Kaolin-Enhanced Superabsorbent Composites: Synthesis, Characterization and Swelling Behaviors
Abstract
:1. Introduction
2. Experimental
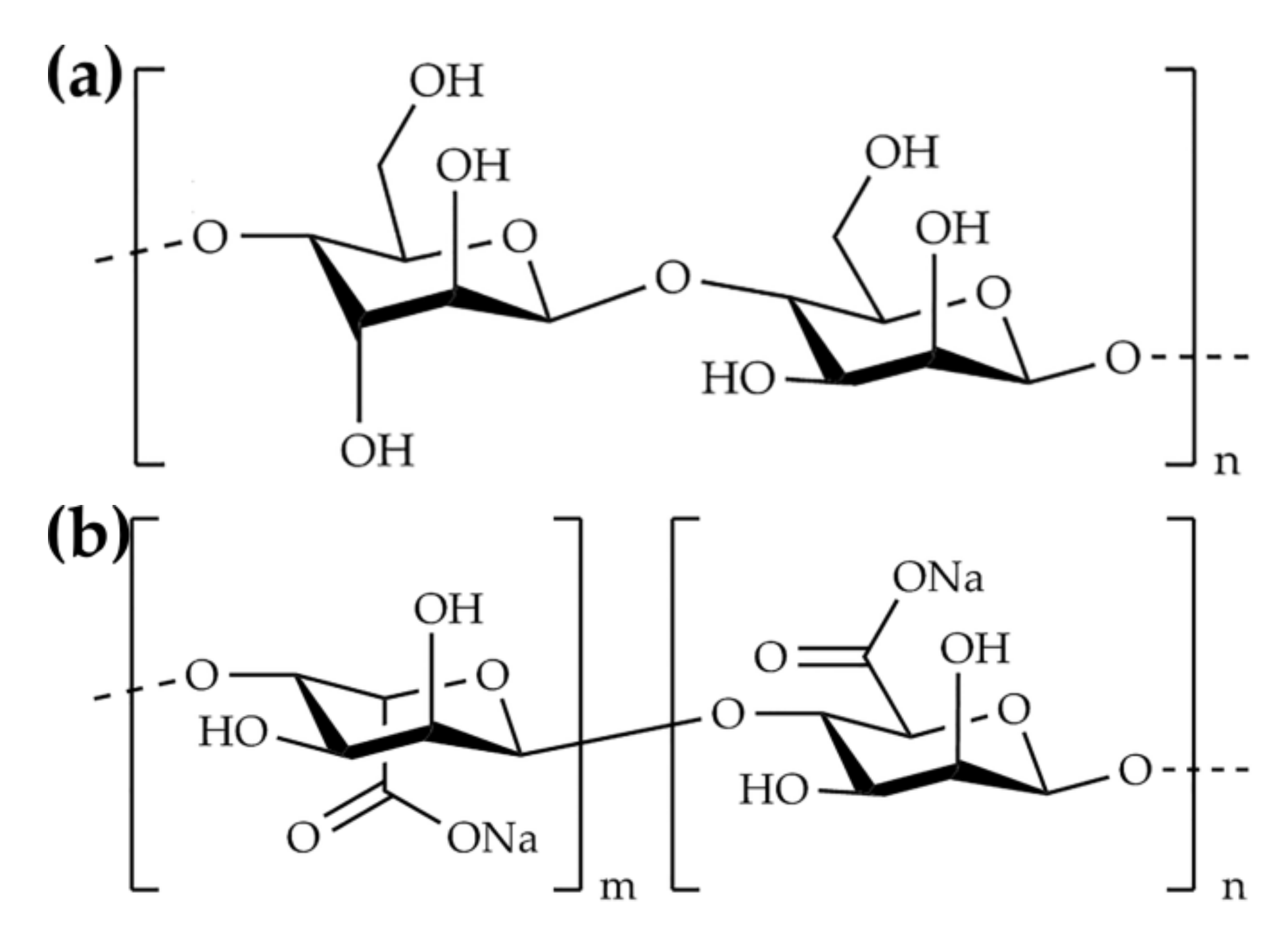
2.1. Materials
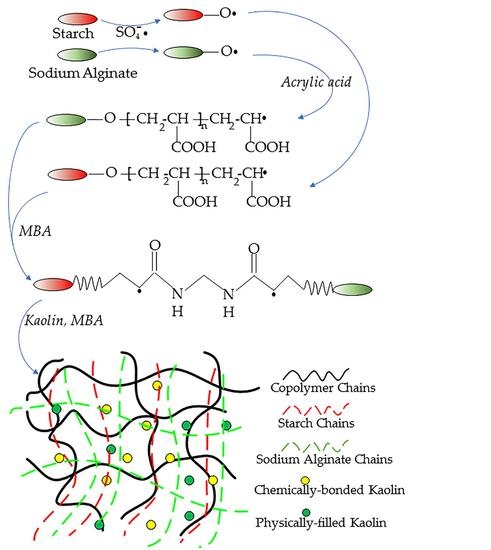
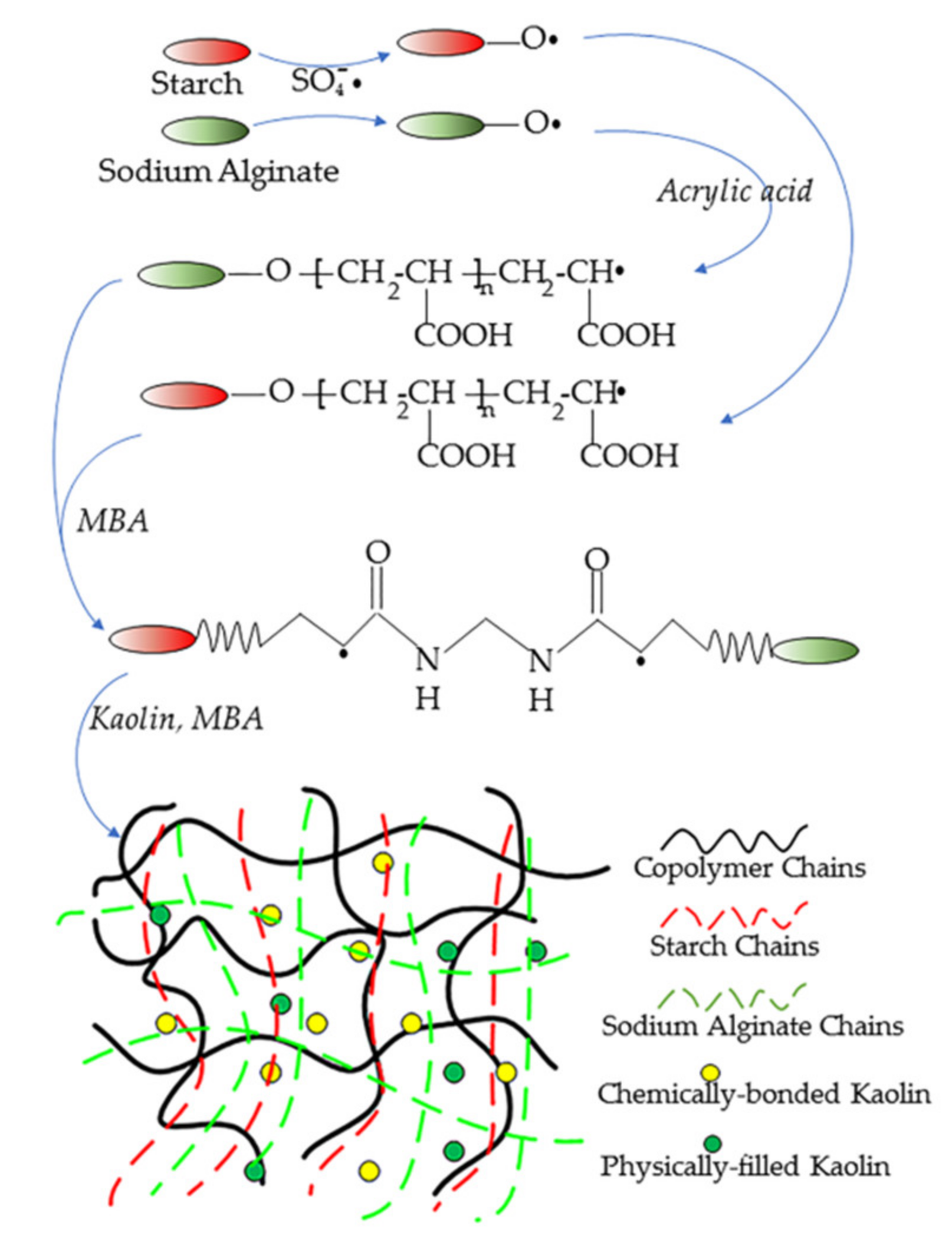
2.2. Synthesis of SAPC
2.3. Characterization
2.4. Performance Test of SAPC
2.4.1. Swelling Ratios
2.4.2. Swelling Kinetics
2.4.3. Swelling in Salt Solutions
2.4.4. Water Retention Measurement
2.4.5. Swelling Measurement in Buffer Solutions
3. Results and Discussion
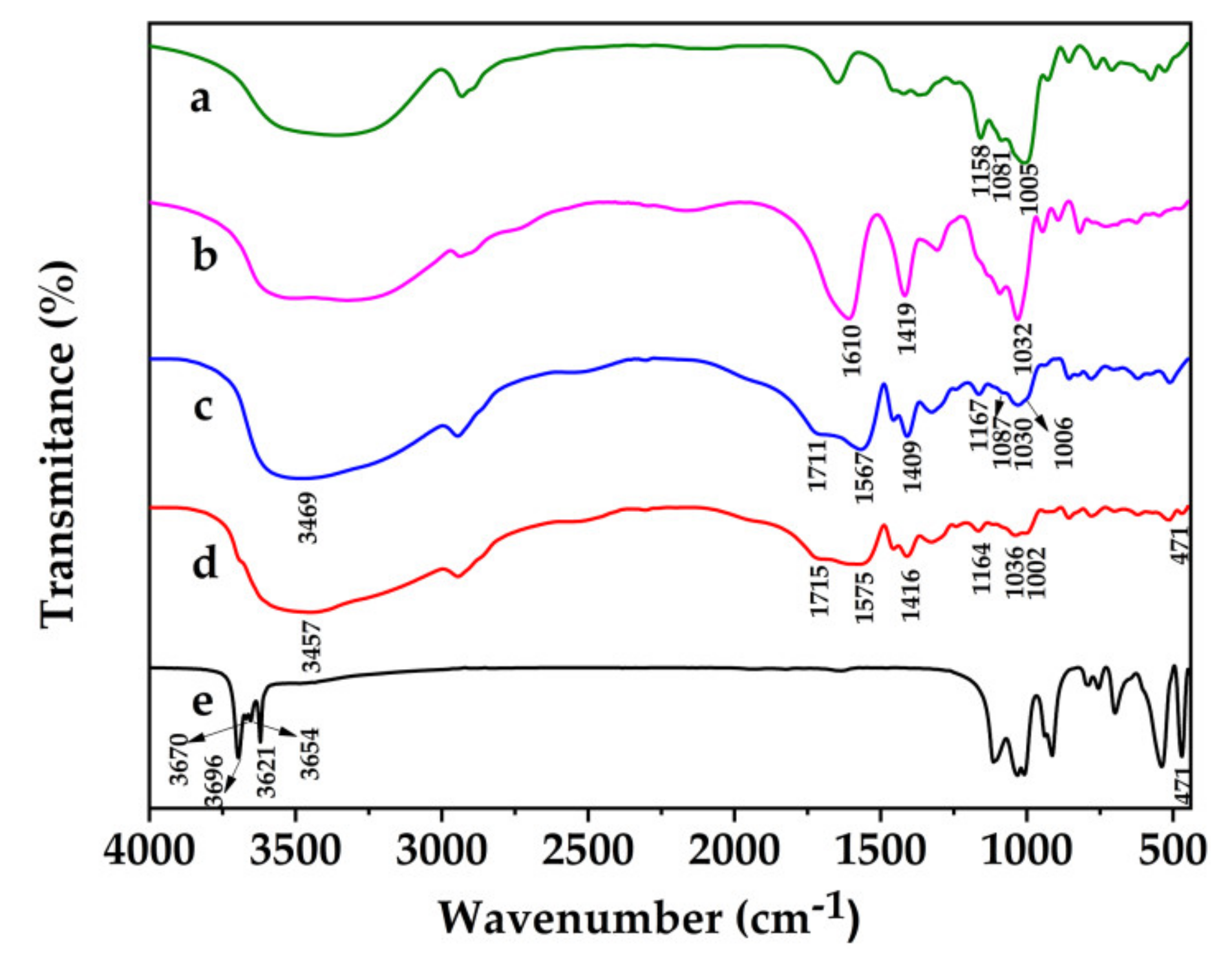
3.1. FT-IR Analysis
3.2. Morphology
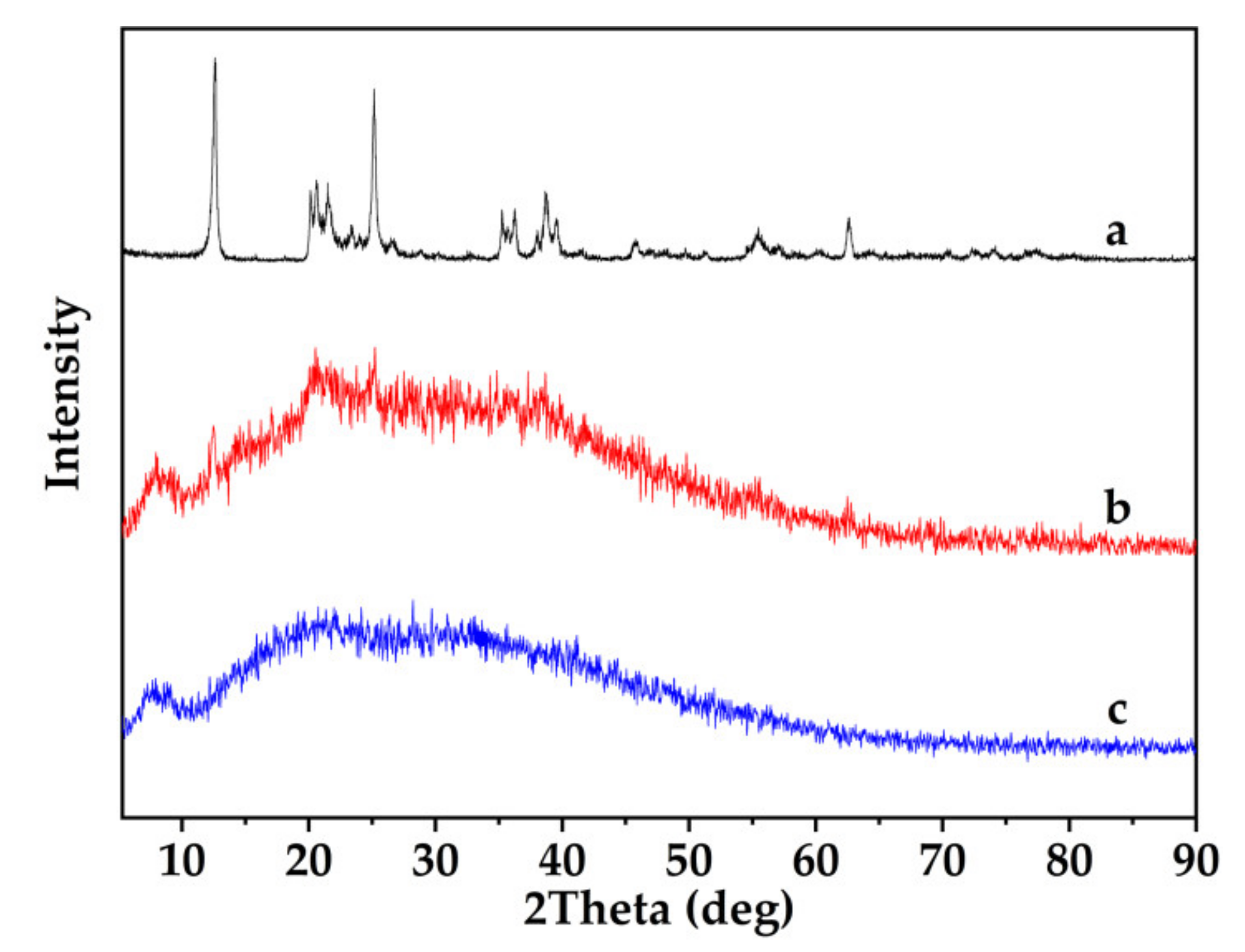
3.3. XRD Analysis
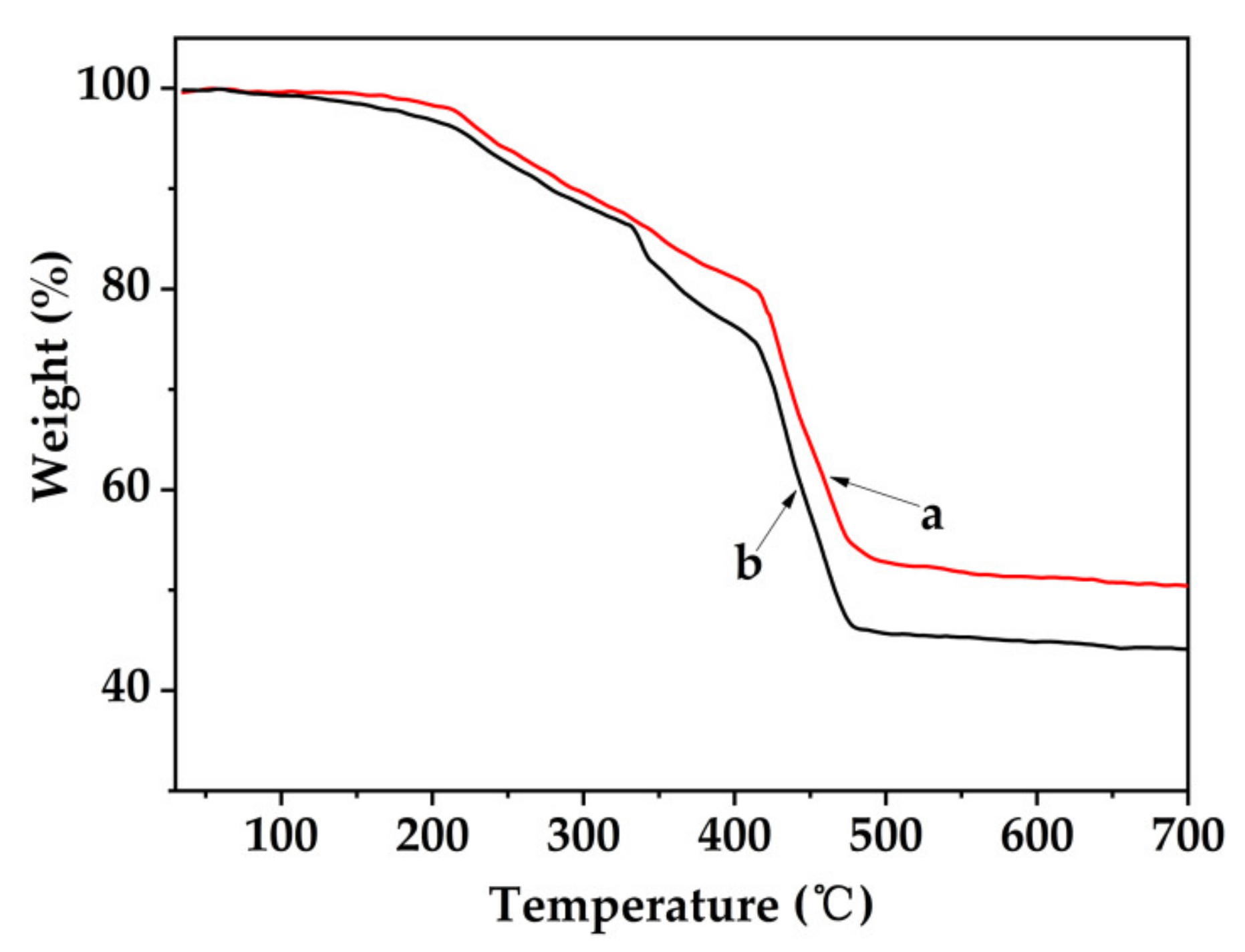
3.4. TGA Analysis
3.5. Effects of Reaction Conditions on SAPC Performance
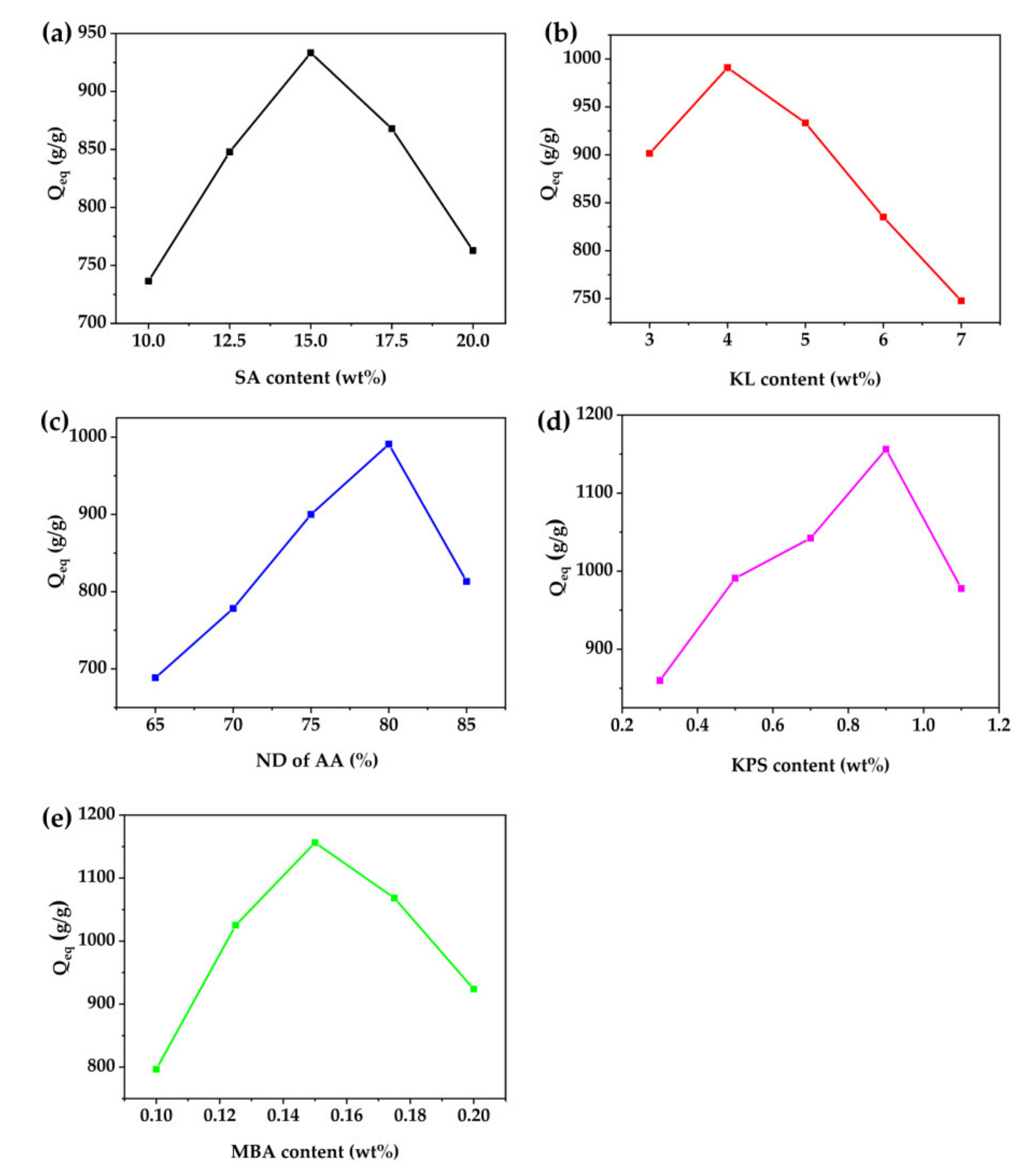
3.5.1. Effect of SA Content
3.5.2. Effect of KL Content
3.5.3. Effect of Neutralization Degree of AA
3.5.4. Effect of KPS Content
3.5.5. Effect of MBA Content
3.6. Performance Tests of SAPC
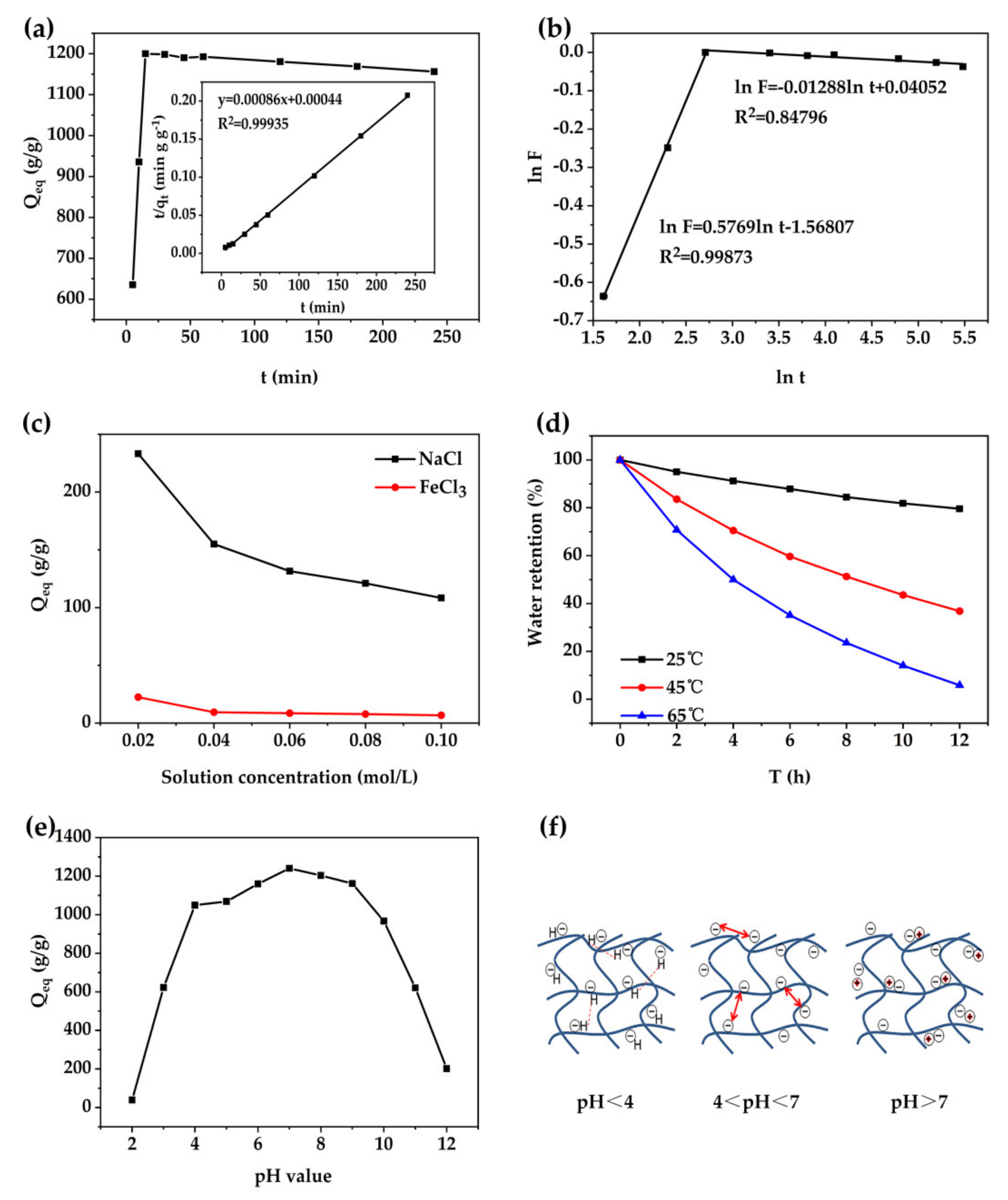
3.6.1. Swelling Kinetics of SAPC in Distilled water
3.6.2. Swelling Behavior in Salt Solutions
3.6.3. Water Retention Properties
3.6.4. Swelling Behavior in Buffer Solutions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fang, S.; Wang, G.; Xing, R.; Chen, X.; Liu, S.; Qin, Y.; Li, K.; Wang, X.; Li, R.; Li, P. Synthesis of superabsorbent polymers based on chitosan derivative graft acrylic acid-co-acrylamide and its property testing. Int. J. Biol. Macromol. 2019, 132, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Adair, A.; Kaesaman, A.; Klinpituksa, P. Superabsorbent materials derived from hydroxyethyl cellulose and bentonite: Preparation, characterization and swelling capacities. Polym. Test. 2017, 64, 321–329. [Google Scholar] [CrossRef]
- Mu, Y.; Du, D.; Yang, R.; Xu, Z. Preparation and performance of poly(acrylic acid-methacrylic acid)/montmorillonite microporous superabsorbent nanocomposite. Mater. Lett. 2015, 142, 94–96. [Google Scholar] [CrossRef]
- Alam, M.N.; Christopher, L.P. Natural Cellulose-Chitosan Cross-Linked Superabsorbent Hydrogels with Superior Swelling Properties. ACS Sustain. Chem. Eng. 2018, 6, 8736–8742. [Google Scholar] [CrossRef]
- Wan, T.; Zhou, Z.; Huang, R.; Zou, C.; Xu, M.; Cheng, W.; Li, R. Synthesis and swelling properties of microcrystal muscovite composite superabsorbent. Appl. Clay Sci. 2014, 101, 199–204. [Google Scholar] [CrossRef]
- Cheng, S.; Liu, X.; Zhen, J.; Lei, Z. Preparation of superabsorbent resin with fast water absorption rate based on hydroxymethyl cellulose sodium and its application. Carbohydr. Polym. 2019, 225, 115214. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Z.; Zhang, C.; Aluko, R.E.; Yuan, J.; Ju, X.; He, R. Structural and functional characterization of rice starch-based superabsorbent polymer materials. Int. J. Biol. Macromol. 2020, 153, 1291–1298. [Google Scholar] [CrossRef]
- Sawut, A.; Yimit, M.; Sun, W.; Nurulla, I. Photopolymerisation and characterization of maleylatedcellulose-g-poly(acrylic acid) superabsorbent polymer. Carbohydr. Polym. 2014, 101, 231–239. [Google Scholar] [CrossRef]
- Essawy, H.A.; Ghazy, M.B.M.; Abd El-Hai, F.; Mohamed, M.F. Superabsorbent hydrogels via graft polymerization of acrylic acid from chitosan-cellulose hybrid and their potential in controlled release of soil nutrients. Int. J. Biol. Macromol. 2016, 89, 144–151. [Google Scholar] [CrossRef]
- Nagarpita, M.V.; Roy, P.; Shruthi, S.B.; Sailaja, R.R.N. Synthesis and swelling characteristics of chitosan and CMC grafted sodium acrylate-co-acrylamide using modified nanoclay and examining its efficacy for removal of dyes. Int. J. Biol. Macromol. 2017, 102, 1226–1240. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, H.; Liu, Y.; Tan, H. Preparation and study on the volume phase transition properties of novel carboxymethyl chitosan grafted polyampholyte superabsorbent polymers. J. Taiwan Inst. Chem. Eng. 2016, 59, 569–577. [Google Scholar] [CrossRef]
- Álvarez-Castillo, E.; Bengoechea, C.; Guerrero, A. Composites from by-products of the food industry for the development of superabsorbent biomaterials. Food Bioprod. Process. 2020, 119, 296–305. [Google Scholar] [CrossRef]
- Fang, S.; Wang, G.; Li, P.; Xing, R.; Liu, S.; Qin, Y.; Yu, H.; Chen, X.; Li, K. Synthesis of chitosan derivative graft acrylic acid superabsorbent polymers and its application as water retaining agent. Int. J. Biol. Macromol. 2018, 115, 754–761. [Google Scholar] [CrossRef]
- Yadav, M.; Rhee, K.Y. Superabsorbent nanocomposite (alginate-g-PAMPS/MMT): Synthesis, characterization and swelling behavior. Carbohydr. Polym. 2012, 90, 165–173. [Google Scholar] [CrossRef]
- Ge, X.; Chang, M.; Jiang, W.; Zhang, B.; Xing, R.; Bulin, C. Selective location of kaolin and effects of maleic anhydride in kaolin/poly(ε-caprolactone)/poly(lactic acid) composites. Appl. Clay Sci. 2020, 189, 105524. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Ayyari, M.; Amini-Fazl, M.S. Taguchi optimized synthesis of collagen-g-poly(acrylic acid)/kaolin composite superabsorbent hydrogel. Eur. Polym. J. 2008, 44, 1209–1216. [Google Scholar] [CrossRef]
- Bao, Y.; Ma, J.; Sun, Y. Swelling behaviors of organic/inorganic composites based on various cellulose derivatives and inorganic particles. Carbohydr. Polym. 2012, 88, 589–595. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, H.; Wang, A. Study on superabsorbent composite. III. Swelling behaviors of polyacrylamide/attapulgite composite based on acidified attapulgite and organo-attapulgite. Eur. Polym. J. 2005, 41, 2434–2442. [Google Scholar] [CrossRef]
- Shalvir, A.; Liu, Q.; Abdekhodaie, M.J.; Wu, X.Y. Novel modified starch-xanthan gum hydrogels for controlled drug delivery: Synthesis and characterization. Carbohydr. Polym. 2010, 79, 898–907. [Google Scholar] [CrossRef]
- Dragan, E.S.; Apopei, D.F. Synthesis and swelling behavior of pH-sensitive semi-interpenetrating polymer network composite hydrogels based on native and modified potatoes starch as potential sorbent for cationic dyes. Chem. Eng. J. 2011, 178, 252–263. [Google Scholar] [CrossRef]
- Phang, Y.; Chee, S.; Lee, C.; Teh, Y. Thermal and microbial degradation of alginate-based superabsorbent polymer. Polym. Degrad. Stabil. 2011, 96, 1653–1661. [Google Scholar] [CrossRef]
- Pereira, R.; Carvalho, A.; Vaz, D.C.; Gil, M.H.; Mendes, A.; Bártolo, P. Development of novel alginate based hydrogel films for wound healing applications. Int. J. Biol. Macromol. 2013, 52, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Olad, A.; Doustdar, F.; Gharekhani, H. Fabrication and characterization of a starch-based superabsorbent hydrogel composite reinforced with cellulose nanocrystals from potato peel waste. Colloid Surf. A-Physicochem. Eng. Asp. 2020, 601, 124962. [Google Scholar] [CrossRef]
- Karadağ, E.; Topag, F.; Kundakci, S.; Üzüm, Ö.B. Novel composite sorbent AAm/MA hydrogels containing starch and kaolin for water sorption and dye uptake. Bull. Mater. Sci. 2014, 37, 1637–1646. [Google Scholar] [CrossRef]
- Wu, J.; Wei, Y.; Lin, J.; Lin, S. Study on starch-graft-acrylamide/mineral powder superabsorbent composite. Polymer. 2003, 44, 6513–6520. [Google Scholar] [CrossRef]
- Zhu, L.; Guan, C.; Zhou, B.; Zhang, Z.; Yang, R.; Tang, Y.; Yang, J. Adsorption of Dyes onto Sodium Alginate Graft Poly(Acrylic Acid-co-2-Acrylamide-2-Methyl Propane Sulfonic Acid)/ Kaolin Hydrogel Composite. Polym. Polym. Compos. 2017, 25, 627–634. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, Q.; Zhou, B.; Ma, D.; Ma, Z.; Zhu, L. Synthesis of Sodium Alginate Graft Poly(Acrylic Acid-Co-Acrylamide)/ Kaolin Composite Hydrogel and the Study on Its Sorption of Rhodamine B. Polym. Polym. Compos. 2015, 23, 467–474. [Google Scholar] [CrossRef]
- Sorour, M.; El-Sayed, M.; El Moneem, N.A.; Talaat, H.; Shaalan, H.; El Marsafy, S. Free radical grafting kinetics of acrylamide onto a blend of starch/chitosan/alginate. Carbohydr. Polym. 2013, 98, 460–464. [Google Scholar] [CrossRef]
- Lee, J.; Park, S.; Roh, H.; Oh, S.; Kim, S.; Kim, M.; Kim, D.; Park, J. Preparation and Characterization of Superabsorbent Polymers Based on Starch Aldehydes and Carboxymethyl Cellulose. Polymers 2018, 10, 605. [Google Scholar] [CrossRef] [Green Version]
- Lan, G.; Zhang, M.; Liu, Y.; Qiu, H.; Xue, S.; Zhang, T.; Xu, Q. Synthesis and Swelling Behavior of Super-Absorbent Soluble Starch-g-poly(AM-co-NaAMC14S) Through Graft Copolymerization and Hydrolysis. Starch/Stärke 2019, 71, 1800272. [Google Scholar] [CrossRef] [Green Version]
- Qiao, D.; Yu, L.; Bao, X.; Zhang, B.; Jiang, F. Understanding the microstructure and absorption rate of starch-based superabsorbent polymers prepared under high starch concentration. Carbohydr. Polym. 2017, 175, 141–148. [Google Scholar] [CrossRef]
- Gharekhani, H.; Olad, A.; Mirmohseni, A.; Bybordi, A. Superabsorbent hydrogel made of NaAlg-g-poly(AA-co-AAm) and rice husk ash: Synthesis, characterization, and swelling kinetic studies. Carbohydr. Polym. 2017, 168, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Wang, A. Synthesis and swelling properties of pH-sensitive semi-IPN superabsorbent hydrogels based on sodium alginate-g-poly(sodium acrylate) and polyvinylpyrrolidone. Carbohydr. Polym. 2010, 80, 1028–1036. [Google Scholar] [CrossRef]
- Shi, X.; Wang, W.; Kang, Y.; Wang, A. Enhanced Swelling Properties of a Novel Sodium Alginate-Based Superabsorbent Composites: NaAlg-g-poly(NaA-co-St)/APT. J. Appl. Polym. Sci. 2012, 125, 1822–1832. [Google Scholar] [CrossRef]
- Feng, E.; Ma, G.; Wu, Y.; Wang, H.; Lei, Z. Preparation and properties of organic-inorganic composite superabsorbent based on xanthan gum and loess. Carbohydr. Polym. 2014, 111, 463–468. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, F. Recycling waste polyethylene film for amphoteric superabsorbent resin Synthesis. Chem. Eng. J. 2018, 331, 169–176. [Google Scholar] [CrossRef]
- Shi, X.; Wang, W.; Wang, A. Effect of surfactant on porosity and swelling behaviors of guar gum-g-poly(sodium acrylate-co-styrene)/attapulgite superabsorbent hydrogels. Colloid Surf. B Biointerfaces 2011, 88, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Rashidzadeh, A.; Olad, A. Slow-released NPK fertilizer encapsulated by NaAlg-g-poly(AA-co-AAm)/MMT superabsorbent nanocomposite. Carbohydr. Polym. 2014, 114, 269–278. [Google Scholar] [CrossRef]
- Behrouzi, M.; Moghadam, P.N. Synthesis of a new superabsorbent copolymer based on acrylic acid grafted onto carboxymethyl tragacanth. Carbohydr. Polym. 2018, 202, 227–235. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, F. A new approach for blending waste plastics processing: Superabsorbent resin synthesis. J. Clean Prod. 2018, 197, 501–510. [Google Scholar] [CrossRef]
- Motamedi, E.; Motesharezedeh, B.; Shirinfekr, A.; Samar, S.M. Synthesis and swelling behavior of environmentally friendly starch-based superabsorbent hydrogels reinforced with natural char nano/micro particles. J. Environ. Chem. Eng. 2020, 8, 103583. [Google Scholar] [CrossRef]
- Diao, M.; Li, Q.; Xiao, H.; Duan, N.; Xu, J. Synthesis and adsorption properties of superabsorbent hydrogel and peanut hull composite. J. Environ. Chem. Eng. 2014, 2, 1558–1567. [Google Scholar] [CrossRef]
- Ahmed, E.M.; Aggor, F.S.; Awad, A.M.; El-Aref, A.T. An innovative method for preparation of nanometal hydroxide superabsorbent Hydrogel. Carbohydr. Polym. 2013, 91, 693–698. [Google Scholar] [CrossRef]
- Dalaran, M.; Emik, S.; Güçlü, G.; İyim, T.B.; Özgümüş, S. Study on a novel polyampholyte nanocomposite superabsorbent hydrogels: Synthesis, characterization and investigation of removal of indigo carmine from aqueous solution. Desalination 2011, 279, 170–182. [Google Scholar] [CrossRef]
- Olad, A.; Doustdar, F.; Gharekhani, H. Starch-based semi-IPN hydrogel nanocomposite integrated with clinoptilolite: Preparation and swelling kinetic study. Carbohydr. Polym. 2018, 200, 516–528. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Ghasemzadeh, H.; Soleyman, R. Synthesis, Characterization, and Swelling Behavior of Alginate-g-Poly(sodium acrylate)/Kaolin Superabsorbent Hydrogel Composites. J. Appl. Polym. Sci. 2007, 105, 2631–2639. [Google Scholar] [CrossRef]
- Ma, G.; Yang, Q.; Ran, F.; Dong, Z.; Lei, Z. High performance and low cost composite superabsorbent based on polyaspartic acid and palygorskite clay. Appl. Clay Sci. 2015, 118, 21–28. [Google Scholar] [CrossRef]
- He, G.; Ke, W.; Chen, X.; Kong, Y.; Zheng, H.; Yin, Y.; Cai, W. Preparation and properties of quaternary ammonium chitosan-g-poly(acrylic acid-co-acrylamide) superabsorbent hydrogels. React. Funct. Polym. 2017, 111, 14–21. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.; Chen, X.; Zhang, C.; Cui, B.; Li, Z.; Zhao, D.; Wang, Z. Kaolin-Enhanced Superabsorbent Composites: Synthesis, Characterization and Swelling Behaviors. Polymers 2021, 13, 1204. https://doi.org/10.3390/polym13081204
Chen M, Chen X, Zhang C, Cui B, Li Z, Zhao D, Wang Z. Kaolin-Enhanced Superabsorbent Composites: Synthesis, Characterization and Swelling Behaviors. Polymers. 2021; 13(8):1204. https://doi.org/10.3390/polym13081204
Chicago/Turabian StyleChen, Mengna, Xuelong Chen, Caiyan Zhang, Baozheng Cui, Zewen Li, Dongyu Zhao, and Zhe Wang. 2021. "Kaolin-Enhanced Superabsorbent Composites: Synthesis, Characterization and Swelling Behaviors" Polymers 13, no. 8: 1204. https://doi.org/10.3390/polym13081204
APA StyleChen, M., Chen, X., Zhang, C., Cui, B., Li, Z., Zhao, D., & Wang, Z. (2021). Kaolin-Enhanced Superabsorbent Composites: Synthesis, Characterization and Swelling Behaviors. Polymers, 13(8), 1204. https://doi.org/10.3390/polym13081204