Comparison of Corn Stover Pretreatments with Lewis Acid Catalyzed Choline Chloride, Glycerol and Choline Chloride-Glycerol Deep Eutectic Solvent
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Pretreatment Solutions
2.3. Pretreatment
2.4. Enzymatic Hydrolysis
2.5. Analytic Methods
2.6. Statistical Analysis
3. Results and Discussion
3.1. Effect of Different Pretreatments on CS Compositions
3.2. Enzymatic Hydrolysis of Differently Pretreated Solids
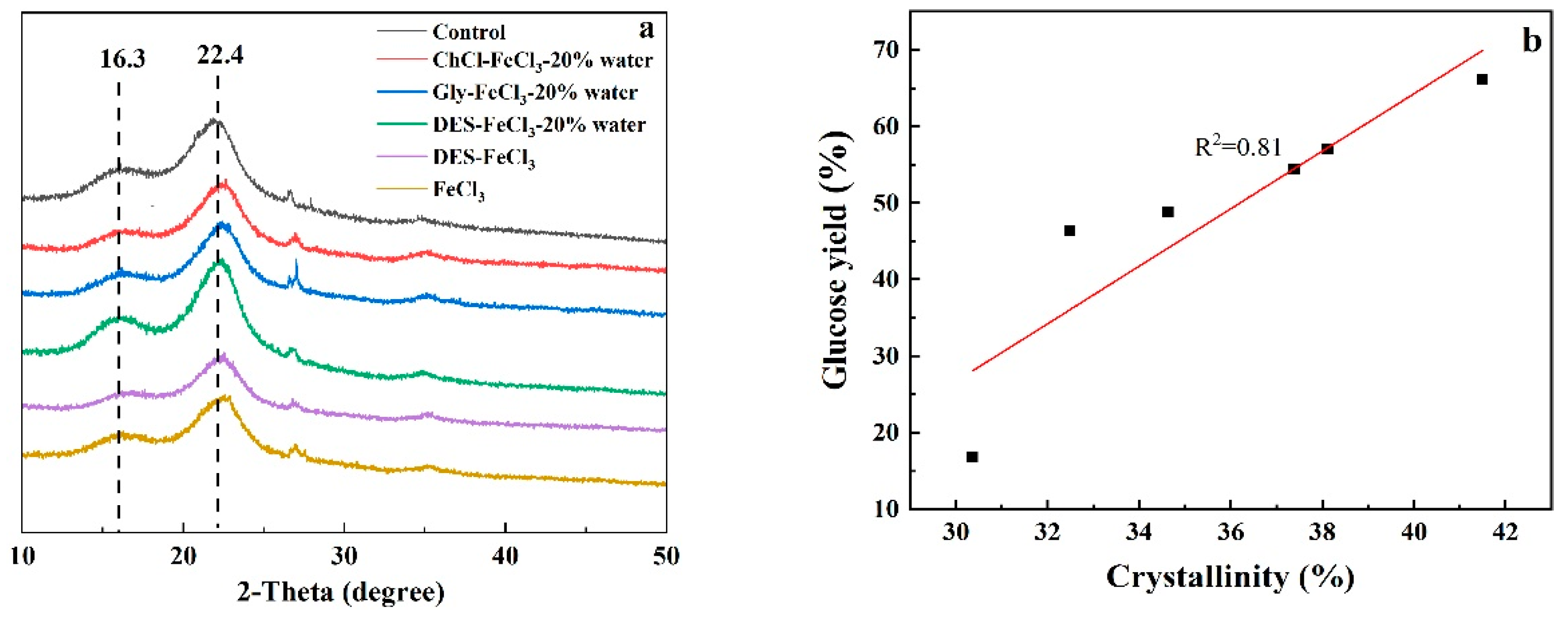
3.3. Characterization of Differently Pretreated Solid Residues
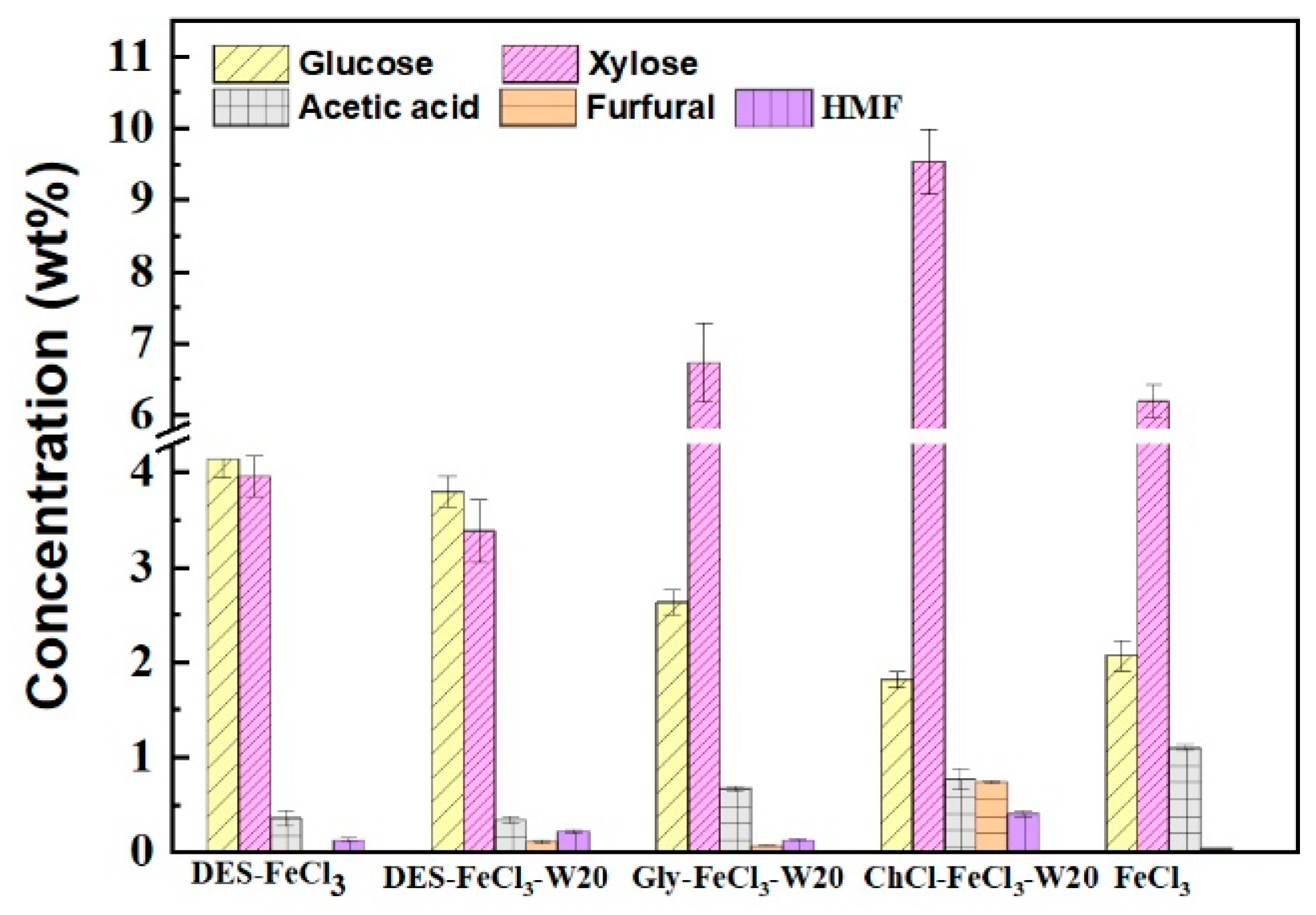
3.4. Monosaccharides and Polysaccharide-derived Inhibitors in Pretreatment Liquids
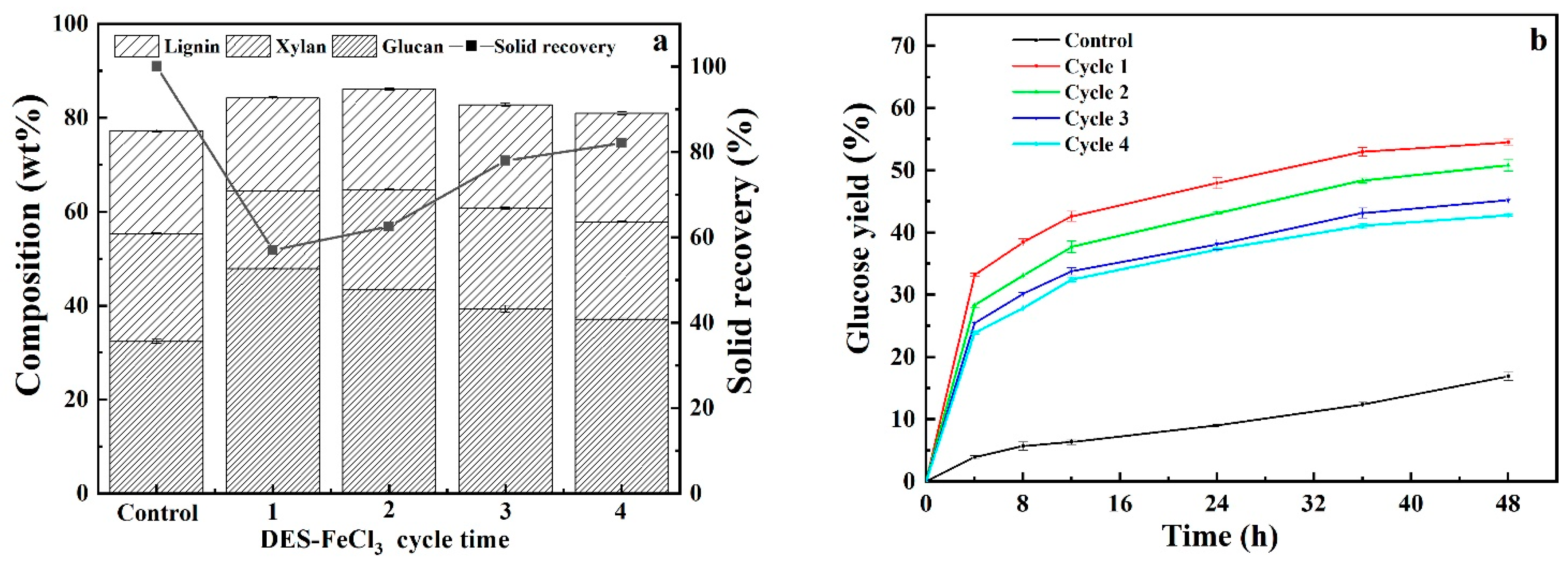
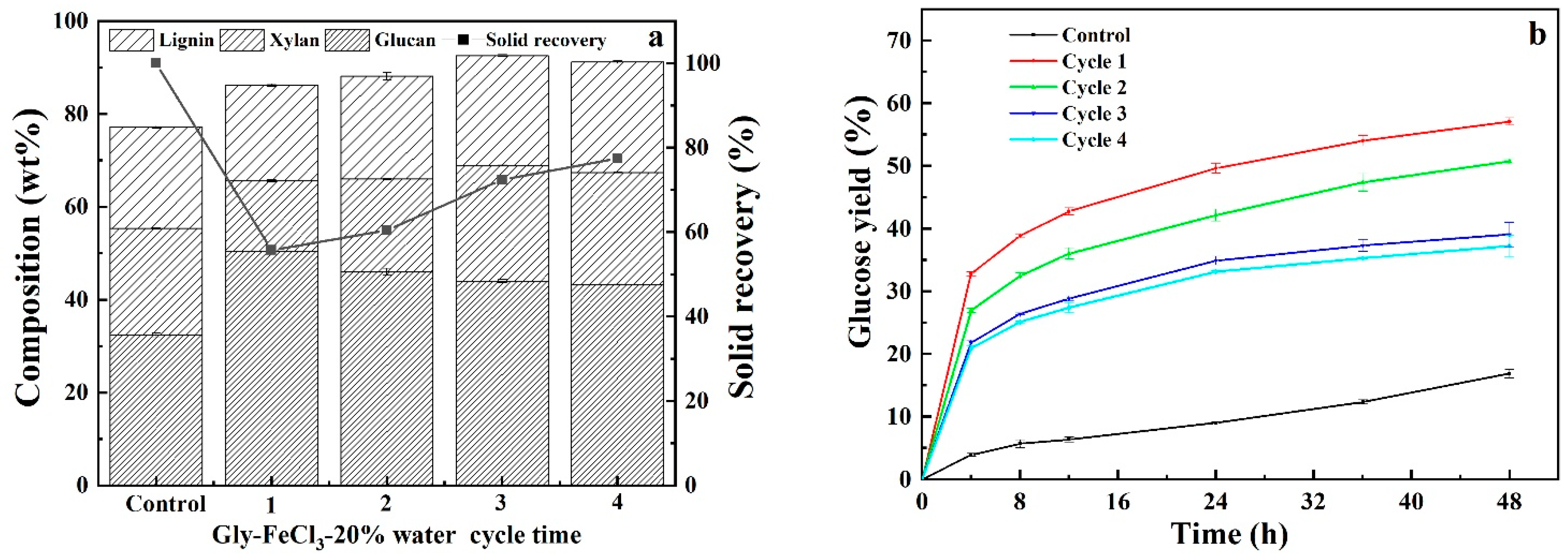
3.5. Recyclability and Reusability of Acidified Solutions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arevalo-Gallegos, A.; Ahmad, Z.; Asgher, M.; Parra-Saldivar, R.; Iqbal, H.M.N. Lignocellulose: A sustainable material to produce value-added products with a zero waste approach—A review. Int. J. Biol. Macromol. 2017, 99, 308–318. [Google Scholar] [CrossRef]
- Jahirul, M.I.; Rasul, M.G.; Chowdhury, A.A.; Ashwath, N. Biofuels Production through Biomass Pyrolysis —A Technological Review. Energ. 2012, 5, 4952–5001. [Google Scholar] [CrossRef]
- Iqbal, H.M.N.; Kyazze, G.; Keshavarz, T. Advances in the valorization of lignocellulosic materials by biotechnology: An overview. BioResources 2013, 8, 3157–3176. [Google Scholar] [CrossRef]
- Nguyen, T.Y.; Cai, C.M.; Kumar, R.; Wyman, C.E. Overcoming factors limiting high-solids fermentation of lignocellulosic biomass to ethanol. Proc. Natl. Acad. Sci. USA 2017, 114, 11673–11678. [Google Scholar] [CrossRef]
- Taherzadeh, M.J.; Karimi, K. Pretreatment of Lignocellulosic Wastes to Improve Ethanol and Biogas Production: A Review. Int. J. Mol. Sci. 2008, 9, 1621–1651. [Google Scholar] [CrossRef]
- Kucharska, K.; Rybarczyk, P.; Hołowacz, I.; Łukajtis, R.; Glinka, M.; Kamiński, M. Pretreatment of Lignocellulosic Materials as Substrates for Fermentation Processes. Molecules 2018, 23, 2937. [Google Scholar] [CrossRef]
- Rastogi, M.; Shrivastava, S. Recent advances in second generation bioethanol production: An insight to pretreatment, saccharification and fermentation processes. Renew. Sust. Energ. Rev. 2017, 80, 330–340. [Google Scholar] [CrossRef]
- Kalhor, P.; Ghandi, K. Deep Eutectic Solvents for Pretreatment, Extraction, and Catalysis of Biomass and Food Waste. Molecules 2019, 24, 4012. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Mu, T. Application of deep eutectic solvents in biomass pretreatment and conversion. Green Energy Environ. 2019, 4, 95–115. [Google Scholar] [CrossRef]
- Zhang, Q.; Vigier, K.D.O.; Royer, S.; Jerome, F. Deep eutectic solvents: Syntheses, properties and applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep eutectic solvents (DESs) and their applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef]
- Grillo, G.; Calcio Gaudino, E.; Rosa, R.; Leonelli, C.; Timonina, A.; Grygiškis, S.; Tabasso, S.; Cravotto, G. Green Deep Eutectic Solvents for Microwave-Assisted Biomass Delignification and Valorisation. Molecules 2021, 26, 798. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Huang, C.; Zhao, Y.; Zheng, C.; Su, H.; Zhang, L.; Luo, W.; Zhao, H.; Wang, S.; Huang, L.-J. Effect of Choline-Based Deep Eutectic Solvent Pretreatment on the Structure of Cellulose and Lignin in Bagasse. Processes 2021, 9, 384. [Google Scholar] [CrossRef]
- Satlewal, A.; Agrawal, R.; Bhagia, S.; Sangoro, J.; Ragauskas, A.J. Natural deep eutectic solvents for lignocellulosic biomass pretreatment: Recent developments, challenges and novel opportunities. Biotechnol. Adv. 2018, 36, 2032–2050. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Peng, J.; Kong, Y.; Liu, Y.; Su, Z.; Li, B.; Song, X.; Liu, S.; Tian, W. Key process parameters for deep eutectic solvents pretreatment of lignocellulosic biomass materials: A review. Bioresour. Technol. 2020, 310, 123416. [Google Scholar] [CrossRef] [PubMed]
- Procentese, A.; Raganati, F.; Olivieri, G.; Russo, M.E.; Rehmann, L.; Marzocchella, A. Low-energy biomass pretreatment with deep eutectic solvents for bio-butanol production. Bioresour. Technol. 2017, 243, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Thomsen, M.H.; Frankær, C.G.; Brudecki, G.P.; Schmidt, J.E.; AlNashef, I.M. Reviving pretreatment effectiveness of deep eutectic solvents on lignocellulosic date palm residues by prior recalcitrance reduction. Ind. Eng. Chem. Res. 2017, 56, 3167–3174. [Google Scholar] [CrossRef]
- Procentese, A.; Johnson, E.; Orr, V.; Campanile, A.G.; Wood, J.A.; Marzocchella, A.; Rehmann, L. Deep eutectic solvent pretreatment and subsequent saccharification of corncob. Bioresour. Technol. 2015, 192, 31–36. [Google Scholar] [CrossRef]
- Alvarez-Vasco, C.; Ma, R.; Quintero, M.; Guo, M.; Geleynse, S.; Ramasamy, K.K.; Wolcott, M.; Zhang, X. Unique low-molecular-weight lignin with high purity extracted from wood by deep eutectic solvents (DES): A source of lignin for valorization. Green Chem. 2016, 18, 5133–5141. [Google Scholar] [CrossRef]
- Chen, Z.; Reznicek, W.D.; Wan, C.X. Deep eutectic solvent pretreatment enabling full utilization of switchgrass. Bioresour. Technol. 2018, 263, 40–48. [Google Scholar] [CrossRef]
- Wang, Z.K.; Li, H.; Lin, X.C.; Tang, L.; Chen, J.J.; Mo, J.W.; Yu, R.S.; Shen, X.J. Novel recyclable deep eutectic solvent boost biomass pretreatment for enzymatic hydrolysis. Bioresour. Technol. 2020, 307, 123237. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.A.; Taherzadeh, M.J. Improving the economy of lignocellulose-based biorefineries with organosolv pretreatment. Bioresour. Technol. 2020, 299, 122695. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Dong, Q.; Fang, Z.; Cong, W.-j.; Miao, Z.-d. High-concentrated substrate enzymatic hydrolysis of pretreated rice straw with glycerol and aluminum chloride at low cellulase loadings. Bioresour. Technol. 2019, 294, 122164. [Google Scholar] [CrossRef] [PubMed]
- Santana, J.C.; Souza Abud, A.K.; Wisniewski, A.; Navickiene, S.; Romão, L.P.C. Optimization of an organosolv method using glycerol with iron catalysts for the pretreatment of water hyacinth. Biomass Bioenerg. 2020, 133, 105454. [Google Scholar] [CrossRef]
- Chen, Z.; Reznicek, W.D.; Wan, C. Aqueous Choline Chloride: A Novel Solvent for Switchgrass Fractionation and Subsequent Hemicellulose Conversion into Furfural. ACS Sustain. Chem. Eng. 2018, 6, 6910–6919. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of structural carbohydrates and lignin in biomass—NREL/TP-510-42618. Lab. Anal. Proced. (LAP) 2008. [Google Scholar]
- Li, Y.; Qi, B.; Wan, Y. Separation of monosaccharides from pretreatment inhibitors by nanofiltration in lignocellulosic hydrolysate: Fouling mitigation by activated carbon adsorption. Biomass Bioenerg. 2020, 136, 105527. [Google Scholar] [CrossRef]
- Ghose, T. Measurement of cellulase activities. Pure Appl. Chem. 1987, 59, 257–268. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.; Martin Jr, A.; Conrad, C. An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Kumar, A.K.; Parikh, B.S.; Pravakar, M. Natural deep eutectic solvent mediated pretreatment of rice straw: Bioanalytical characterization of lignin extract and enzymatic hydrolysis of pretreated biomass residue. Environ. Sci. Pollut. Res. 2016, 23, 9265–9275. [Google Scholar] [CrossRef]
- Tarasov, D.; Leitch, M.; Fatehi, P. Lignin–carbohydrate complexes: Properties, applications, analyses, and methods of extraction: A review. Biotechnol. Biofuels 2018, 11, 1–28. [Google Scholar] [CrossRef]
- Cai, J.; He, Y.; Yu, X.; Banks, S.W.; Yang, Y.; Zhang, X.; Yu, Y.; Liu, R.; Bridgwater, A.V. Review of physicochemical properties and analytical characterization of lignocellulosic biomass. Renew. Sust. Energ. Rev. 2017, 76, 309–322. [Google Scholar] [CrossRef]
- Yoo, C.G.; Meng, X.; Pu, Y.; Ragauskas, A.J. The critical role of lignin in lignocellulosic biomass conversion and recent pretreatment strategies: A comprehensive review. Bioresour. Technol. 2020, 301, 122784. [Google Scholar] [CrossRef] [PubMed]
- Saini, J.K.; Patel, A.K.; Adsul, M.; Singhania, R.R. Cellulase adsorption on lignin: A roadblock for economic hydrolysis of biomass. Renew. Energ. 2016, 98, 29–42. [Google Scholar] [CrossRef]
- Zakaria, M.R.; Hirata, S.; Hassan, M.A. Hydrothermal pretreatment enhanced enzymatic hydrolysis and glucose production from oil palm biomass. Bioresour. Technol. 2015, 176, 142–148. [Google Scholar] [CrossRef] [PubMed]
- El Hage, R.; Brosse, N.; Chrusciel, L.; Sanchez, C.; Sannigrahi, P.; Ragauskas, A. Characterization of milled wood lignin and ethanol organosolv lignin from miscanthus. Polym. Degrad. Stab. 2009, 94, 1632–1638. [Google Scholar] [CrossRef]
- Cai, Q.; Fan, Z.S.; Chen, J.B.; Guo, W.J.; Ma, F.; Sun, S.Q.; Hu, L.M.; Zhou, Q. Dissolving process of bamboo powder analyzed by FT-IR spectroscopy. J. Mol. Struct. 2018, 1171, 639–643. [Google Scholar] [CrossRef]
- Zhang, C.W.; Xia, S.Q.; Ma, P.S. Facile pretreatment of lignocellulosic biomass using deep eutectic solvents. Bioresour. Technol. 2016, 219, 1–5. [Google Scholar] [CrossRef]
- Li, X.; Wei, Y.; Xu, J.; Xu, N.; He, Y. Quantitative visualization of lignocellulose components in transverse sections of moso bamboo based on FTIR macro- and micro-spectroscopy coupled with chemometrics. Biotechnol. Biofuels 2018, 11, 263. [Google Scholar] [CrossRef]
- Zhuang, J.; Li, M.; Pu, Y.; Ragauskas, A.J.; Yoo, C.G. Observation of Potential Contaminants in Processed Biomass Using Fourier Transform Infrared Spectroscopy. Appl. Sci. 2020, 10, 4345. [Google Scholar] [CrossRef]
- Kim, D. Physico-Chemical Conversion of Lignocellulose: Inhibitor Effects and Detoxification Strategies: A Mini Review. Molecules 2018, 23, 309. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Yadav, S.K.; Kumar, J.; Ahluwalia, V. A critical review on current strategies and trends employed for removal of inhibitors and toxic materials generated during biomass pretreatment. Bioresour. Technol. 2020, 299, 122633. [Google Scholar] [CrossRef] [PubMed]
- van der Pol, E.C.; Bakker, R.R.; Baets, P.; Eggink, G. By-products resulting from lignocellulose pretreatment and their inhibitory effect on fermentations for (bio)chemicals and fuels. Appl. Microbiol. Biotechnol. 2014, 98, 9579–9593. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Liu, L.; Li, W.-C.; Zhu, J.-Q.; Li, B.-Z.; Yuan, Y.-J. Evaluation of soluble fraction and enzymatic residual fraction of dilute dry acid, ethylenediamine, and steam explosion pretreated corn stover on the enzymatic hydrolysis of cellulose. Bioresour. Technol. 2016, 209, 172–179. [Google Scholar] [CrossRef]
- Román-Leshkov, Y.; Davis, M.E. Activation of Carbonyl-Containing Molecules with Solid Lewis Acids in Aqueous Media. ACS Catalysis 2011, 1, 1566–1580. [Google Scholar] [CrossRef]
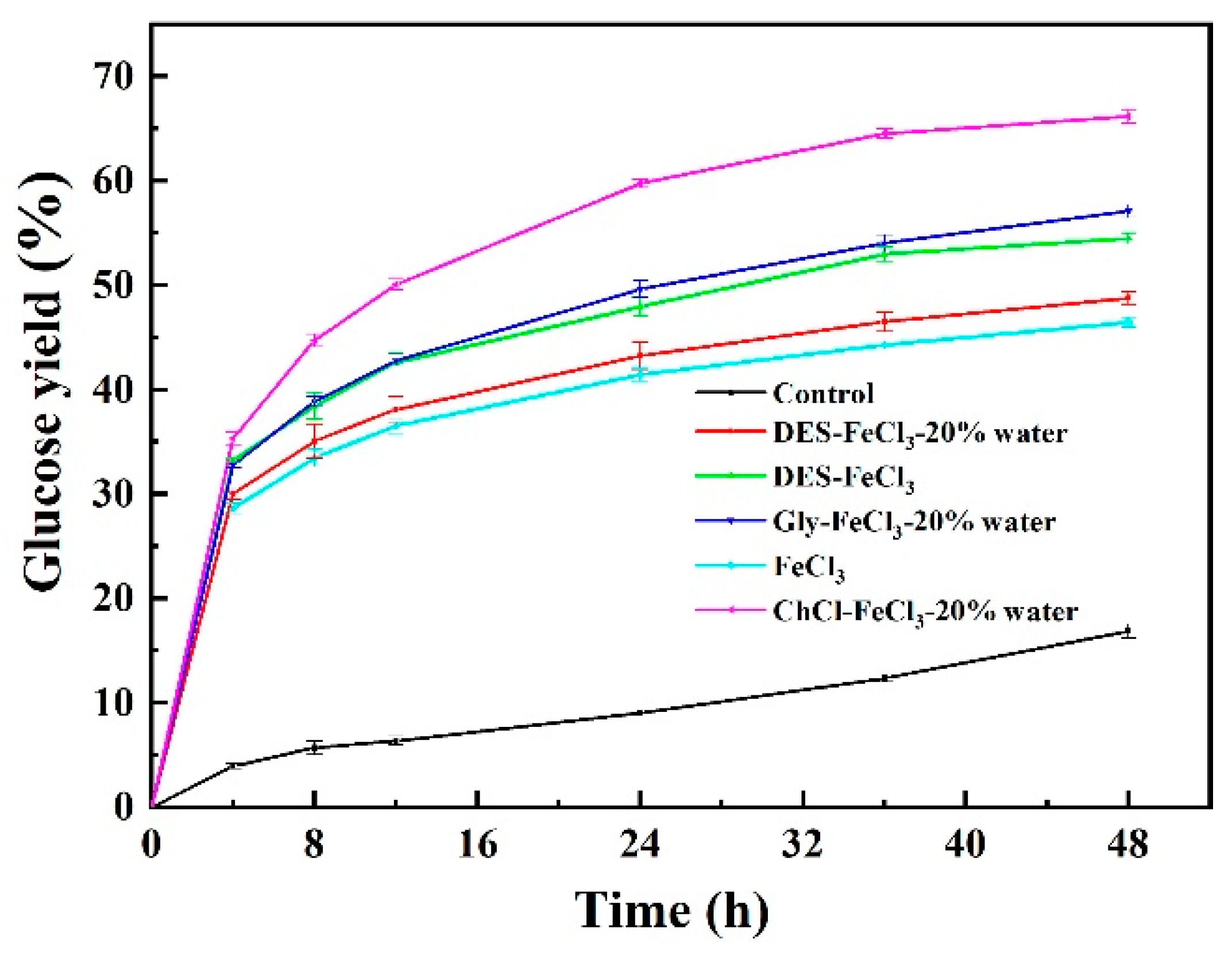
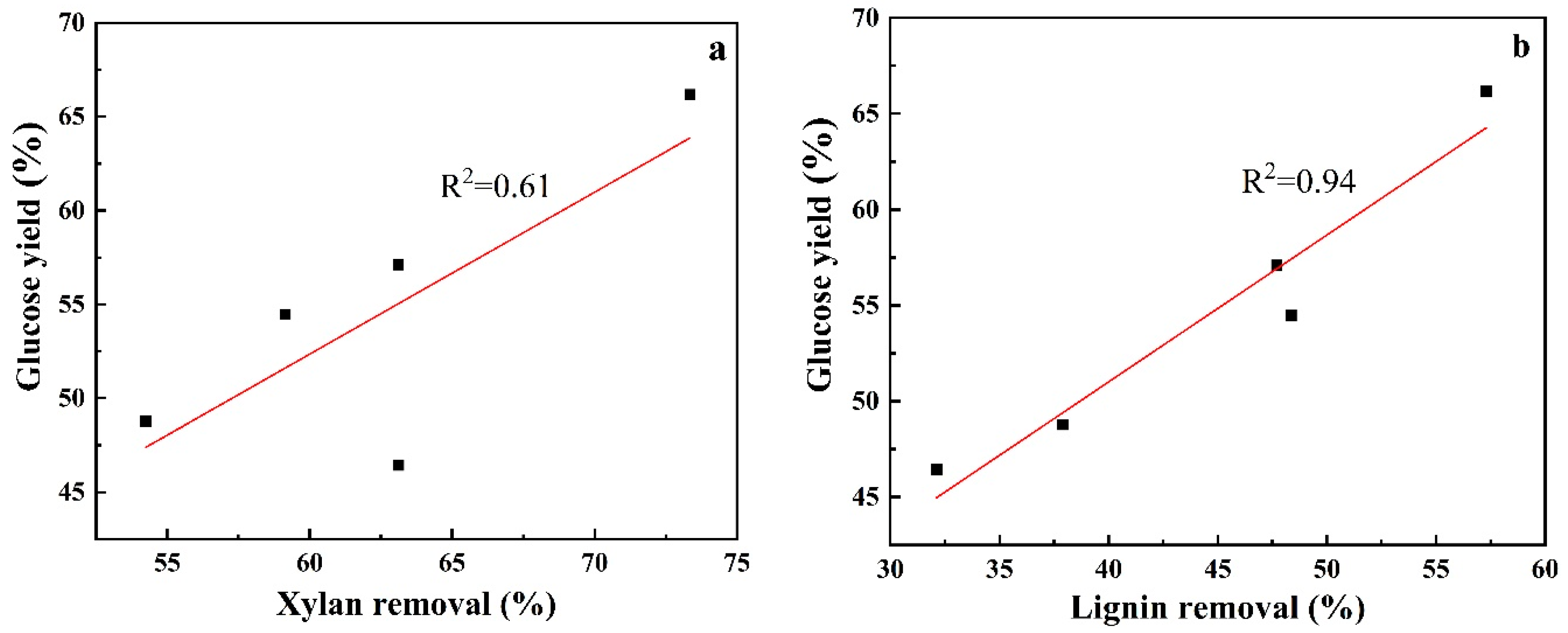
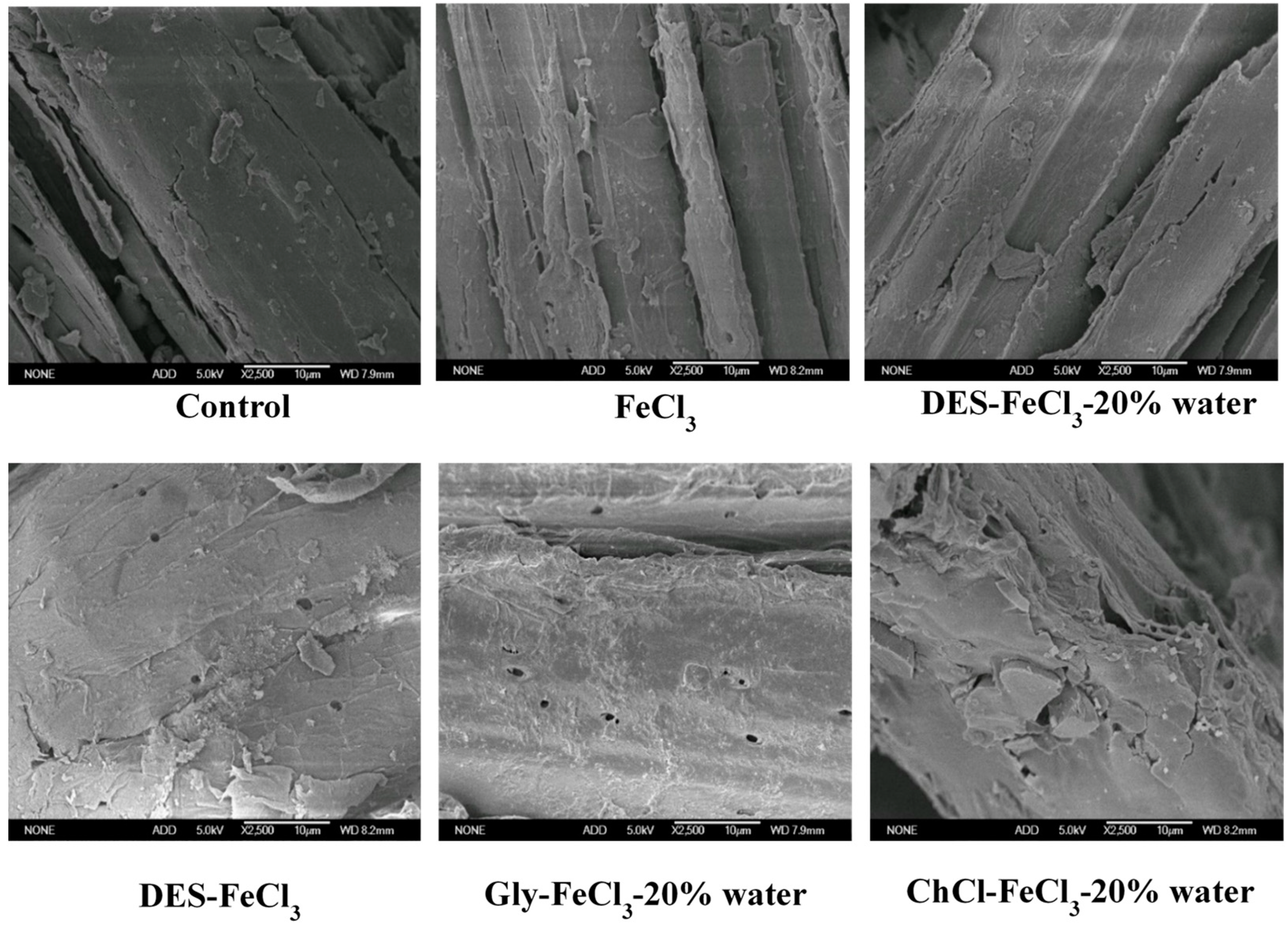
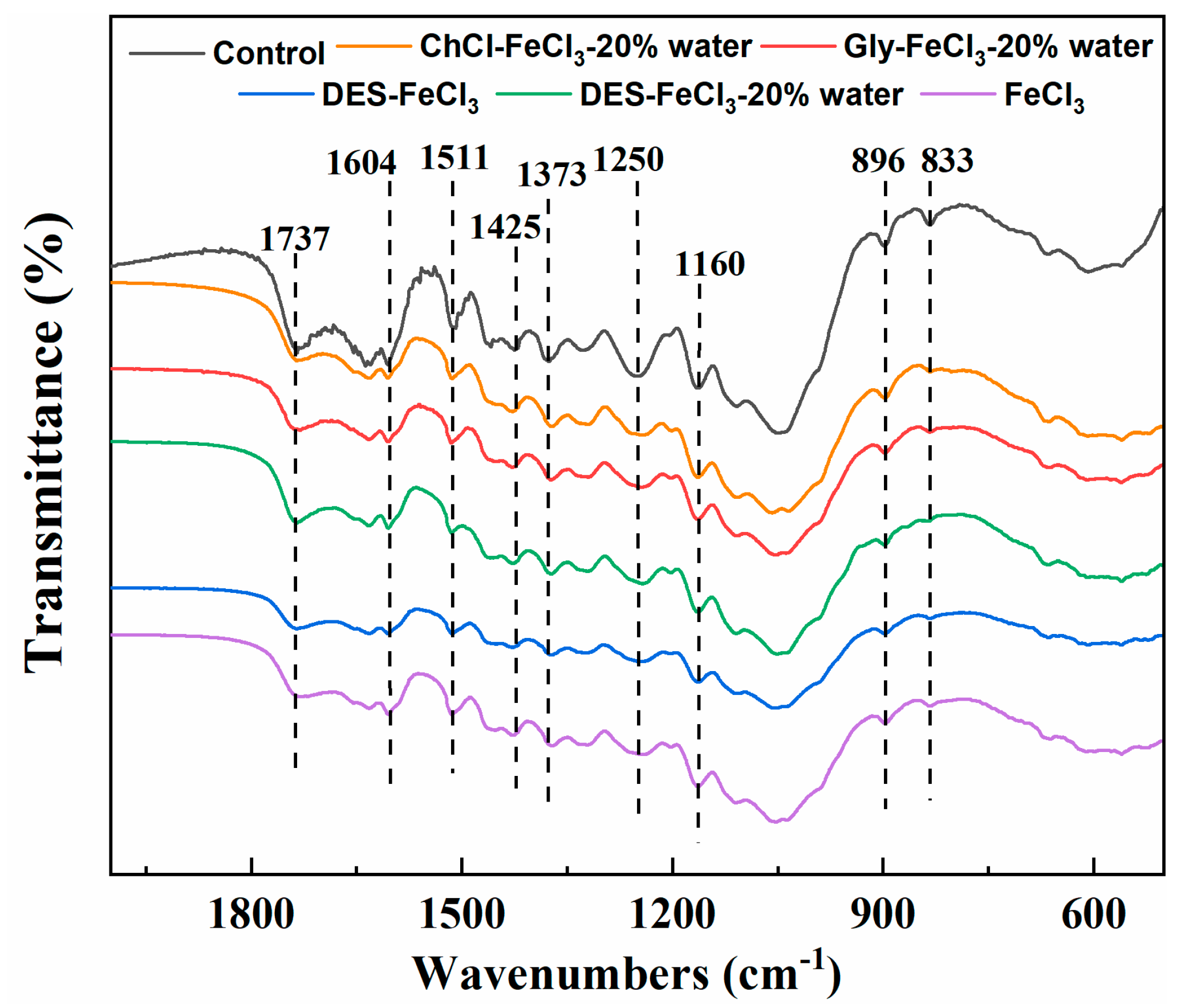
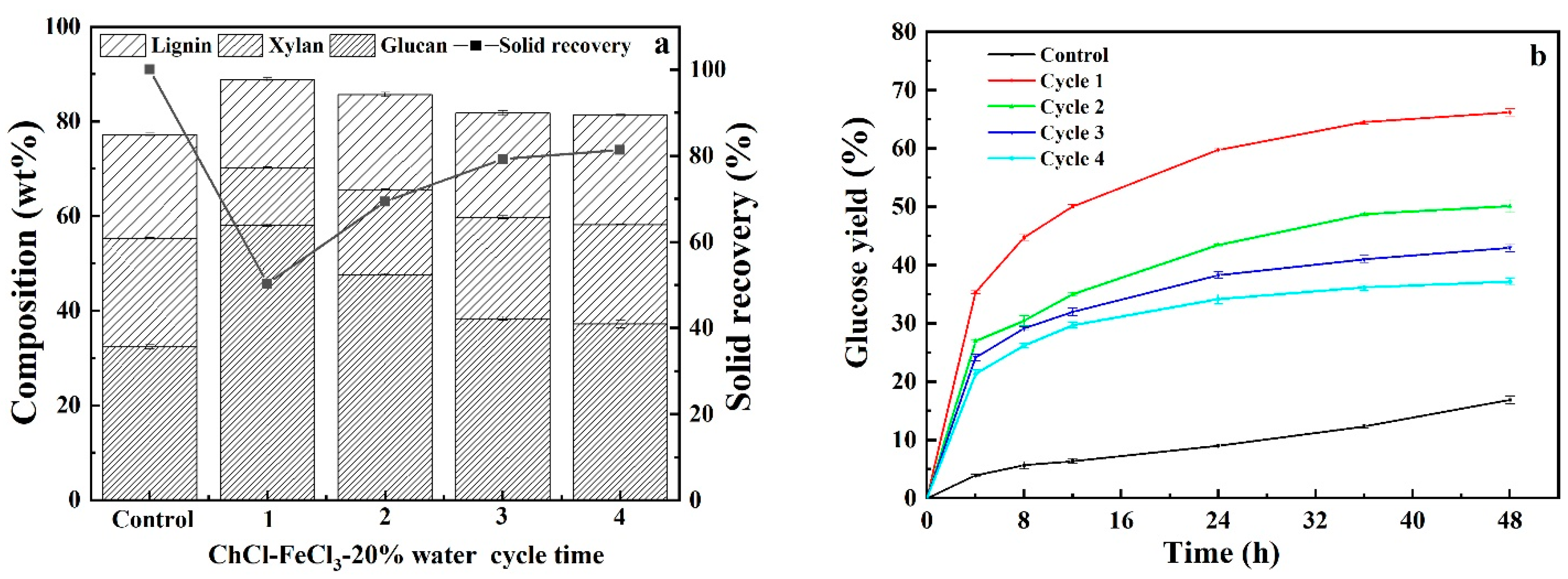
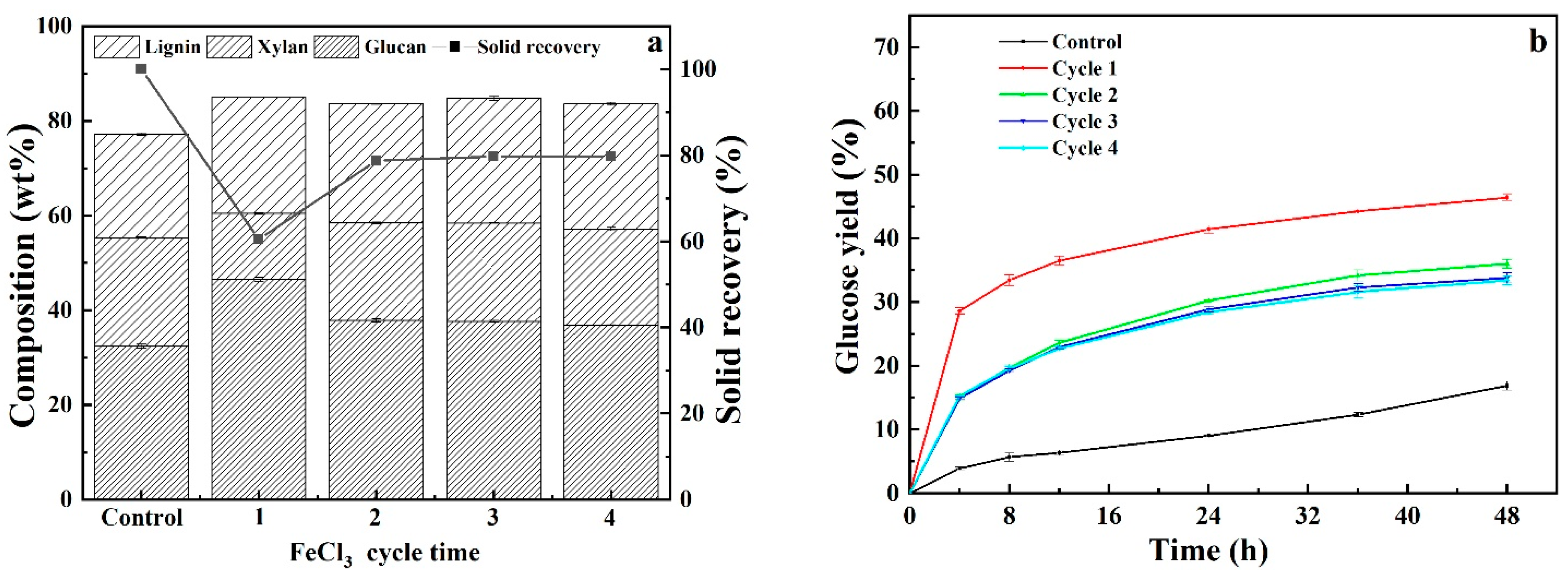
| Pretreatment Solvent | Solid Recovery (%) | Removal (%) | ||
|---|---|---|---|---|
| Glucan | Xylan | Lignin | ||
| ChCl-Gly-FeCl3 | 56.98 ± 0.86 | 15.75 ± 0.62 | 59.15 ± 0.28 | 48.35 ± 0.75 |
| ChCl-Gly-FeCl3-20% water | 60.66 ± 1.53 | 15.41 ± 1.48 | 54.25 ± 1.38 | 37.90 ± 0.75 |
| FeCl3 | 60.51 ± 0.98 | 13.18 ± 0.83 | 63.12 ± 0.26 | 32.12 ± 0.18 |
| Gly-FeCl3-20% water | 55.71 ± 1.12 | 13.23 ± 0.41 | 63.11 ± 0.71 | 47.70 ± 0.69 |
| ChCl-FeCl3-20% water | 50.31 ± 1.34 | 9.83 ± 0.48 | 73.35 ± 0.82 | 57.29 ± 0.83 |
| Pretreatment Solvent | Crystallinity (%) | 48 h Glucose Yield (%) |
|---|---|---|
| Control | 30.36 | 16.87 ± 0.68 |
| FeCl3 | 32.49 | 46.43 ± 0.47 |
| DES-FeCl3-20% water | 34.63 | 48.78 ± 0.63 |
| DES-FeCl3 | 37.38 | 54.47 ± 0.52 |
| Gly-FeCl3-20% water | 38.11 | 57.10 ± 0.60 |
| ChCl-FeCl3-20% water | 41.49 | 66.18 ± 0.66 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Qi, B.; Liang, X.; Luo, J.; Wan, Y. Comparison of Corn Stover Pretreatments with Lewis Acid Catalyzed Choline Chloride, Glycerol and Choline Chloride-Glycerol Deep Eutectic Solvent. Polymers 2021, 13, 1170. https://doi.org/10.3390/polym13071170
Zhu Y, Qi B, Liang X, Luo J, Wan Y. Comparison of Corn Stover Pretreatments with Lewis Acid Catalyzed Choline Chloride, Glycerol and Choline Chloride-Glycerol Deep Eutectic Solvent. Polymers. 2021; 13(7):1170. https://doi.org/10.3390/polym13071170
Chicago/Turabian StyleZhu, Yuan, Benkun Qi, Xinquan Liang, Jianquan Luo, and Yinhua Wan. 2021. "Comparison of Corn Stover Pretreatments with Lewis Acid Catalyzed Choline Chloride, Glycerol and Choline Chloride-Glycerol Deep Eutectic Solvent" Polymers 13, no. 7: 1170. https://doi.org/10.3390/polym13071170
APA StyleZhu, Y., Qi, B., Liang, X., Luo, J., & Wan, Y. (2021). Comparison of Corn Stover Pretreatments with Lewis Acid Catalyzed Choline Chloride, Glycerol and Choline Chloride-Glycerol Deep Eutectic Solvent. Polymers, 13(7), 1170. https://doi.org/10.3390/polym13071170





