Polyaniline/Reduced Graphene Oxide Composites for Hole Transporting Layer of High-Performance Inverted Perovskite Solar Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PANI/Graphene Nanocomposite Solution
2.3. Device Fabrication and Characterization
2.4. Characterizations
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stranks, S.D.; Eperon, G.E.; Grancini, G.; Menelaou, C.; Alcocer, M.J.P.; Leijtens, T.; Herz, L.M.; Petrozza, A.; Snaith, H.J. Electron-Hole Diffusion Lengths Exceeding 1 Micrometer in an Organometal Trihalide Perovskite Absorber. Science 2013, 342, 341–344. [Google Scholar] [CrossRef]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- Wu, Y.; Xie, F.; Chen, H.; Yang, X.; Su, H.; Cai, M.; Zhou, Z.; Noda, T.; Han, L. Thermally stable MAPbI3 perovskite solar cells with efficiency of 19.19% and area over 1 cm2 achieved by additive engineering. Adv. Mater. 2017, 29, 1701073. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Chen, C.C.; Wu, Y.; Li, X.; Cai, M.; Liu, X.; Yang, X.; Han, L. Vertical recrystallization for highly efficient and stable formamidinium-based inverted-structure perovskite solar cells. Energy Environ. Sci. 2017, 10, 1942–1949. [Google Scholar] [CrossRef]
- Chiang, C.H.; Wu, C.G. Bulk heterojunction perovskite–PCBM solar cells with high fill factor. Nat. Photonics 2016, 10, 196–200. [Google Scholar] [CrossRef]
- Ye, F.; Yang, W.; Luo, D.; Zhu, R.; Gong, Q. Applications of cesium in the perovskite solar cells. J. Semicond. 2017, 38, 011003. [Google Scholar] [CrossRef]
- Fu, F.; Pisoni, S.; Weiss, T.P.; Feurer, T.; Wackerlin, A.; Fuchs, P.; Nishiwaki, S.; Zortea, L.; Tiwari, A.N.; Buecheler, S. Compositionally graded absorber for efficient and stable near-infrared-transparent perovskite solar cells. Adv. Sci. 2018, 5, 1700675. [Google Scholar] [CrossRef]
- Zhang, H.; Cheng, J.; Li, D.; Lin, F.; Mao, J.; Liang, C.; Jen, A.K.; Grätzel, M.; Choy, W.C. Toward all room-temperature, solution-processed, high-performance planar perovskite solar cells: A new scheme of pyridine-promoted perovskite formation. Adv. Mater. 2017, 29, 1604695. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Wang, X.; Zhao, L.; Jiu, T.; Fang, J. Polyelectrolyte based hole-transporting materials for high performance solution processed planar perovskite solar cells. J. Mater. Chem. A 2015, 3, 15024–15029. [Google Scholar] [CrossRef]
- Huang, D.; Goh, T.; Kong, J.; Zheng, Y.; Zhao, S.; Xu, Z.; Taylor, A.D. Perovskite solar cells with a DMSO-treated PEDOT:PSS hole transport layer exhibit higher photovoltaic performance and enhanced durability. Nanoscale 2017, 9, 4236–4243. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Shi, Z.; Li, T.; Chen, Y.; Huang, W. Stability of perovskite solar cells: A prospective on the substitution of the A cation and X anion. Angew. Chem. Int. Ed. 2017, 56, 1190–1212. [Google Scholar] [CrossRef]
- Zhang, J.; Mao, W.; Hou, X.; Duan, J.; Zhou, J.; Huang, S.; Ou-Yang, W.; Zhang, X.; Sun, Z.; Chen, X. Solution-processed Sr-doped NiOx as hole transport layer for efficient and stable perovskite solar cells. Sol. Energy 2018, 174, 1133–1141. [Google Scholar] [CrossRef]
- Chen, W.; Wu, Y.; Fan, J.; Djurišić, A.B.; Liu, F.; Tam, H.W.; Ng, A.; Surya, C.; Chan, W.K.; Wang, D.; et al. Understanding the Doping Effect on NiO: Toward High-Performance Inverted Perovskite Solar Cells. Adv. Energy Mater. 2018, 8, 1703519. [Google Scholar] [CrossRef]
- Shin, S.S.; Lee, S.J.; Seok, S.I. Metal Oxide Charge Transport Layers for Efficient and Stable Perovskite Solar Cells. Adv. Funct. Mater. 2019, 29, 1900455. [Google Scholar] [CrossRef]
- Bi, C.; Shao, Y.; Yuan, Y.; Xiao, Z.; Huang, J. Non-wetting surface-driven high-aspect-ratio crystalline grain growth for efficient hybrid perovskite solar cells. Nat. Commun. 2015, 6, 7747. [Google Scholar] [CrossRef] [PubMed]
- Murad, A.R.; Iraqi, A.; Aziz, S.B. l Abdullah, S.N.; Brza, M.A. Conducting Polymers for Optoelectronic Devices and Organic Solar Cells: A Review. Polymers 2020, 12, 2627. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Ye, L.; Zhang, S.; Fan, B.; Sun, M.; Hou, J. Ultrathin Polyaniline-based Buffer Layer for Highly Efficient Polymer Solar Cells with Wide Applicability. Sci. Rep. 2014, 4, 6570. [Google Scholar] [CrossRef]
- Xiao, Y.; Han, G.; Chang, Y.; Zhou, H.; Li, M.; Li, Y. An all-solid-state perovskite-sensitized solar cell based on the dual function polyaniline as the sensitizer and p-type hole-transporting material. J. Power Sources 2014, 267, 1–8. [Google Scholar] [CrossRef]
- Ameen, S.; Akhtar, M.S.; Seo, H.-K.; Shin, H.-S. Photocurrent induced by conducting channels of hole transporting layer to adjacent photoactive perovskite sensitized TiO2 thin film: Solar cell paradigm. Langmuir 2014, 30, 12786–12794. [Google Scholar] [CrossRef]
- Abdelmagid, A.; Tahan, A.E.; Habib, M.; Anas, M.; Soliman, M. Effect of different ratios of polyaniline:poly(styrene sulfonate) on the hole extraction ability in perovskite solar cells. Syn. Met. 2020, 259, 16232. [Google Scholar] [CrossRef]
- Mabrouk, S.; Bahrami, B.; Elbohy, H.; Reza, K.M.; Gurung, A.; Liang, M.; Wu, F.; Wang, M.; Yang, S.; Qiao, Q. Synergistic engineering of hole transport materials in perovskite solar cells. InfoMat 2020, 2, 928–941. [Google Scholar] [CrossRef]
- Huang, X.; Guo, H.; Yang, J.; Wang, K.; Niu, X.; Liu, X. Moderately reduced graphene oxide/PEDOT:PSS as hole transport layer to fabricate efficient perovskite hybrid solar cells. Org. Electron. 2016, 39, 288–295. [Google Scholar] [CrossRef]
- Xie, B.; Zhang, Y.; Li, Y.; Chen, W.; Hu, X.; Zhang, S. Solution preparation of molybdenum oxide on graphene: A hole transport layer for efficient perovskite solar cells with a 1.12 V high open-circuit voltage. J. Mater. Sci. Mater. Electron. 2020, 31, 6248–6254. [Google Scholar] [CrossRef]
- Hsu, Y.-C.; Tseng, L.-C.; Lee, R.-H. Graphene oxide sheet–polyaniline nanohybrids for enhanced photovoltaic performance of dye-sensitized solar cells. J. Polym. Sci. Part B Polym. Phys. 2014, 52, 321–332. [Google Scholar] [CrossRef]
- Tarcan, R.; Todor-Boer, O.; Petrovai, I.; Leordean, C.; Astilean, S.; Botiz, I. Reduced graphene oxide today. J. Mater. Chem. C 2020, 8, 1198–1224. [Google Scholar] [CrossRef]
- Bae, W.J.; Kim, K.H.; Park, Y.H.; Jo, W.H. A novel water-soluble and self-doped conducting polyaniline graft copolymer. Chem. Commun. 2003, 22, 2768–2769. [Google Scholar] [CrossRef]
- Hummers W.S., W.S., Jr.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Yun, J.M.; Yeo, J.S.; Kim, J.; Jeong, H.G.; Kim, D.Y.; Noh, N.J.; Kim, S.S.; Ku, B.C.; Na, S.I. Solution-Processable Reduced Graphene Oxide as a Novel Alternative to PEDOT:PSS Hole Transport Layers for Highly Efficient and Stable Polymer Solar Cells. Adv. Mater. 2011, 23, 4923–4928. [Google Scholar] [CrossRef]
- Jung, J.W.; Lee, J.U.; Jo, W.H. High-efficiency polymer solar cells with watersoluble and self-doped conducting polyaniline graft copolymer as hole transport layer. J. Phys. Chem. C 2010, 114, 633–639. [Google Scholar] [CrossRef]
- Jung, J.W.; Park, J.S.; Han, I.K.; Lee, Y.; Park, C.; Kwon, W.; Park, M. Flexible and highly efficient perovskite solar cells with a large active area incorporating cobalt-doped poly(3-hexylthiophene) for enhanced open-circuit voltage. J. Mater. Chem. A 2017, 5, 12158–12167. [Google Scholar] [CrossRef]
- Jung, J.W.; Chueh, C.-C.; Jen, A.K.-Y. A Low-Temperature, Solution-Processable, Cu-Doped Nickel Oxide Hole-Transporting Layer via the Combustion Method for High-Performance Thin-Film Perovskite Solar Cells. Adv. Mater. 2015, 27, 7874–7880. [Google Scholar] [CrossRef] [PubMed]
- Noh, Y.W.; Jin, I.S.; Kim, K.S.; Park, S.H.; Jung, J.W. Reduced energy loss in SnO2/ZnO bilayer electron transport layer-based perovskite solar cells for achieving high efficiencies in outdoor/indoor environments. J. Mater. Chem. A 2020, 8, 17163–17173. [Google Scholar] [CrossRef]
- Lee, J.H.; Jin, I.S.; Noh, Y.W.; Park. S., H.; Jung, J.W. A Solution-Processed Spinel CuCo2O4 as an Effective Hole Transport Layer for Efficient Perovskite Solar Cells with Negligible Hysteresis. ACS Sustain. Chem. Eng. 2019, 7, 17661–17670. [Google Scholar] [CrossRef]
- Han, W.; Ren, G.; Liu, J.; Li, Z.; Bao, H.; Liu, C.; Guo, W. Recent Progress of Inverted Perovskite Solar Cells with a Modified PEDOT:PSS Hole Transport Layer. ACS Appl. Mater. Interfaces 2020, 12, 49297–49322. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Noh, Y.W.; Jin, I.S.; Park, S.H.; Jung, J.W. Facile Surface Engineering of Nickel Oxide Thin Film for Enhanced Power Conversion Efficiency of Planar Heterojunction Perovskite Solar Cells. ACS Sustain. Chem. Eng. 2019, 7, 15495–15503. [Google Scholar] [CrossRef]
- Palma, A.L.; Cinà, L.; Pescetelli, S.; Agresti, A.; Raggio, M.; Paolesse, R.; Bonaccorso, F.; Carlo, A.D. Reduced graphene oxide as efficient and stable hole transporting material in mesoscopic perovskite solar cells. Nano Energy 2016, 22, 349–360. [Google Scholar] [CrossRef]
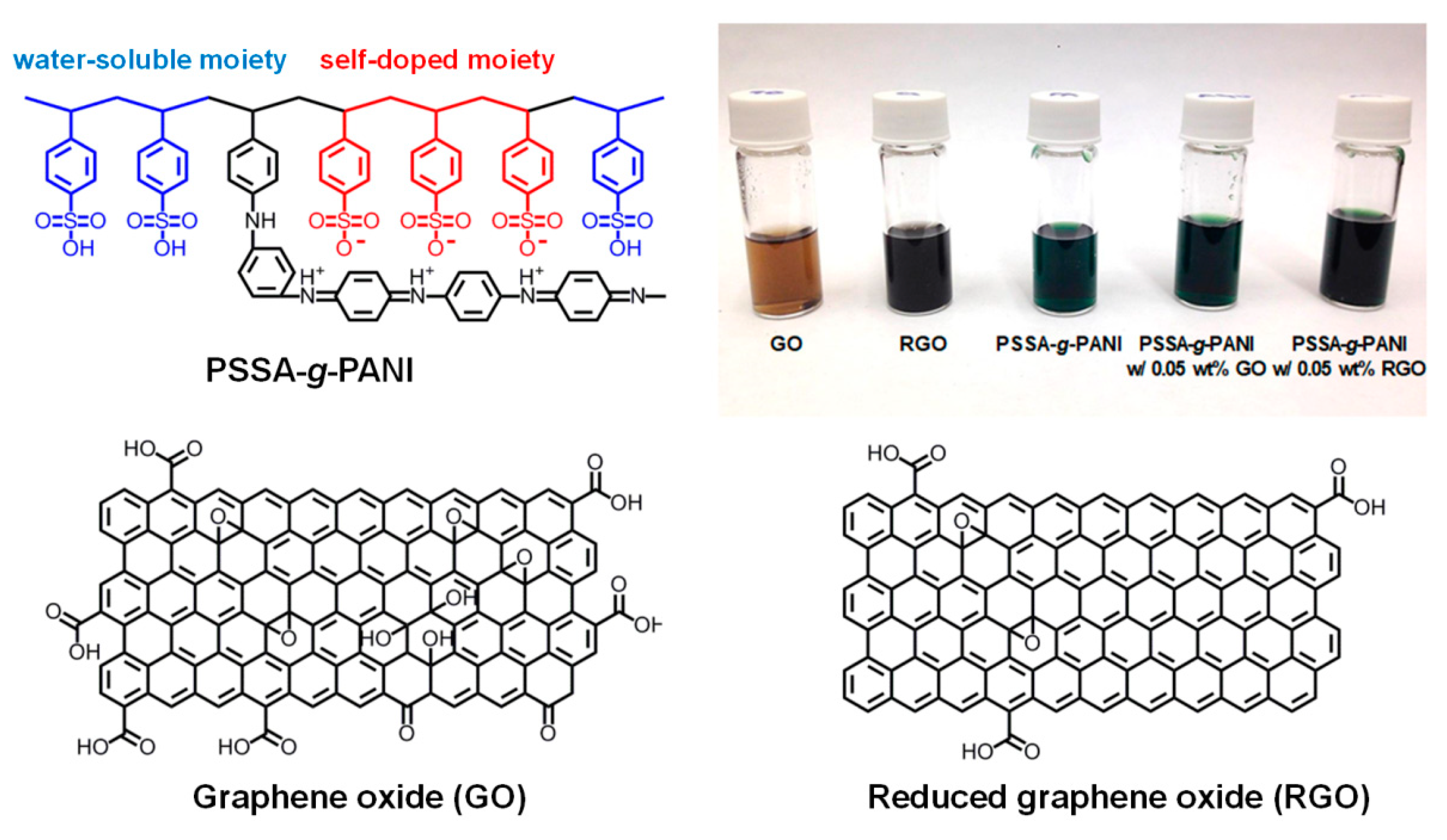
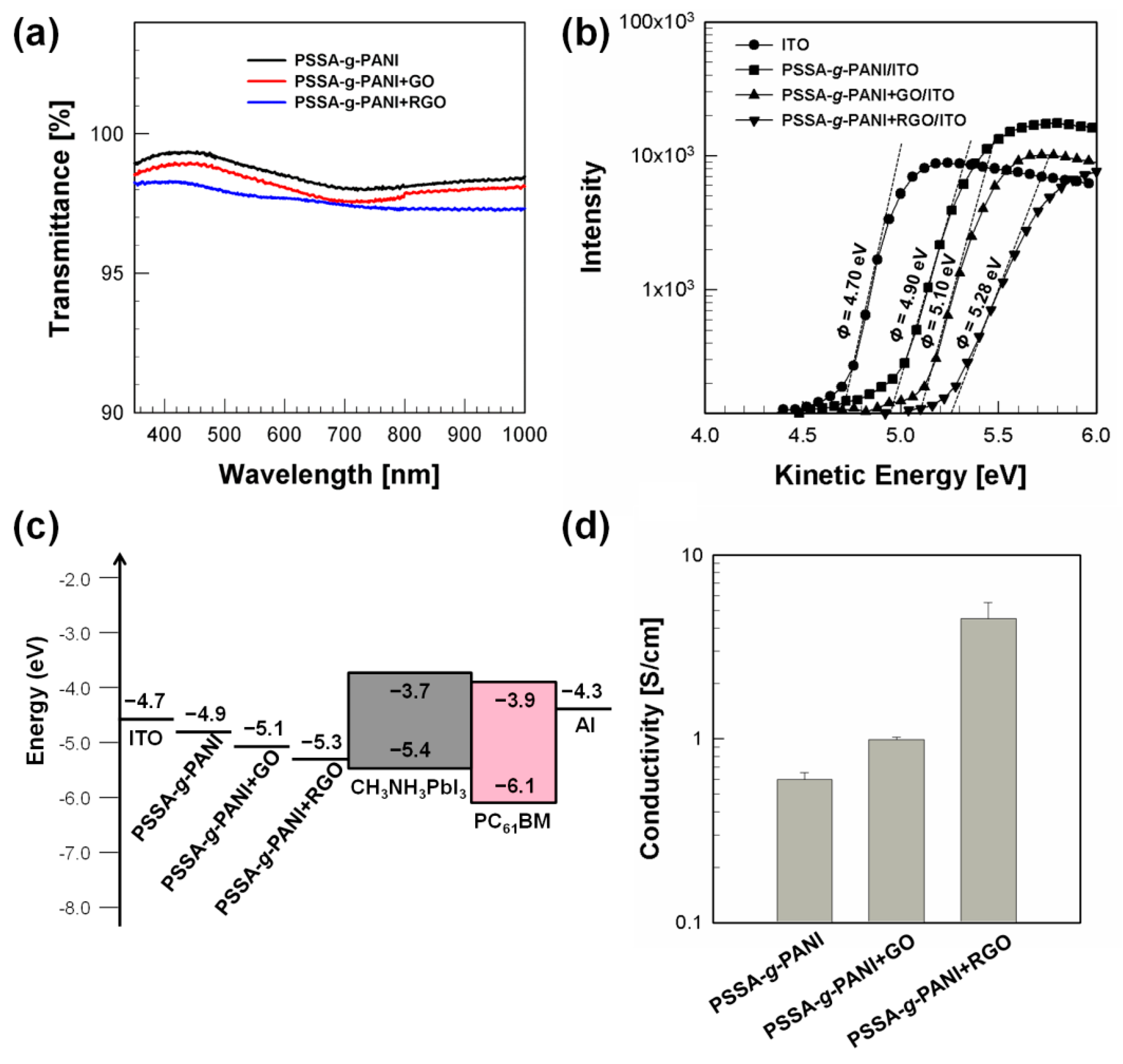

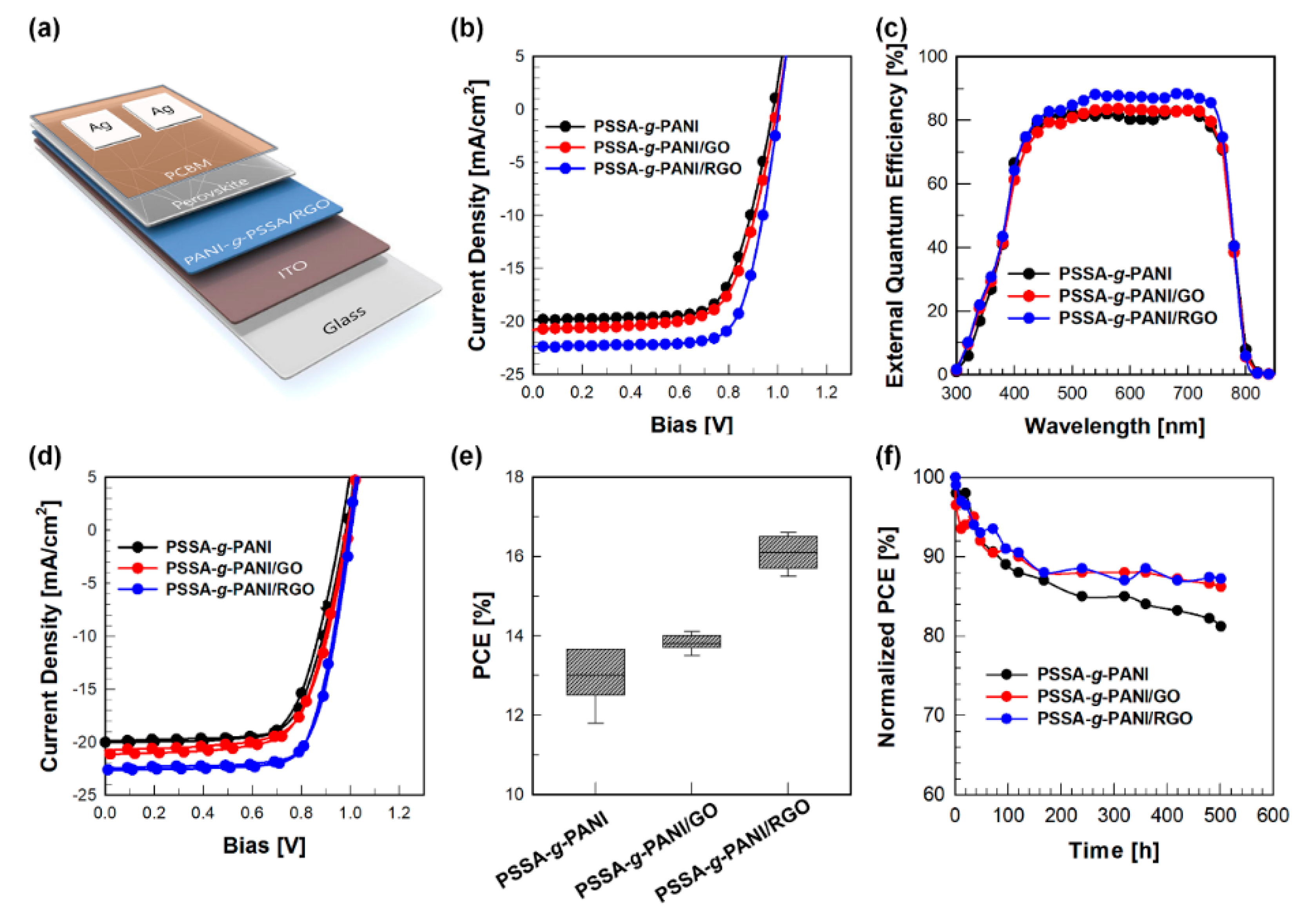
| Hole Transport Layer | VOC [V] | JSC [mA/cm2] | FF | PCE [%] | Rs [Ω] |
|---|---|---|---|---|---|
| PSSA-g-PANI | 0.99 | 19.86 | 0.69 | 13.66 | 2.68 |
| PSSA-g-PANI/GO | 1.00 | 20.78 | 0.68 | 14.11 | 1.74 |
| PSSA-g-PANI/RGO | 1.01 | 22.38 | 0.74 | 16.61 | 0.66 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, J.W.; Son, S.H.; Choi, J. Polyaniline/Reduced Graphene Oxide Composites for Hole Transporting Layer of High-Performance Inverted Perovskite Solar Cells. Polymers 2021, 13, 1281. https://doi.org/10.3390/polym13081281
Jung JW, Son SH, Choi J. Polyaniline/Reduced Graphene Oxide Composites for Hole Transporting Layer of High-Performance Inverted Perovskite Solar Cells. Polymers. 2021; 13(8):1281. https://doi.org/10.3390/polym13081281
Chicago/Turabian StyleJung, Jae Woong, Seung Hwan Son, and Jun Choi. 2021. "Polyaniline/Reduced Graphene Oxide Composites for Hole Transporting Layer of High-Performance Inverted Perovskite Solar Cells" Polymers 13, no. 8: 1281. https://doi.org/10.3390/polym13081281
APA StyleJung, J. W., Son, S. H., & Choi, J. (2021). Polyaniline/Reduced Graphene Oxide Composites for Hole Transporting Layer of High-Performance Inverted Perovskite Solar Cells. Polymers, 13(8), 1281. https://doi.org/10.3390/polym13081281






