Influence of the Epoxy Resin Process Parameters on the Mechanical Properties of Produced Bidirectional [±45°] Carbon/Epoxy Woven Composites
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. [±45°] WCFF/Aerotuf Composite Laminates
2.3. Aerotuf Curing Process
3. Results and Discussion
3.1. Functional Groups Analysis of Aerotuf
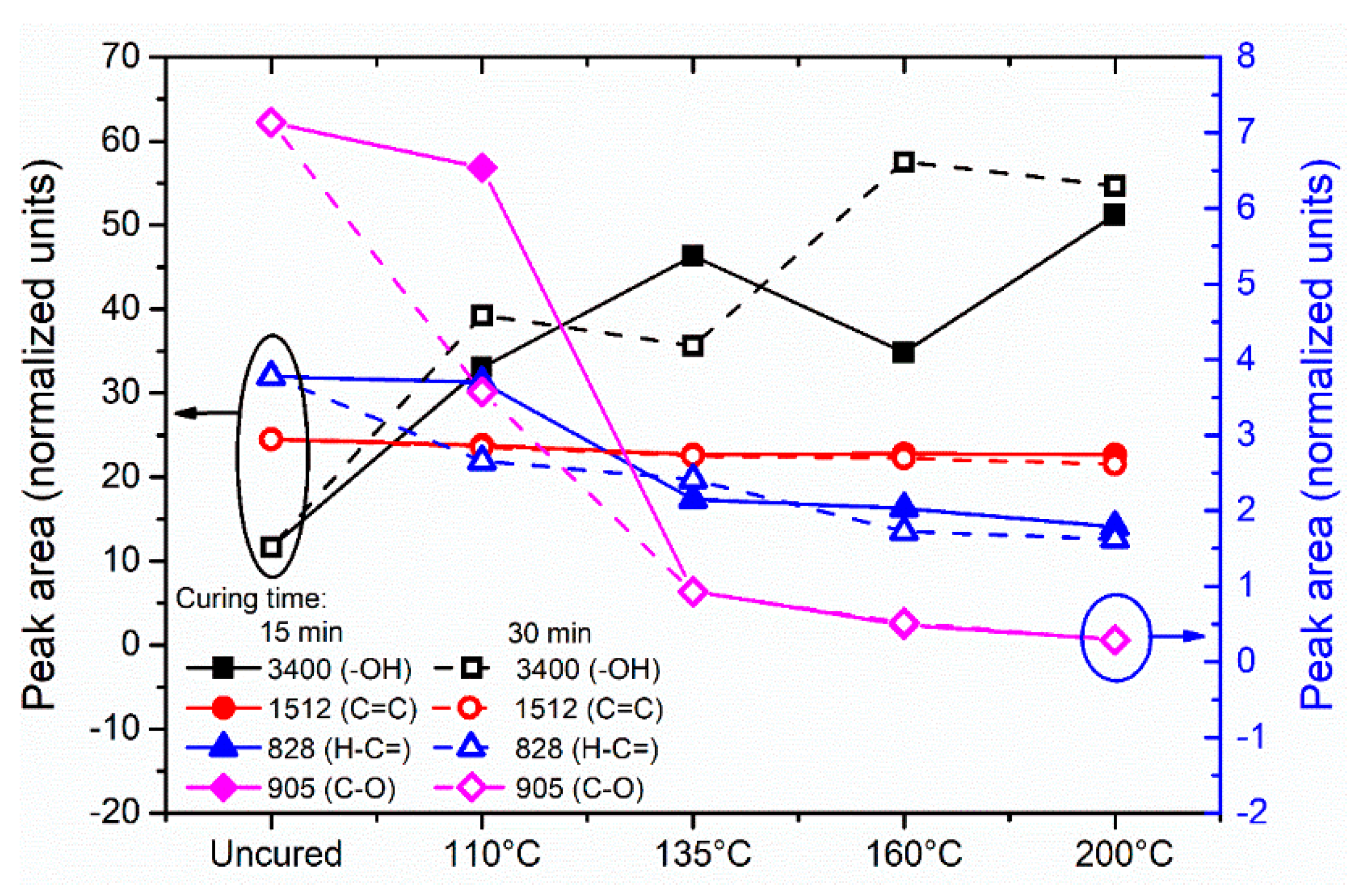
3.2. Resin Curing Reaction Behavior
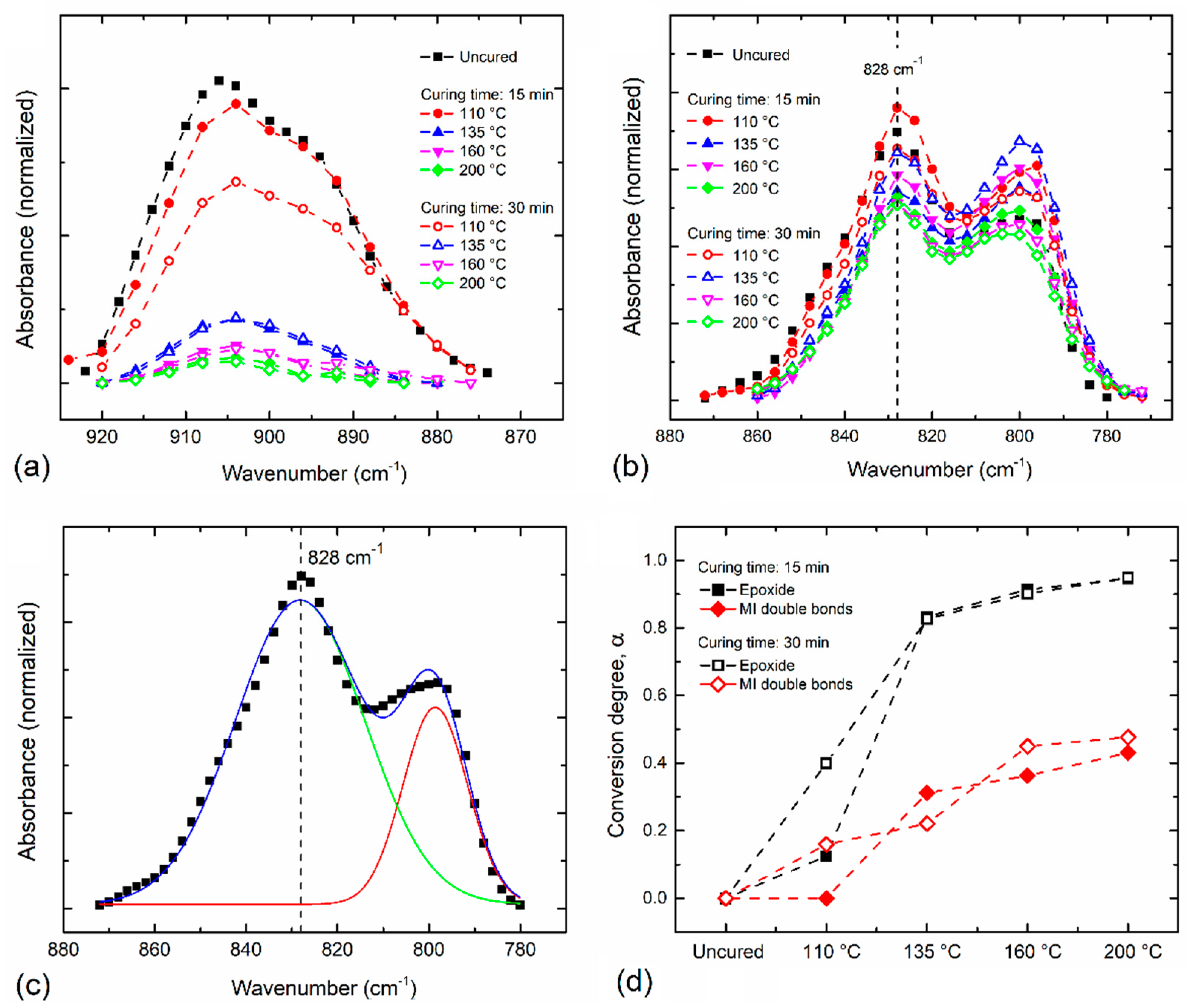
3.3. Curing Degree Quantitative Analysis
3.4. Influence of the Curing Temperature and Time on the Mechanical Properties of [±45°] WCFF/Aerotuf Composite Laminates
4. Conclusions
- The reaction mechanism that occurs in Aerotuf resin curing process consists of two independent reactions: (a) the consumption of the epoxide groups and (b) the reaction of the =C–H unsaturation of MI units either with the amine groups or by homopolymerization, which are influenced by the reaction temperature and the curing time.
- The baseline band absorption located at 905 cm−1 can be used determine the resin curing/cross-linking degree in terms of epoxide groups.
- An epoxy resin curing temperature of 160 °C during 15 min is enough to achieve an adequate conversion degree of 0.91 in terms of epoxide groups representing a good approximation of the real conversion of Aerotuf.
- Tensile tests confirmed that the ultimate tensile strength values of the produced [±45°] WCFF/Aerotuf composite laminates samples achieved the best mechanical performance when using an epoxy resin curing temperature of 160 °C during 15 min.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mouritz, A.P. Introduction to Aerospace Materials, 1st ed.; Woodhead Publishing Limited: Cambridge, UK, 2012; pp. 268–302. [Google Scholar]
- Cañavate, J.; Colom, X.; Pagès, P.; Carrasco, F. Study of the curing process of an epoxy resin by FTIR spectroscopy. Polym. Plast. Technol. Eng. 2000, 39, 937–943. [Google Scholar] [CrossRef]
- Mphahlele, K.; Ray, S.S.; Kolesnikov, A. Cure kinetics, morphology development, and rheology of a high-performance carbon-fiber-reinforced epoxy composite. Compos. Part B Eng. 2019, 176, 107300. [Google Scholar] [CrossRef]
- Wu, F.; Zhou, X.; Yu, X. Reaction mechanism, cure behavior and properties of a multifunctional epoxy resin, TGDDM, with latent curing agent dicyandiamide. RSC Adv. 2018, 8, 8248–8258. [Google Scholar] [CrossRef]
- May, C.A. Epoxy Resins-Chemistry and Technology, 2nd ed.; Marcel Dekker, Inc.: New York, NY, USA, 1988. [Google Scholar]
- Massingill, J.L., Jr.; Bauer, R.S. Epoxy Resins. In Applied Polymer Science 21st Century; Craver, C.D., Carraher, C.E., Jr., Eds.; Elsevier Science Ltd: Amsterdam, The Netherlands, 2000; pp. 393–424. [Google Scholar]
- Erdmann, M.; Trappe, V.; Sturm, H.; Braun, U.; Duemichen, E. Cure conversion of structural epoxies by cure state analysis and in situ cure kinetics using nondestructive NIR spectroscopy. Thermochim. Acta 2017, 650, 8–17. [Google Scholar] [CrossRef]
- Wang, L.; Fernando, G.F. Cure monitoring of epoxy resin by simultaneous DSC / FTIR. Adv. Mater. Res. 2014, 883, 905–908. [Google Scholar] [CrossRef]
- González, M.G.; Cabanelas, J.C.; Baselga, J. Applications of FTIR on epoxy resins—Identification, monitoring the curing process, phase separation and water uptake. In Infrared Spectroscopy—Materials Science, Engineering and Technology; Theophile, T., Ed.; InTech: Rijeka, Croatia, 2012; pp. 261–284. [Google Scholar]
- Rogers, M.G. The structure of epoxy resins using NMR and GPC techniques. J. Appl. Polym. Sci. 1972, 16, 1953–1958. [Google Scholar] [CrossRef]
- Sojka, S.A.; Moniz, W.B. The curing of an epoxy resin as followed by carbon-13 NMR spectroscopy. J. Appl. Polym. Sci. 1976, 20, 1977–1982. [Google Scholar] [CrossRef]
- Merad, L.; Cochez, M.; Margueron, S.; Jauchem, F.; Ferriol, M.; Benyoucef, B.; Bourson, P. In-situ monitoring of the curing of epoxy resins by Raman spectroscopy. Polym. Test. 2009, 28, 42–45. [Google Scholar] [CrossRef]
- Rigail-Cedeño, A.; Sung, C.S.P. Fluorescence and IR characterization of epoxy cured with aliphatic amines. Polymer 2005, 46, 9378–9384. [Google Scholar] [CrossRef]
- McHugh, J.; Stark, W.; Döring, J. Evaluation of the cure behaviour of epoxy resin using rheometric and ultrasonic techniques. In Nondestructive Characterization of Materials XI. Advances in the Statistical Sciences; Green, R.E., Djordjevic, B.B., Hentschel, M.P., Eds.; Springer: Berlin/Heidelberg, Germany, 2003; Volume 6, pp. 651–657. [Google Scholar]
- Billaud, C.; Vandeuren, M.; Legras, R.; Carlier, V. Quantitative analysis of epoxy resin cure reaction: A study by near-infrared spectroscopy. Appl. Spectrosc. 2002, 56, 1413–1421. [Google Scholar] [CrossRef]
- Musto, P.; Martuscelli, E.; Ragosta, G.; Russo, P.; Villano, P. Tetrafunctional epoxy resins: Modeling the curing kinetics based on FTIR spectroscopy data. J. Appl. Polym. Sci. 1999, 74, 532–540. [Google Scholar] [CrossRef]
- Poisson, N.; Lachenal, G.; Sautereau, H. Near- and mid-infrared spectroscopy studies of an epoxy reactive system. Vib. Spectrosc. 1996, 12, 237–247. [Google Scholar] [CrossRef]
- Nikolic, G.; Zlatkovic, S.; Cakic, M.; Cakic, S.; Lacnjevac, C.; Rajic, Z. Fast Fourier transform IR characterization of epoxy GY systems crosslinked with aliphatic and cycloaliphatic EH polyamine adducts. Sensors 2010, 10, 684–696. [Google Scholar] [CrossRef]
- Fernàndez-Francos, X.; Kazarian, S.G.; Ramis, X.; Serra, À. Simultaneous monitoring of curing shrinkage and degree of cure of thermosets by attenuated total reflection Fourier transform infrared (ATR FT-IR) spectroscopy. Appl. Spectrosc. 2013, 67, 1427–1436. [Google Scholar] [CrossRef] [PubMed]
- Drakonakis, V.M.; Seferis, J.C.; Doumanidis, C.C. Curing pressure influence of out-of-autoclave processing on structural composites for commercial aviation. Adv. Mater. Sci. Eng. 2013, 356824. [Google Scholar] [CrossRef]
- Musto, P.; Martuscelli, E.; Ragosta, G.; Russo, P.; Scarinzi, G. An interpenetrated system based on a tetrafunctional epoxy resin and a thermosetting bismaleimide: Structure—Properties correlation. J. Appl. Polym. Sci. 1998, 69, 1029–1042. [Google Scholar] [CrossRef]
- Gu, A.; Liang, G.; Lan, L. High-performance bismaleimide resin with good processing characteristics. J. Appl. Polym. Sci. 1996, 62, 799–803. [Google Scholar] [CrossRef]
- Chandra, R.; Rajabi, L. Recent advances in bismaleimides and epoxy-imide/bismaleimide formulations and composites. J. Macromol. Sci. Rev. Macromol. Chem. Phys. 1997, 37, 61–96. [Google Scholar] [CrossRef]
- Kanimozhi, K.; Sethuraman, K.; Selvaraj, V.; Alagar, M. Development of ricehusk ash reinforced bismaleimide toughened epoxy nanocomposites. Front. Chem. 2014, 2, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jena, R.K.; Yue, C.Y.; Sk, M.M.; Ghosh, K. A novel high performance bismaleimide/diallyl bisphenol A (BMI/DBA)-epoxy interpenetrating network resin for rigid riser application. RSC Adv. 2015, 5, 79888–79897. [Google Scholar] [CrossRef]
- Abbate, M.; Martuscelli, E.; Musto, P.; Ragosta, G. Thermosetting bismaleimide/reactive rubber blends: Curing kinetics and mechanical behavior. J. Appl. Polym. Sci. 1997, 65, 979–990. [Google Scholar] [CrossRef]
- Finzel, M.C.; Delong, J.; Hawley, M.C. Effect of stoichiometry and diffusion on an epoxy/amine reaction mechanism. J. Polym. Sci. Part A Polym. Chem. 1995, 33, 673–689. [Google Scholar] [CrossRef]
- Kumar, A.A.; Alagar, M.; Rao, R.M.V.G.K. Studies on thermal and morphological behavior of siliconized epoxy bismaleimide matrices. J. Appl. Polym. Sci. 2001, 81, 2330–2346. [Google Scholar] [CrossRef]
- Cholake, S.T.; Mada, M.R.; Raman, R.K.S.; Bai, Y.; Zhao, X.L.; Rizkalla, S.; Bandyopadhyay, S. Quantitative analysis of curing mechanisms of epoxy resin by mid- and near-Fourier transform infrared spectroscopy. Def. Sci. J. 2014, 64, 314–321. [Google Scholar] [CrossRef]
- Zhuang, W.; Ao, W. Effect of stacking angles on mechanical properties and damage propagation of plain woven carbon fiber laminates. Mater. Res. Express 2018, 5, 035603. [Google Scholar] [CrossRef]
- Rahmani, H.; Najafi, S.H.M.; Ashori, A. Mechanical performance of epoxy/carbon fiber laminated composites. J. Reinf. Plast. Compos. 2014, 33, 733–740. [Google Scholar] [CrossRef]
- Jin, H.; Lu, W.; Nelson, K.; Nissen, A.; Briggs, T. Mechanical Properties of Woven Composites at Ambient Temperature; Sandia National Laboratories: Albuquerque, NW, USA, 2018. [Google Scholar]
- De Paiva, J.M.F.; Mayer, S.; Rezende, M.C. Evaluation of mechanical properties of four different carbon/epoxy composites used in aeronautical field. Mater. Res. 2005, 8, 91–97. [Google Scholar] [CrossRef]
- Carbas, R.J.C.; Marques, E.A.S.; Da Silva, L.F.M.; Lopes, A.M. Effect of cure temperature on the glass transition temperature and mechanical properties of epoxy adhesives. J. Adhes. 2014, 90, 104–119. [Google Scholar] [CrossRef]
- Zhang, K.; Gu, Y.; Li, M.; Zhang, Z. Effect of rapid curing process on the properties of carbon fiber/epoxy composite fabricated using vacuum assisted resin infusion molding. Mater. Des. 2014, 54, 624–631. [Google Scholar] [CrossRef]
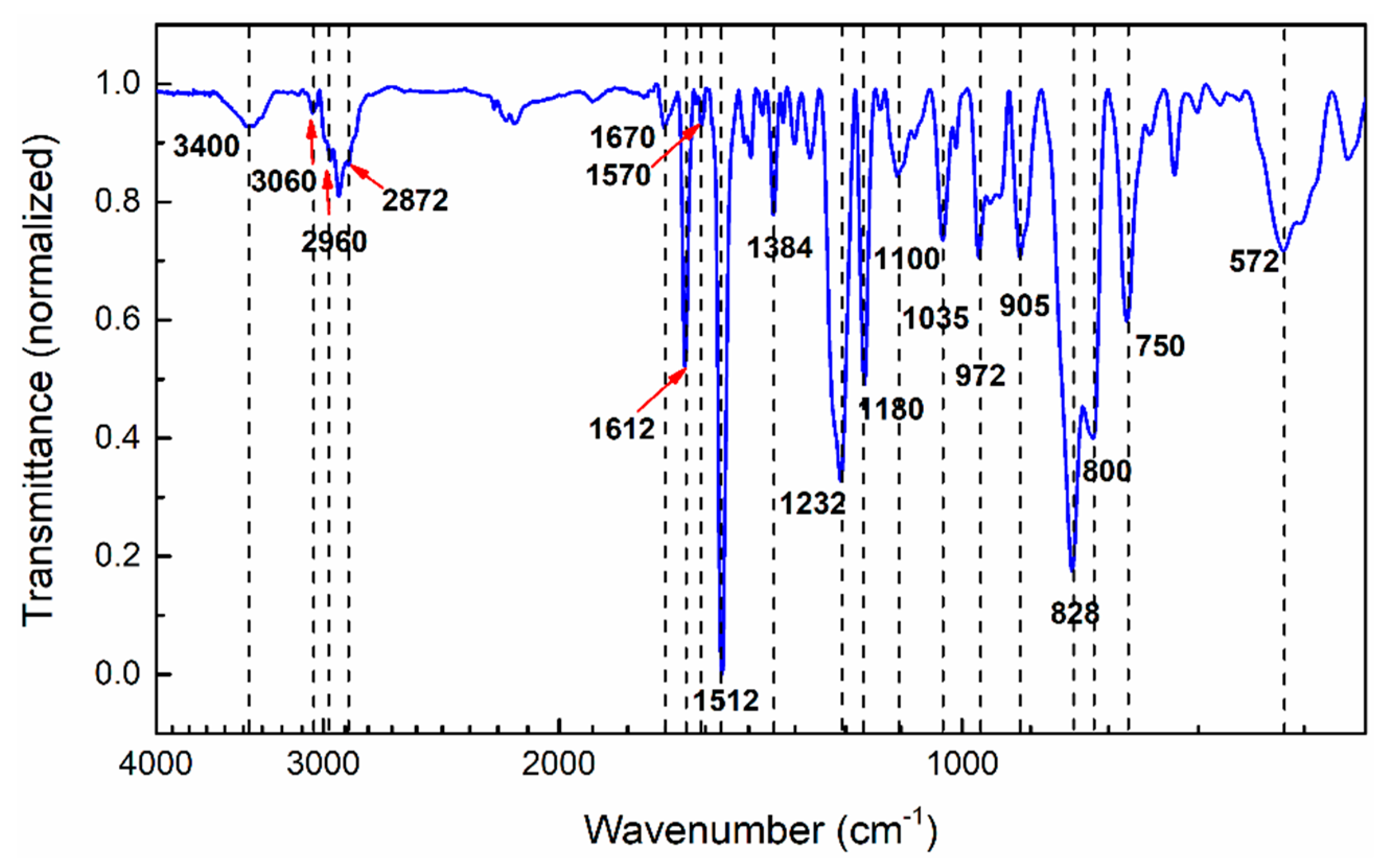


| Wavenumber (cm−1) | Band Assignment |
|---|---|
| 3400 | O–H stretching and symmetric stretching of primary amine |
| 3060 | C–H stretching of epoxide group |
| 2960, 2920, 2872 | C–H stretching of CH2 and CH3 aromatic and aliphatic |
| 1670 | N–H bending of primary amine |
| 1612 | C=C stretching of aromatic ring, N–H bending of primary amine |
| 1570 | N–H bending of primary amine |
| 1512 | C–C stretching of aromatic ring |
| 1384 | C–N stretching of imide |
| 1232 | C–O–C stretching of ether linkage |
| 1180 | C–O stretching of aromatic ring |
| 1100 | Aromatic stretching |
| 1035 | C–O–C stretching of ether linkage |
| 972 | Glycidyl ether of oxirane ring |
| 905 | C–O stretching of oxirane ring |
| 828–800 | H–C= out-of-plane bending of MI ring and 1–4 substituted aromatic ring |
| 750–572 | C–H out-of-plane of aromatic ring |
| Curing Time (min) | Curing Temperature (°C) | UTS (MPa) | E (GPa) | ||||
|---|---|---|---|---|---|---|---|
| Average | SD | CV (%) | Average | SD | CV (%) | ||
| 15 | 110 | 45.1 | 1.5 | 3.3 | 6.4 | 0.2 | 2.5 |
| 135 | 149.7 | 7.4 | 4.9 | 6.1 | 0.2 | 3.6 | |
| 160 | 172.0 | 5.0 | 2.9 | 6.4 | 0.2 | 3.7 | |
| 200 | 180.7 | 8.7 | 4.8 | 6.2 | 0.3 | 4.7 | |
| 30 | 110 135 160 200 | 131.3 141.3 153.7 174.3 | 1.2 6.7 13.3 4.6 | 2.6 4.4 7.7 2.6 | 6.4 6.1 6.0 5.9 | 0.1 0.4 0.2 0.4 | 1.3 6.3 3.6 7.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez-Herrera, C.A.; Cruz-Cruz, I.; Jiménez-Cedeño, I.H.; Martínez-Romero, O.; Elías-Zúñiga, A. Influence of the Epoxy Resin Process Parameters on the Mechanical Properties of Produced Bidirectional [±45°] Carbon/Epoxy Woven Composites. Polymers 2021, 13, 1273. https://doi.org/10.3390/polym13081273
Ramírez-Herrera CA, Cruz-Cruz I, Jiménez-Cedeño IH, Martínez-Romero O, Elías-Zúñiga A. Influence of the Epoxy Resin Process Parameters on the Mechanical Properties of Produced Bidirectional [±45°] Carbon/Epoxy Woven Composites. Polymers. 2021; 13(8):1273. https://doi.org/10.3390/polym13081273
Chicago/Turabian StyleRamírez-Herrera, Claudia A., Isidro Cruz-Cruz, Isaac H. Jiménez-Cedeño, Oscar Martínez-Romero, and Alex Elías-Zúñiga. 2021. "Influence of the Epoxy Resin Process Parameters on the Mechanical Properties of Produced Bidirectional [±45°] Carbon/Epoxy Woven Composites" Polymers 13, no. 8: 1273. https://doi.org/10.3390/polym13081273
APA StyleRamírez-Herrera, C. A., Cruz-Cruz, I., Jiménez-Cedeño, I. H., Martínez-Romero, O., & Elías-Zúñiga, A. (2021). Influence of the Epoxy Resin Process Parameters on the Mechanical Properties of Produced Bidirectional [±45°] Carbon/Epoxy Woven Composites. Polymers, 13(8), 1273. https://doi.org/10.3390/polym13081273








