Cross-Linked Polymer Brushes Containing N-Halamine Groups for Antibacterial Surface Applications
Abstract
:1. Introduction
2. Experimental
2.1. Materials
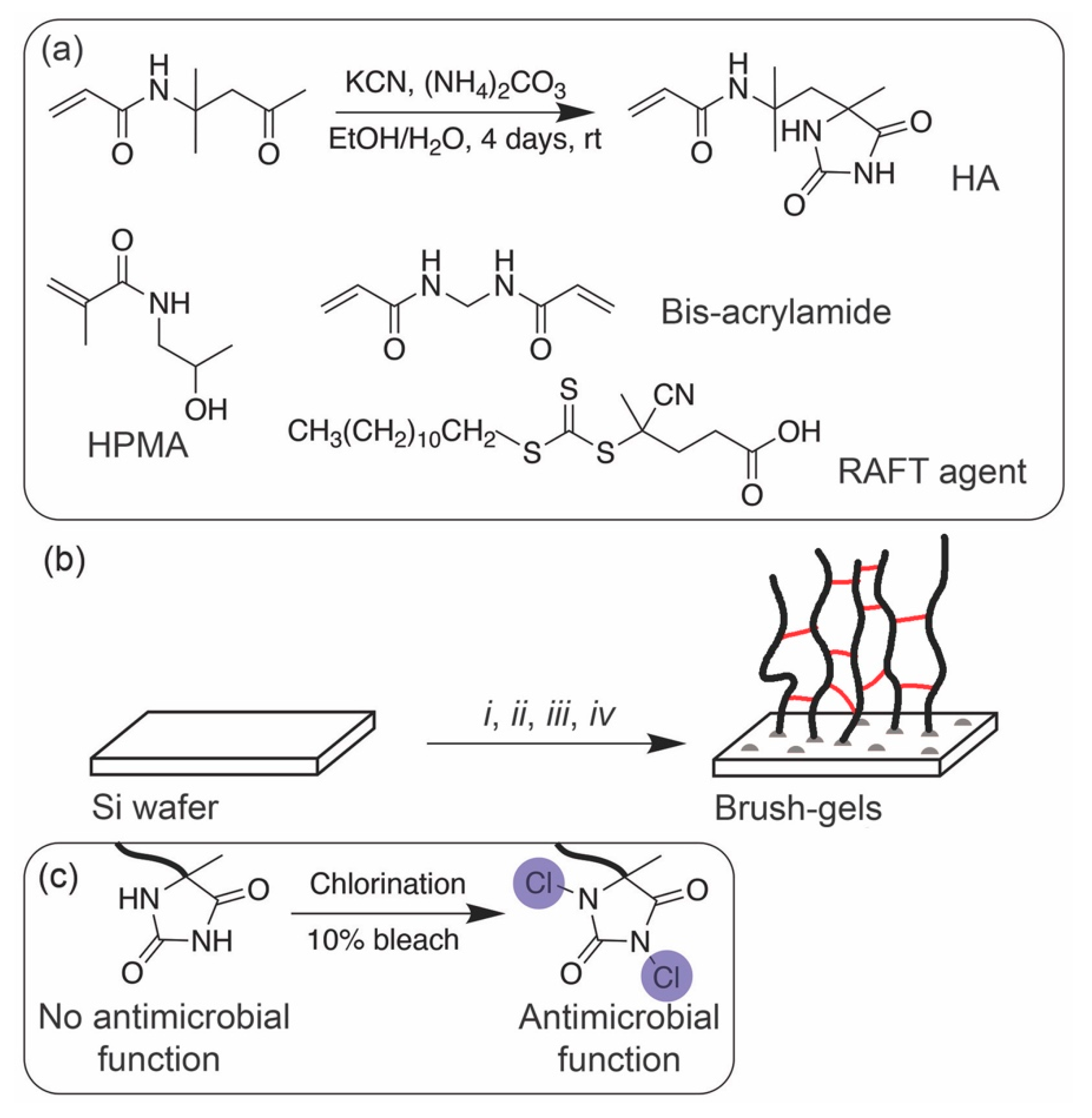
2.2. Synthesis of Halamine Vinyl Monomer (HA)
2.3. Surface-Initiated Polymerization and Activation of N-Halamine Precursor
2.4. Antibacterial Surface
2.5. Characterization
3. Results and Discussions
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Friedman, M. Chemistry, antimicrobial mechanisms, and antibiotic activities of cinnamaldehyde against pathogenic bacteria in animal feeds and human foods. J. Agric. Food Chem. 2017, 65, 10406–10423. [Google Scholar] [CrossRef]
- Notermans, S.; Hoogenboom-Verdegaal, A. Existing and emerging foodborne diseases. Int. J. Food Microbiol. 1992, 15, 197–205. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Lu, X. Molecular imprinting technology for sensing foodborne pathogenic bacteria. Anal. Bioanal. Chem. 2021, 1–18. [Google Scholar] [CrossRef]
- Ochmian, I.; Blaszak, M.; Lachowicz, S.; Piwowarczyk, R. The impact of cultivation systems on the nutritional and phytochemical content, and microbiological contamination of highbush blueberry. Sci. Rep. 2020, 10, 16696. [Google Scholar] [CrossRef] [PubMed]
- Salin, V.; Hooker, N.H. Stock market reaction to food recalls. Rev. Agric. Econ. 2001, 23, 33–46. [Google Scholar] [CrossRef]
- Sung, S.-Y.; Sin, L.T.; Tee, T.-T.; Bee, S.-T.; Rahmat, A.R.; Rahman, W.A.; Tan, A.-C.; Vikhraman, M. Antimicrobial agents for food packaging applications. Trends Food Sci. Technol. 2013, 33, 110–123. [Google Scholar] [CrossRef]
- Donsi, F.; Ferrari, G. Essential oil nanoemulsions as antimicrobial agents in food. J. Biotechnol. 2016, 233, 106–120. [Google Scholar] [CrossRef]
- Gyawali, R.; Ibrahim, S.A. Natural products as antimicrobial agents. Food Control 2014, 46, 412–429. [Google Scholar] [CrossRef]
- Tako, M.; Kerekes, E.B.; Zambrano, C.; Kotogan, A.; Papp, T.; Krisch, J.; Vagvölgyi, C. Plant phenolics and phenolic-enriched extracts as antimicrobial agents against food-contaminating microorganisms. Antioxidants 2020, 9, 165. [Google Scholar] [CrossRef] [Green Version]
- Bahrami, A.; Delshadi, R.; Assadpour, E.; Jafari, A.M.; Williams, L. Antimicrobial-loaded nanocarriers for food packaging applications. Adv. Colloid Interface 2020, 278, 102140. [Google Scholar] [CrossRef]
- Kinali-Demirci, S.; Disli, A.; Idil, A.; Demirci, S. Adenine derivatives for regenerable antibacterial surface applications based on A−T base pairing. ChemistrySelect 2020, 5, 10128–10134. [Google Scholar] [CrossRef]
- Dong, Y.-S.; Xiong, X.-H.; Lu, X.-W.; Wu, Z.-Q.; Chen, H. Antibacterial surfaces based on poly(cationic liquid) brushes: Switchability between killing and releasing via anion counterion switching. J. Mater. Chem. B 2016, 4, 6111–6116. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Sun, Y. N-Halamine-based antimicrobial additives for polymers: Preparation, characterization, and antimicrobial activity. Ind. Eng. Chem. Res. 2006, 45, 2634–2640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kocer, H.B.; Akdag, A.; Worley, S.D.; Acevedo, O.; Broughton, R.M.; Wu, Y. Mechanism of photolytic decomposition of n-halamine antimicrobial siloxane coatings. ACS Appl. Mater. Interfaces 2010, 2, 2456–2464. [Google Scholar] [CrossRef]
- Hui, F.; Debiemme-Chouvy, C. Antimicrobial N-halamine polymers and coatings: A review of their synthesis, characterization, and applications. Biomacromolecules 2013, 14, 585–601. [Google Scholar] [CrossRef]
- Kocer, H.B.; Cerkez, I.; Worley, S.D.; Broughton, R.M.; Huang, T.S. Polymeric antimicrobial N-halamine epoxides. ACS Appl. Mater. Interfaces 2011, 3, 2845–2850. [Google Scholar] [CrossRef]
- Kocer, H.B.; Cerkez, I.; Worley, S.D.; Broughton, R.M.; Huang, T.S. N-Halamine copolymers for use in antimicrobial paints. ACS Appl. Mater. Interfaces 2011, 3, 3189–3194. [Google Scholar] [CrossRef] [PubMed]
- Denis-Rohr, A.; Bastarrachea, L.J.; Goddard, J.M. Antimicrobial efficacy of N-halamine coatings prepared via dip and spray layer-by-layer deposition. Food Bioprod. Process. 2015, 96, 12–19. [Google Scholar] [CrossRef]
- Qiao, M.; Liu, Q.; Yong, Y.; Pardo, Y.; Worobo, R.; Liu, Z.; Jiang, S.; Ma, M. Scalable and rechargeable antimicrobial coating for food safety applications. J. Agric. Food Chem. 2018, 66, 11441–11450. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Li, J.; Si, Y.; Huang, K.; Nitin, N.; Sun, G. Rechargeable antibacterial N-halamine films with antifouling function for food packaging applications. ACS Appl. Mater. Interfaces 2019, 11, 17814–17822. [Google Scholar] [CrossRef]
- Simoncic, B.; Tomsic, B. Strucures of novel antimicrobial agents for textiles—A review. Text. Res. J. 2010, 80, 1721–1737. [Google Scholar] [CrossRef]
- Wang, F.; Huang, L.; Zhang, P.; Si, Y.; Yu, J.; Ding, B. Antibacterial N-halamine fibrous materials. Compos. Commun. 2020, 22, 100487. [Google Scholar] [CrossRef]
- Wang, Y.; Yin, M.; Lin, X.; Li, L.; Li, Z.; Ren, X.; Sun, Y. Tailored synthesis of polymer-brush-grafted mesoporous silicas with N-halamine and quaternary ammonium groups for antimicrobial applications. J. Colloid Interface Sci. 2019, 533, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Milner, S.T. Polymer brushes. Science 1991, 251, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Barbey, R.; Lavanant, L.; Paripovic, D.; Schüwer, N.; Sugnaux, C.; Tugulu, S.; Klok, H.-A. Polymer brushes via surface-initiated controlled radical polymerization: Synthesis, characterization, properties, and applications. Chem. Rev. 2009, 109, 5437–5527. [Google Scholar] [CrossRef] [PubMed]
- Demirci, S.; Kinali-Demirci, S.; Jiang, S. A switchable polymer brush system for antifouling and controlled detection. Chem. Commun. 2017, 53, 3713–3716. [Google Scholar] [CrossRef] [PubMed]
- Demirci, S.; Kinali-Demirci, S.; VanVeller, B. Controlled supramolecular complexation of cyclodextrin- functionalized polymeric ionic liquid brushes. ACS Appl. Polym. Mater. 2020, 2, 751–757. [Google Scholar] [CrossRef]
- Kim, M.; Schmitt, S.K.; Choi, J.W.; Krutty, J.D.; Gopalan, P. From self-assembled monolayers to coatings: Advances in the synthesis and nanobio applications of polymer brushes. Polymers 2015, 7, 1346–1378. [Google Scholar] [CrossRef]
- Krishnamoorthy, M.; Hakobyan, S.; Ramstedt, M.; Gautrot, J.E. Surface-initiated polymer brushes in the biomedical field: Applications in membrane science, biosensing, cell culture, regenerative medicine and antibacterial coatings. Chem. Rev. 2014, 114, 10976–11026. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Yu, B.; Ye, Q.; Zhou, F. Tapping the potential of polymer brushes through synthesis. Acc. Chem. Res. 2015, 48, 229–237. [Google Scholar] [CrossRef]
- Zoppe, J.O.; Ataman, N.C.; Mocny, P.; Wang, J.; Moraes, J.; Klok, H.-A. Surface-initiated controlled radical polymerization: State-of-the-art, opportunities, and challenges in surface and interface engineering with polymer brushes. Chem. Rev. 2017, 117, 1105–1318. [Google Scholar] [CrossRef] [Green Version]
- Braunecker, W.A.; Matyjaszewski, K. Controlled/living radical polymerization: Features, developments, and perspectives. Prog. Polym. Sci. 2007, 32, 93–146. [Google Scholar] [CrossRef]
- Edmondson, S.; Osborne, V.L.; Huck, W.T.S. Polymer brushes via surface-initiated polymerizations. Chem. Soc. Rev. 2004, 33, 14–22. [Google Scholar] [CrossRef]
- Satoh, R.; Homma, S.; Arafune, H.; Shomura, R.; Kamijo, T.; Morinaga, T.; Sato, T. In situ surface-initiated atom-transfer radical polymerization utilizing the nonvolatile nature of ionic liquids: A first attempt. Polymers 2021, 13, 61. [Google Scholar] [CrossRef]
- Demirci, S.; Kinali-Demirci, S.; Caykara, T. A new selenium-based RAFT agent for surface-initiated RAFT polymerization of 4-vinylpyridine. Polymer 2013, 54, 5345–5350. [Google Scholar] [CrossRef]
- Wei, W.; Balamurugan, A.; Dwyer, J.H.; Gopalan, P. Substrate-independent approach to dense cleavable polymer brushes by nitroxide-mediated polymerization. ACS Macro Lett. 2018, 7, 100–104. [Google Scholar] [CrossRef]
- Hoffmann, M.; Lang, M.; Sommer, J.-U. Gelation threshold of cross-linked polymer brushes. Phys. Rev. E 2011, 83, 021803. [Google Scholar] [CrossRef] [PubMed]
- Demirci, S. Crosslinked-polymer brushes with switchable capture and release capabilities. Polymers 2018, 10, 956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demirci, S.; Kinali-Demirci, S.; VanVeller, B. Surface-grafted polymeric ionic liquids with tunable morphology via in/ex situ cross-linking methods. ACS Macro Lett. 2020, 9, 1806–1811. [Google Scholar] [CrossRef]
- Kocer, H.B.; Worley, S.D.; Broughton, R.M.; Huang, T.S. A novel N-halamine acrylamide monomer and its copolymersfor antimicrobial coating. React. Funct. Polym. 2011, 71, 561–568. [Google Scholar] [CrossRef]
- Jung, K.-H.; Huh, M.-W.; Meng, W.; Yuan, J.; Hyun, S.H.; Bae, J.-S.; Hudson, S.M.; Kang, I.-K. Preparation and antibacterial activity of PET/chitosan nanofibrous mats using an electrospinning technique. J. Appl. Polym. Sci. 2007, 105, 2816–2823. [Google Scholar] [CrossRef]
- Yildirim, E.; Cimen, D.; Zengin, A.; Caykara, T. Synthesis of poly(N-(2-hydroxypropyl) methacrylamide) brushes by interface-mediated RAFT polymerization. RSC Adv. 2016, 6, 45259–45264. [Google Scholar] [CrossRef]





| Entry | O 1s | N 1s | C 1s | Cl 2p | S 2p | Si 2p | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| N-C = O | C-O | C-N | C-C | |||||||
| Si-NH2 | Energy (eV) Conc. (%) | 532.2 13.2 | 400.0 16.1 | – 52.8 | – | 285.6 | 285.0 | – – | – – | 99.1 17.9 |
| Si-RAFT | Energy (eV) Conc. (%) | 532.2 21.5 | 399.8 8.3 | 288.6 52.6 | – | 286.2 | 285.0 | – – | 163.1 9.3 | 99.1 8.3 |
| CB-0 | Energy (eV) Conc. (%) | 532.5 22.7 | 400.1 8.9 | 288.9 66.8 | 286.8 | 285.7 | 285.0 | – – | 163.1 1.6 | – – |
| CB-25 2 | Energy (eV) Conc. (%) | 532.3 22.6 | 399.8 10.1 | 288.7 65.9 | 286.7 | 285.6 | 285.0 | – – | 163.2 1.4 | – – |
| CB-50 2 | Energy (eV) Conc. (%) | 532.2 21.4 | 399.9 11.4 | 288.7 66.3 | 286.8 | 285.7 | 285.0 | – – | 163.1 0.9 | – – |
| CB-75 2 | Energy (eV) Conc. (%) | 532.1 20.8 | 399.8 13.7 | 289.0 64.8 | 286.8 | 285.5 | 285.0 | – – | 162.9 0.7 | – – |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kinali-Demirci, S. Cross-Linked Polymer Brushes Containing N-Halamine Groups for Antibacterial Surface Applications. Polymers 2021, 13, 1269. https://doi.org/10.3390/polym13081269
Kinali-Demirci S. Cross-Linked Polymer Brushes Containing N-Halamine Groups for Antibacterial Surface Applications. Polymers. 2021; 13(8):1269. https://doi.org/10.3390/polym13081269
Chicago/Turabian StyleKinali-Demirci, Selin. 2021. "Cross-Linked Polymer Brushes Containing N-Halamine Groups for Antibacterial Surface Applications" Polymers 13, no. 8: 1269. https://doi.org/10.3390/polym13081269





