Modeling and Simulation in Capacity Degradation and Control of All-Solid-State Lithium Battery Based on Time-Aging Polymer Electrolyte
Abstract
:1. Introduction
2. Methodology
2.1. Basic Electro-Chemical Model
2.2. Discharging Capacity of a Cell with Time-Aging SPE
2.3. Solving Conditions and Model Parameters
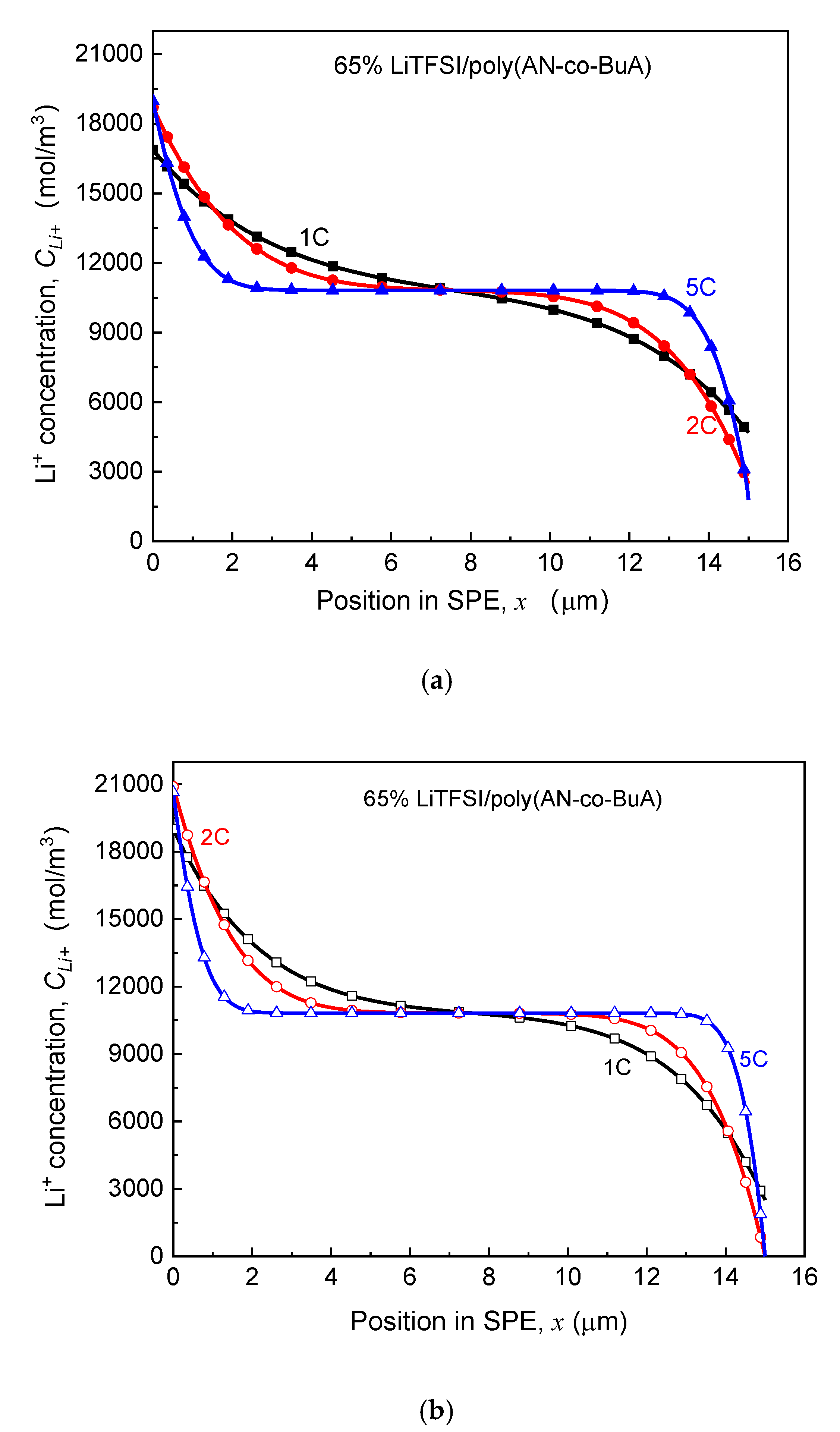
3. Results and Discussion
3.1. Dependency of Cell Discharging Capacity on Time-Aging SPE
3.2. Effect of Discharge Rate and Operation Temperature
3.3. Effect of Lithium Salt Content of SPE
3.4. Effect of Electrolyte Thickness
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix B
References
- Zhang, Q.; Liu, K.; Ding, F.; Liu, X. Recent advances in solid polymer electrolytes for lithium batteries. Nano Res. 2017, 10, 4139–4174. [Google Scholar] [CrossRef]
- Glenneberg, J.; Kasiri, G.; Bardenhagen, I.; La Mantia, F.; Busse, M.; Kun, R. Investigations on morphological and electrochemical changes of all-solid-state thin film battery cells under dynamic mechanical stress conditions. Nano Energy 2019, 57, 549–557. [Google Scholar] [CrossRef]
- Choudhury, S.; Stalin, S.; Vu, D.; Warren, A.; Deng, Y.; Biswal, P.; Archer, L.A. Solid-state polymer electrolytes for high-performance lithium metal batteries. Nat. Commun. 2019, 10, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yue, L.; Ma, J.; Zhang, J.; Zhao, J.; Dong, S.; Liu, Z.; Cui, G.; Chen, L. All solid-state polymer electrolytes for high-performance lithium ion batteries. Energy Storage Mater. 2016, 5, 139–164. [Google Scholar] [CrossRef]
- Sengwa, R.; Dhatarwal, P.; Choudhary, S. Study of time-ageing effect on the ionic conduction and structural dynamics in solid polymer electrolytes by dielectric relaxation spectroscopy. Solid State Ionics 2018, 324, 247–259. [Google Scholar] [CrossRef]
- Imrie, C.T.; Ingram, M.D.; McHattie, G.S. Ion Transport in Glassy Polymer Electrolytes. J. Phys. Chem. B 1999, 103, 4132–4138. [Google Scholar] [CrossRef]
- Kumar, B.; Koka, S.; Rodrigues, S.J.; Nookala, M. Physical aging effects on conductivity in polymer electrolytes. Solid State Ionics 2003, 156, 163–170. [Google Scholar] [CrossRef]
- Łasińska, A.; Marzantowicz, M.; Dygas, J.; Krok, F.; Florjańczyk, Z.; Tomaszewska, A.; Zygadło-Monikowska, E.; Żukowska, Z.; Lafont, U. Study of ageing effects in polymer-in-salt electrolytes based on poly(acrylonitrile-co-butyl acrylate) and lithium salts. Electrochim. Acta 2015, 169, 61–72. [Google Scholar] [CrossRef]
- Sengwa, R.; Dhatarwal, P.; Choudhary, S. Effects of plasticizer and nanofiller on the dielectric dispersion and relaxation behaviour of polymer blend based solid polymer electrolytes. Curr. Appl. Phys. 2015, 15, 135–143. [Google Scholar] [CrossRef]
- Appetecchi, G.; Croce, F.; Persi, L.; Ronci, F.; Scrosati, B. Transport and interfacial properties of composite polymer electrolytes. Electrochim. Acta 2000, 45, 1481–1490. [Google Scholar] [CrossRef]
- Li, Q.; Sun, H.; Takeda, Y.; Imanishi, N.; Yang, J.; Yamamoto, O. Interface properties between a lithium metal electrode and a poly(ethylene oxide) based composite polymer electrolyte. J. Power Sources 2001, 94, 201–205. [Google Scholar] [CrossRef]
- Liu, S.; Imanishi, N.; Zhang, T.; Hirano, A.; Takeda, Y.; Yamamoto, O.; Yang, J. Effect of nano-silica filler in polymer electrolyte on Li dendrite formation in Li/poly(ethylene oxide)–Li(CF3SO2)2N/Li. J. Power Sources 2010, 195, 6847–6853. [Google Scholar] [CrossRef]
- Appetecchi, G.B.; Croce, F.; Dautzenberg, G.; Mastragostino, M.; Ronci, F.; Scrosati, B.; Soavi, F.; Zanelli, A.; Alessandrini, F.; Prosini, P.P. Composite Polymer Electrolytes with Improved Lithium Metal Electrode Interfacial Properties: I. Elechtrochemical Properties of Dry PEO-LiX Systems. J. Electrochem. Soc. 1998, 145, 4126–4132. [Google Scholar] [CrossRef]
- Masoud, E.M.; El-Bellihi, A.-A.; Bayoumy, W.; Mousa, M. Effect of LiAlO2 nanoparticle filler concentration on the electrical properties of PEO–LiClO4 composite. Mater. Res. Bull. 2013, 48, 1148–1154. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Seki, S.; Yamanaka, A.; Miyashiro, H.; Mita, Y.; Iwahori, T. Development of high-voltage and high-capacity all-solid-state lithium secondary batteries. J. Power Sources 2005, 146, 719–722. [Google Scholar] [CrossRef]
- Nair, J.R.; Destro, M.; Bella, F.; Appetecchi, G.B.; Gerbaldi, C. Thermally cured semi-interpenetrating electrolyte networks (s-IPN) for safe and aging-resistant secondary lithium polymer batteries. J. Power Sources 2016, 306, 258–267. [Google Scholar] [CrossRef]
- Tian, H.-K.; Qi, Y. Simulation of the Effect of Contact Area Loss in All-Solid-State Li-Ion Batteries. J. Electrochem. Soc. 2017, 164, E3512–E3521. [Google Scholar] [CrossRef]
- Struik, L.C.E. Physical Aging in Amorphous Polymers and Other Materials; Elsevier Scientific Publishing Company: New York, NY, USA, 1978. [Google Scholar] [CrossRef]
- Hutchinson, J.M. Physical aging of polymers. Prog. Polym. Sci. 1995, 20, 703–760. [Google Scholar] [CrossRef]
- Hu, H.; Fan, X.; He, Y. A Coupled Thermodynamic Model for Transport Properties of Thin Films during Physical Aging. Polymers 2019, 11, 387. [Google Scholar] [CrossRef] [Green Version]
- Grillon, N.; Bouyssou, E.; Jacques, S.; Gautier, G. Failure Mechanisms Analysis of All-Solid-State Thin Film Microbatteries from an Extended Electrochemical Reliability Study. J. Electrochem. Soc. 2015, 162, A2847–A2853. [Google Scholar] [CrossRef]
- Wang, Z.; Lee, J.Z.; Xin, H.L.; Han, L.; Grillon, N.; Guy-Bouyssou, D.; Bouyssou, E.; Proust, M.; Meng, Y.S. Effects of cathode electrolyte interfacial (CEI) layer on long term cycling of all-solid-state thin-film batteries. J. Power Sources 2016, 324, 342–348. [Google Scholar] [CrossRef] [Green Version]
- Danilov, D.D.; Niessen, R.R.; Notten, P.P. Modeling All-Solid-State Li-Ion Batteries. J. Electrochem. Soc. 2011, 158, A215–A222. [Google Scholar] [CrossRef] [Green Version]
- Grillon, N.; Bouyssou, É.; Jacques, S.; Gautier, G. Cycle life and statistical predictive reliability model for all-solid-state thin film microbatteries. Microelectron. Reliab. 2019, 93, 102–108. [Google Scholar] [CrossRef]
- Grazioli, D.; Zadin, V.; Brandell, D.; Simone, A. Electrochemical-mechanical modeling of solid polymer electrolytes: Stress development and non-uniform electric current density in trench geometry microbatteries. Electrochim. Acta 2019, 296, 1142–1162. [Google Scholar] [CrossRef]
- Grazioli, D.; Verners, O.; Zadin, V.; Brandell, D.; Simone, A. Electrochemical-mechanical modeling of solid polymer electrolytes: Impact of mechanical stresses on Li-ion battery performance. Electrochim. Acta 2019, 296, 1122–1141. [Google Scholar] [CrossRef]
- Zhu, Z.; Hu, H.; He, Y.; Tao, B. Buckling analysis and control of layered electrode structure at finite deformation. Compos. Struct. 2018, 204, 822–830. [Google Scholar] [CrossRef]
- Watanabè, M.; Sanui, K.; Ogata, N.; Kobayashi, T.; Ohtaki, Z. Ionic conductivity and mobility in network polymers from poly(propylene oxide) containing lithium perchlorate. J. Appl. Phys. 1985, 57, 123–128. [Google Scholar] [CrossRef]
- Haldar, B.; Singru, R.M.; Maurya, K.K.; Chandra, S. Temperature dependence of positron-annihilation lifetime, free volume, conductivity, ionic mobility, and number of charge carriers in a polymer electrolyte polyethylene oxide complexed withNH4ClO4. Phys. Rev. B 1996, 54, 7143–7150. [Google Scholar] [CrossRef]
- Oradd, G.; Edman, L.; Ferry, A. Diffusion: A comparison between liquid and solid polymer LiTFSI electrolytes. Solid State Ionics 2002, 152, 131–136. [Google Scholar] [CrossRef]
- McCaig, M.; Paul, D.; Barlow, J. Effect of film thickness on the changes in gas permeability of a glassy polyarylate due to physical agingPart II. Mathematical model. Polymer 2000, 41, 639–648. [Google Scholar] [CrossRef]
- Singh, P.; Bharati, D.C.; Gupta, P.; Saroj, A. Vibrational, thermal and ion transport properties of PVA-PVP-PEG-MeSO4Na based polymer blend electrolyte films. J. Non Cryst. Solids 2018, 494, 21–30. [Google Scholar] [CrossRef]
- Chandra, M.V.L.; Karthikeyan, S.; Selvasekarapandian, S.; Pandi, D.V.; Monisha, S.; Packiaseeli, S.A. Characterization of high ionic conducting PVAc–PMMA blend-based polymer electrolyte for electrochemical applications. Ionics 2016, 22, 2409–2420. [Google Scholar] [CrossRef]
- Li, X.; Faghri, A. Optimization of the Cathode Structure of Lithium-Air Batteries Based on a Two-Dimensional, Transient, Non-Isothermal Model. J. Electrochem. Soc. 2012, 159, A1747–A1754. [Google Scholar] [CrossRef]
- Liu, Q.; Peng, B.; Shen, M.; Hu, B.; Chen, Q. Polymer chain diffusion and Li+ hopping of poly(ethylene oxide)/LiAsF6 crystalline polymer electrolytes as studied by solid state NMR and ac impedance. Solid State Ionics 2014, 255, 74–79. [Google Scholar] [CrossRef]
- Choi, B.; Kim, Y. Thermal history effects on the ionic conductivity of PEO-salt electrolytes. Mater. Sci. Eng. B 2004, 107, 244–250. [Google Scholar] [CrossRef]
- Money, B.K.; Hariharan, K.; Swenson, J.K. Relation between structural and conductivity relaxation in PEO and PEO based electrolytes. Solid State Ionics 2014, 262, 785–789. [Google Scholar] [CrossRef]
- Yang, J.; Wang, X.; Zhang, G.; Ma, A.; Chen, W.; Shao, L.; Shen, C.; Xie, K. High-Performance Solid Composite Polymer Electrolyte for all Solid-State Lithium Battery Through Facile Microstructure Regulation. Front. Chem. 2019, 7, 388. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xu, H.; Wang, A.; Liu, X.; Chen, J.; Wang, Z.; Feng, D.; Zeng, Q.; Zhang, L.Y. Nanocomposite All-Solid-State Polymer Electrolyte for High-Performance Lithium Batteries. Energy Technol. 2018, 7, 122–130. [Google Scholar] [CrossRef]











| Domains | SPE | Positive Electrode |
|---|---|---|
| State variables | , , | |
| Initial conditions | , | |
| BCs at x = 0 | , | |
| BCs at x = LSPE | , | |
| BCs at x = LSPE + LPos | ||
| Symbol | Description | Value |
|---|---|---|
| Fraction of free Li ions in equilibrium | 0.18 [17,23] | |
| Diffusion coefficient for Li, positive electrode | 1.76 × 10−15 m2/s [17,23] | |
| Ionic diffusion coefficients of Li+ and TFSI− | [30] | |
| Selected maximal concentration of Li, positive electrode | 2.33 × 104 [17,23] | |
| Rate constant charge transfer reaction, positive electrode | 5.1 × 10−4 [17] | |
| Rate constant charge transfer reaction, negative electrode | 1 × 10−2 [17,23] | |
| Charge transfer coefficient in positive electrode | 0.6 [17,23] | |
| Charge transfer coefficient in negative electrode | 0.5 [23] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, X.; He, Y.; Fan, X.; Zhang, D.; Hu, H. Modeling and Simulation in Capacity Degradation and Control of All-Solid-State Lithium Battery Based on Time-Aging Polymer Electrolyte. Polymers 2021, 13, 1206. https://doi.org/10.3390/polym13081206
Fang X, He Y, Fan X, Zhang D, Hu H. Modeling and Simulation in Capacity Degradation and Control of All-Solid-State Lithium Battery Based on Time-Aging Polymer Electrolyte. Polymers. 2021; 13(8):1206. https://doi.org/10.3390/polym13081206
Chicago/Turabian StyleFang, Xuansen, Yaolong He, Xiaomin Fan, Dan Zhang, and Hongjiu Hu. 2021. "Modeling and Simulation in Capacity Degradation and Control of All-Solid-State Lithium Battery Based on Time-Aging Polymer Electrolyte" Polymers 13, no. 8: 1206. https://doi.org/10.3390/polym13081206
APA StyleFang, X., He, Y., Fan, X., Zhang, D., & Hu, H. (2021). Modeling and Simulation in Capacity Degradation and Control of All-Solid-State Lithium Battery Based on Time-Aging Polymer Electrolyte. Polymers, 13(8), 1206. https://doi.org/10.3390/polym13081206







