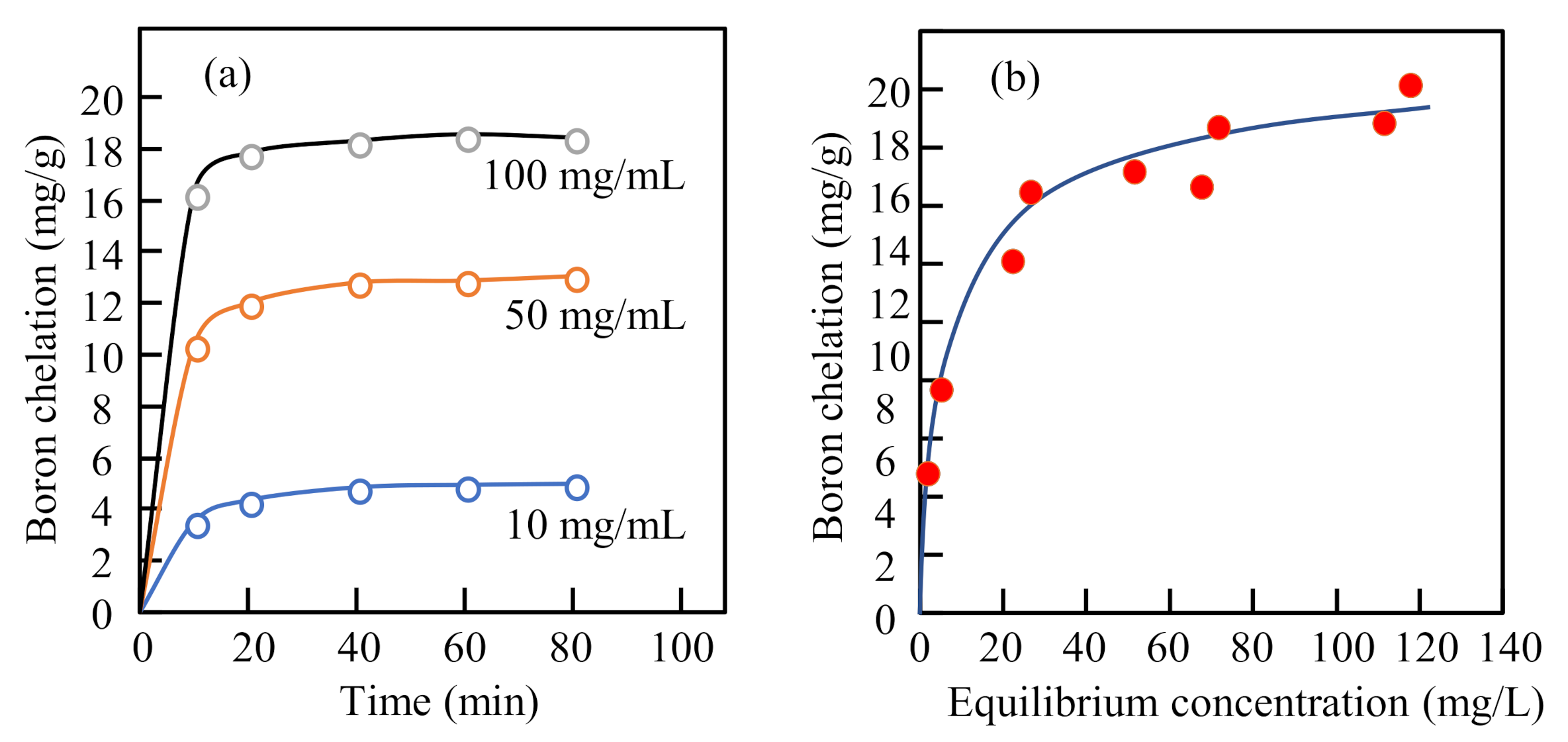
3.6. Boron Chelation in Aqueous Solution
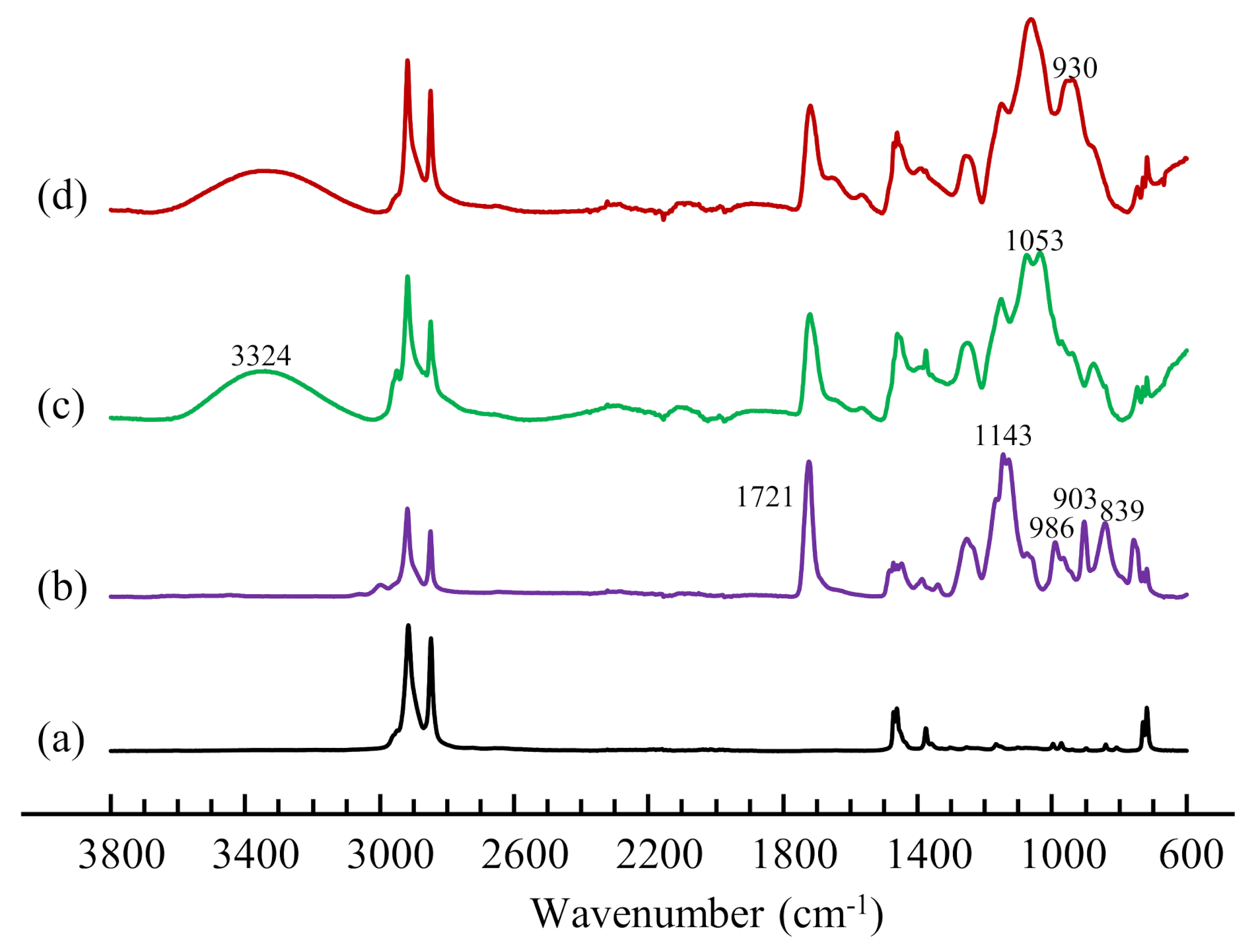
3.7. FTIR Characterization
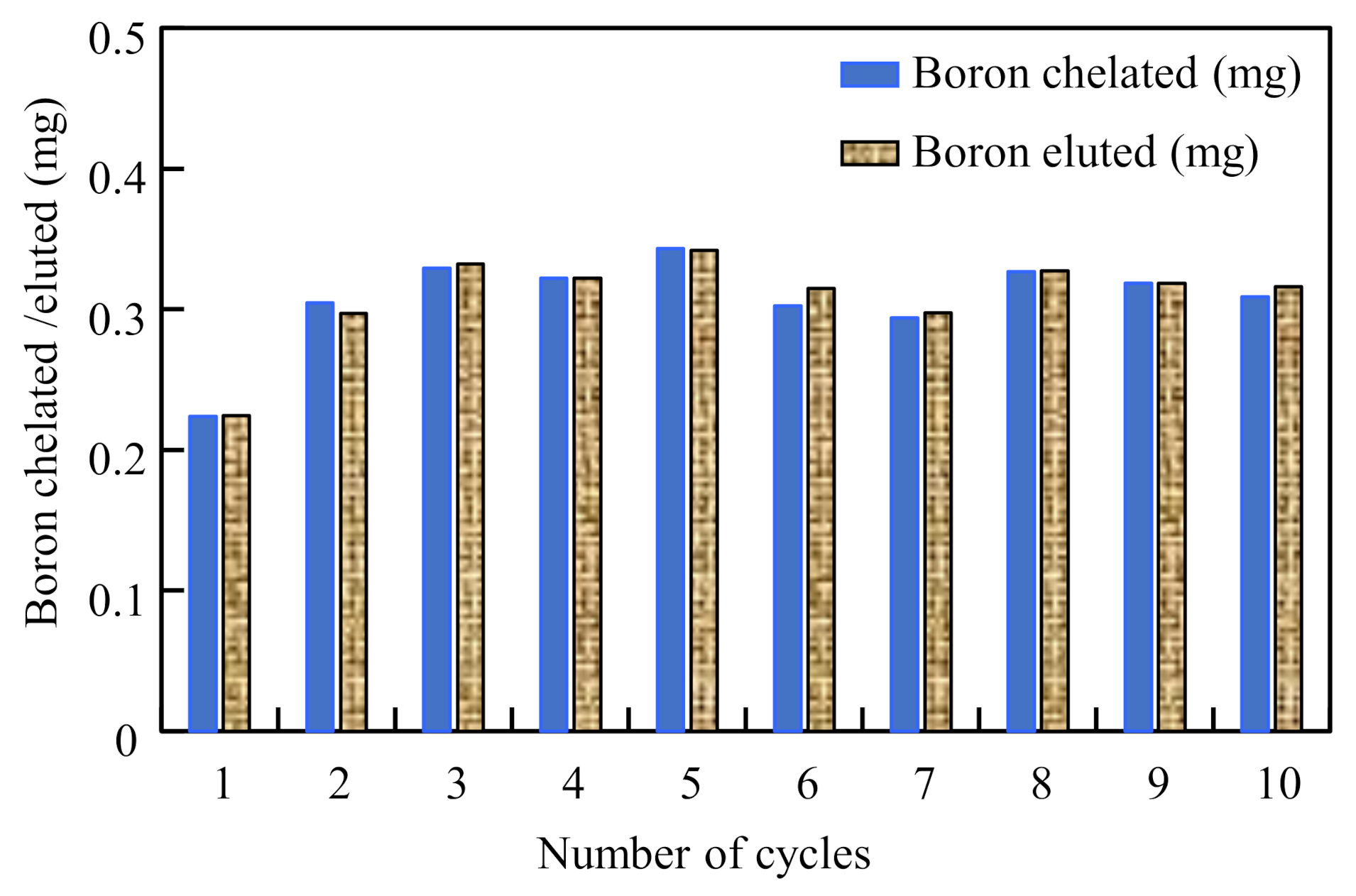
3.8. Stability of the Chelating Fabric
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kochkodan, V.; Darwish, N.; Hilal, N. The Chemistry of Boron in Water; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Brdar-Jokanović, M. Boron toxicity and deficiency in agricultural plants. Int. J. Mol. Sci. 2020, 21, 1424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolska, J.; Bryjak, M. Methods for boron removal from aqueous solutions—A review. Desalination 2013, 310, 18–24. [Google Scholar] [CrossRef]
- Hilal, N.; Kim, G.; Somerfield, C. Boron removal from saline water, a comprehensive review. Desalination 2011, 273, 23–35. [Google Scholar] [CrossRef]
- Guan, Z.; Lv, J.; Bai, P.; Guo, X. Boron removal from aqueous solutions by adsorption—A review. Desalination 2016, 383, 29–37. [Google Scholar] [CrossRef]
- Xu, Y.; Jiang, J. Technologies for boron removal. Indus. Chem. Res. 2008, 47, 16–24. [Google Scholar] [CrossRef]
- Samatya, S.; Tuncel, S.; Kabay, N. Boron removal from RO permeate of geothermal water by monodisperse poly (vinylbenzyl chloride-co-divinylbenzene) beads containing N-methyl-D-glucamine. Desalination 2015, 364, 75–81. [Google Scholar] [CrossRef]
- Tu, K.; Long, D.; Allan, R. Boron removal by reverse osmosis membranes in seawater desalination applications. Separ. Purif. Tech. 2010, 75, 87–101. [Google Scholar] [CrossRef]
- Isaacs-Paez, E.; Leyva-Ramos, R.; Jacobo-Azuara, A.; Martinez-Rosales, J.; Flores-Cano, J. Adsorption of boron on calcined AlMg layered double hydroxide from aqueous solutions. Mechanism and effect of operating conditions. Chem. Eng. J. 2014, 245, 248–257. [Google Scholar] [CrossRef]
- Bıçak, N.; Özbelge, H.; Yilmaz, L.; Senkal, B. Crosslinked polymer gels for boron extraction derived from N-glucidol-N-methyl-2-hydroxypropyl methacrylate. Macromol. Chem. Phys. 2000, 201, 577–584. [Google Scholar] [CrossRef]
- Köse, D.A.; Zümreoglu-Karan, B. Complexation of boric acid with vitamin C. New J. Chem. 2009, 33, 1874–1881. [Google Scholar] [CrossRef]
- Manjunatha, R.; Gerbec, J.; Shimizu, F.; Chmelka, B. Nanoscale surface compositions and structures influence boron adsorption properties of anion exchange resins. Langmuir 2019, 35, 15661–15673. [Google Scholar] [CrossRef]
- Kamcev, J.; Taylor, M.; Shin, D.; Jarenwattananon, N.; Colwell, K.; Long, J. Functionalized porous aromatic frameworks as high-performance adsorbents for the rapid removal of boric acid from water. Adv. Mater. 2019, 31, 1808027. [Google Scholar] [CrossRef]
- Wang, L.; Qi, T.; Gao, Z.; Zhang, Y.; Chu, J. Synthesis of N-methylglucamine modified macroporous poly(GMA-co-TRIM) and its performance as a boron sorbent. React. Funct. Polym. 2007, 67, 202–209. [Google Scholar] [CrossRef]
- Ting, T.; Nasef, M.; Hashim, K. Tuning N-methyl-D-glucamine density in a new radiation grafted poly (vinyl benzyl chloride)/nylon-6 fibrous boron-selective adsorbent using the response surface method. RSC Adv. 2015, 5, 37869–37880. [Google Scholar] [CrossRef]
- Wei, Y.T.; Zheng, Y.M.; Chen, J.P. Functionalization of regenerated cellulose membrane via surface-initiated atom transfer radical polymerization for boron removal from aqueous solution. Langmuir 2011, 27, 6018–6025. [Google Scholar] [CrossRef]
- Kang, J.; Tang, Y.; Gao, S.; Liu, L. One-dimensional controllable crosslinked polymers grafted with N-methyl-D-glucamine for effective boron adsorption. New J. Chem. 2018, 42, 11334–11340. [Google Scholar] [CrossRef]
- Sabarudin, A.; Oshita, K.; Oshima, M.; Motomizu, S. Synthesis of cross-linked chitosan possessing N-methyl-d-glucamine moiety (CCTS-NMDG) for adsorption/concentration of boron in water samples and its accurate measurement by ICP-MS and ICP-AES. Talanta 2005, 66, 136–144. [Google Scholar] [CrossRef] [Green Version]
- Xiao, B.; Zhang, Y.; Wang, Y.; Jiang, G.; Liang, M.; Chen, X.; Long, G. A fractal model for Kozeny–Carman constant and dimensionless permeability of fibrous porous media with roughened surfaces. Fractals 2019, 27, 1950116. [Google Scholar] [CrossRef]
- Gu, J.; Xiao, P.; Chen, P.; Zhang, L.; Wang, H.; Dai, L.; Song, L.; Huang, Y.; Zhang, J.; Chen, T. Functionalization of biodegradable PLA nonwoven fabric as superoleophilic and superhydrophobic material for efficient oil absorption and oil/water separation. ACS Appl. Mater. Interfaces 2017, 9, 5968–5973. [Google Scholar] [CrossRef]
- Recepoğlu, Y.; Kabay, N.; Ipek, I.; Arda, M.; Yüksel, M.; Yoshizuka, K.; Nishihama, S. Packed bed column dynamic study for boron removal from geothermal brine by a chelating fiber and breakthrough curve analysis by using mathematical models. Desalination 2018, 437, 1–6. [Google Scholar] [CrossRef]
- Nallappan, M.; Nasef, M.; Ting, T.; Ahmad, A. An optimized covalent Immobilization of glucamine on electrospun nanofibrous poly (vinylidene fluoride) sheets grafted with oxirane groups for higher boron adsorption. Fibers Polym. 2018, 19, 1694–1705. [Google Scholar] [CrossRef]
- Nasef, M.; Güven, O. Radiation-grafted copolymers for separation and purification purposes, Status, challenges and future directions. Prog. Polym. Sci. 2012, 37, 1597–1656. [Google Scholar] [CrossRef]
- Hayashi, N.; Chen, J.; Seko, N. Nitrogen-containing fabric adsorbents prepared by radiation grafting for removal of chromium from wastewater. Polymers 2018, 10, 744. [Google Scholar] [CrossRef] [Green Version]
- Kodama, Y.; Barsbay, M.; Güven, O. Poly (2-hydroxyethyl methacrylate) (PHEMA) grafted polyethylene/polypropylene (PE/PP) nonwoven fabric by γ-initiation, Synthesis, characterization and benefits of RAFT mediation. Radia. Phys. Chem. 2014, 105, 31–38. [Google Scholar] [CrossRef]
- Dong, Z.; Zhao, L. Covalently bonded ionic liquid onto cellulose for fast adsorption and efficient separation of Cr (VI), Batch, column and mechanism investigation. Carbohyd. Polym. 2018, 189, 190–197. [Google Scholar] [CrossRef]
- Muzammil, E.; Khan, A.; Stuparu, M. Post-polymerization modification reactions of poly (glycidyl methacrylate)s. RSC Adv. 2017, 7, 55874–55884. [Google Scholar] [CrossRef] [Green Version]
- Kimmins, S.; Wyman, P.; Cameron, N. Amine-functionalization of glycidyl methacrylate-containing emulsion-templated porous polymers and immobilization of proteinase K for biocatalysis. Polymer 2014, 55, 416–425. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Asano, M.; Yamaki, T.; Yoshida, M. Preparation and characterization of chemically stable polymer electrolyte membranes by radiation-induced graft copolymerization of four monomers into ETFE films. J. Membr. Sci. 2006, 269, 194–204. [Google Scholar] [CrossRef]
- Darwish, N.B.; Kochkodan, V.; Hilal, N. Microfiltration of micro-sized suspensions of boron-selective resin with PVDF membranes. Desalination 2017, 403, 161–171. [Google Scholar] [CrossRef] [Green Version]
- Ehlers, J.; Rondan, N.; Huynh, L.; Pham, H.; Marks, M.; Truong, T. Theoretical study on mechanisms of the epoxy− amine curing reaction. Macromolecules 2007, 40, 4370–4377. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Shabanian, M.; Khaleghi, M.; Paran, S.; Ghiyasi, S.; Vahabi, H.; Formela, K.; Puglia, D.; Saeb, M. Acid-aided epoxy-amine curing reaction as reflected in epoxy/Fe3O4 nanocomposites, Chemistry, mechanism, and fracture behavior. Prog. Org. Coat. 2018, 125, 384–392. [Google Scholar] [CrossRef]
- Mijovic, J.; Fishbain, A.; Wijaya, J. Mechanistic modeling of epoxy-amine kinetics. 1. Model compound study. Macromolecules 1992, 25, 979–985. [Google Scholar] [CrossRef]
- Ikeda, K.; Umeno, D.; Saito, K.; Koide, F.; Miyata, E.; Sugo, T. Removal of boron using nylon-based chelating fibers. Indus. Eng. Chem. Res. 2011, 50, 5727–5732. [Google Scholar] [CrossRef]
- Seko, N.; Ninh, N.; Tamada, M. Emulsion grafting of glycidyl methacrylate onto polyethylene fiber. Rad. Phys. Chem. 2010, 79, 22–26. [Google Scholar] [CrossRef]
- Hoshina, H.; Seko, N.; Ueki, Y.; Tamada, M. Synthesis of graft adsorbent with N-methyl-D-glucamine for boron adsorption. J. Ion Exch. 2007, 18, 236–239. [Google Scholar] [CrossRef]
- Forster, A.; Tsinas, Z.; Al-sheikhly, M. Effect of irradiation and detection of long-lived polyenyl radicals in highly crystalline ultra-high molar mass polyethylene (UHMMPE) fibers. Polymers 2019, 11, 924. [Google Scholar] [CrossRef] [Green Version]
- Mora, A.S.; Tayouo, R.; Boutevin, B.; David, G.; Caillol, S. A perspective approach on the amine reactivity and the hydrogen bonds effect on epoxy-amine systems. Eur. Polym. J. 2020, 123, 109460. [Google Scholar] [CrossRef]
- Mun, D.; Huynh, N.; Shin, S.; Kim, Y.; Kim, S.; Shul, Y.; Cho, J. Facile isomerization of glucose into fructose using anion-exchange resins in organic solvents and application to direct conversion of glucose into furan compounds. Res. Chem. Intermed. 2017, 43, 5495–5506. [Google Scholar] [CrossRef]
- Sar, P.; Ghosh, A.; Scarso, A.; Saha, B. Surfactant for better tomorrow, applied aspect of surfactant aggregates from laboratory to industry. Res. Chem. Intermed. 2019, 45, 6021–6041. [Google Scholar] [CrossRef]
- Eddingsaas, N.; Vandervelde, D.; Wennberg, P. Kinetics and products of the acid-catalyzed ring-opening of atmospherically relevant butyl epoxy alcohols. J. Phys. Chem. A 2010, 114, 8106–8113. [Google Scholar] [CrossRef] [Green Version]
- Stropoli, S.; Elrod, M. Assessing the potential for the reactions of epoxides with amines on secondary organic aerosol particles. J. Phys. Chem. A 2015, 119, 10181–10189. [Google Scholar] [CrossRef]
- Innocenzi, P.; Kidchob, T.; Yoko, T. Hybrid organic-inorganic sol-gel materials based on epoxy-amine systems. J. Sol-Gel Sci. Techn. 2005, 35, 225–235. [Google Scholar] [CrossRef]
- Blank, W.; He, Z.; Picci, M. Catalysis of the epoxy-carboxyl reaction. J. Coat. Tech. 2002, 74, 33–41. [Google Scholar] [CrossRef]
- Ting, T.; Nasef, M. Modification of polyethylene-polypropylene fibers by emulsion and solvent radiation grafting systems for boron removal. Fibers. Polym. 2017, 18, 1048–1055. [Google Scholar] [CrossRef]
- Joshi, M.; Chalumot, G.; Kim, Y.; Anderson, J. Synthesis of glucaminium-based ionic liquids and their application in the removal of boron from water. Chem. Commun. 2012, 48, 1410–1412. [Google Scholar] [CrossRef]
- Joshi, M.; Steyer, D.; Anderson, J. Evaluating the complexation behavior and regeneration of boron selective glucaminium-based ionic liquids when used as extraction solvents. Anal. Chim. Acta 2012, 740, 66–73. [Google Scholar] [CrossRef]
- Akan, Z.; Demiroglu, H.; Avcibasi, U.; Oto, G.; Ozdemir, H.; Deniz, S.; Basak, A.S. Complexion of Boric Acid with 2-Deoxy-D-glucose (DG) as a novel boron carrier for BNCT. Med. Sci. Discov. 2014, 1, 65–71. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Guo, X.; Bai, P. Removal technology of boron dissolved in aqueous solutions–a review. Coll. Surf. A Phys. Eng. Asp. 2014, 444, 338–344. [Google Scholar] [CrossRef]

| Grafting Conditions | Grafting Results | |||||
|---|---|---|---|---|---|---|
| Starting fabric | Preirradiation (kGy) | Monomer * | Temp. (°C) | Time (h) | Grafting (%) | Epoxy density (mmol/g) |
| PE/PP | 24.0 | GMA | 40 | 4.0 | 179.5 | 4.52 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoshina, H.; Chen, J.; Amada, H.; Seko, N. Chelating Fabrics Prepared by an Organic Solvent-Free Process for Boron Removal from Water. Polymers 2021, 13, 1163. https://doi.org/10.3390/polym13071163
Hoshina H, Chen J, Amada H, Seko N. Chelating Fabrics Prepared by an Organic Solvent-Free Process for Boron Removal from Water. Polymers. 2021; 13(7):1163. https://doi.org/10.3390/polym13071163
Chicago/Turabian StyleHoshina, Hiroyuki, Jinhua Chen, Haruyo Amada, and Noriaki Seko. 2021. "Chelating Fabrics Prepared by an Organic Solvent-Free Process for Boron Removal from Water" Polymers 13, no. 7: 1163. https://doi.org/10.3390/polym13071163
APA StyleHoshina, H., Chen, J., Amada, H., & Seko, N. (2021). Chelating Fabrics Prepared by an Organic Solvent-Free Process for Boron Removal from Water. Polymers, 13(7), 1163. https://doi.org/10.3390/polym13071163









