New Hyaluronic Acid/Polyethylene Oxide-Based Electrospun Nanofibers: Design, Characterization and In Vitro Biological Evaluation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of HA-PEO-ACs Solutions
2.3. The Viscosity Measurements of HA-PEO-ACs Solutions
2.4. Preparation of HA-PEO-NFs
2.5. Characterization of the HA-PEO-NFs
2.5.1. Surface Morphology and Fiber Diameter
2.5.2. Fourier Transform Infrared Spectroscopy (FTIR)
2.5.3. Water-Vapor Transmission Rate (WVTR)
2.5.4. In Vitro Antioxidant Assays
2.5.5. In Vitro Cytotoxicity (MTS) Assay
2.5.6. Antimicrobial Assay
2.5.7. Data Analysis
3. Results and Discussion
3.1. Viscosity Measurements of HA-PEO-ACs Solutions
3.2. Physicochemical Characterization of HA-PEO-NFs
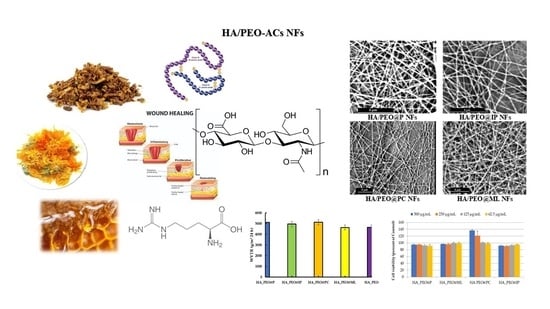
3.2.1. SEM Morphology and Fiber Diameter
3.2.2. Fourier Transform Infrared Spectroscopy (FTIR)
3.2.3. Water-Vapor Transmission Rate (WVTR)
3.2.4. In Vitro Antioxidant Assays
3.2.5. In Vitro Cytotoxicity Assay
3.2.6. Antimicrobial Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Veith, A.P.; Henderson, K.; Spencer, A.; Sligar, A.D.; Baker, A.B. Therapeutic strategies for enhancing angiogenesis in wound healing. Adv. Drug Deliv. Rev. 2019, 146, 97–125. [Google Scholar] [CrossRef] [PubMed]
- Halstead, F.D.; Rauf, M.; Bamford, A.; Wearn, C.M.; Bishop, J.R.B.; Burt, R.; Fraise, A.P.; Moiemen, N.S.; Oppenheim, B.A.; Webber, M.A. Antimicrobial dressings: Comparison of the ability of a panel of dressings to prevent biofilm formation by key burn wound pathogens. Burns 2015, 41, 1683–1694. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Lan, X.; Liang, C.; Zhong, Z.; Xie, R.; Zhou, Y.; Miao, X.; Wang, H.; Wang, W. Honey loaded alginate/PVA nanofibrous membrane as potential bioactive wound dressing. Carbohydr. Polym. 2019, 219, 113–120. [Google Scholar] [CrossRef]
- Xiao, B.; Huang, Q.; Chen, H.; Chen, X.; Long, G. A Fractal model for capillary flow through a single tortuous capillary with roughened surfaces in fibrous porous media. Fractals 2021, 29, 2150017. [Google Scholar] [CrossRef]
- Petrova, V.A.; Chernyakov, D.D.; Poshina, D.N.; Gofman, I.V.; Romanov, D.P.; Mishanin, A.I.; Golovkin, A.S.; Skorik, Y.A. Electrospun bilayer chitosan/hyaluronan material and its compatibility with mesenchymal stem cells. Materials 2019, 12, 2016. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Beekman, J.; Hew, J.; Jackson, S.; Issler-Fisher, A.C.; Parungao, R.; Lajevardi, S.S.; Li, Z.; Maitz, P.K.M. Burn injury: Challenges and advances in burn wound healing, infection, pain and scarring. Adv. Drug Deliv. Rev. 2018, 123, 3–17. [Google Scholar] [CrossRef]
- Boateng, J.; Catanzano, O. Advanced Therapeutic Dressings for Effective Wound Healing—A Review. J. Pharm. Sci. 2015, 104, 3653–3680. [Google Scholar] [CrossRef] [Green Version]
- Wade, R.J.; Bassin, E.J.; Rodell, C.B.; Burdick, J.A. Protease-degradable electrospun fibrous hydrogels. Nat. Commun. 2015, 6, 6639. [Google Scholar] [CrossRef] [Green Version]
- Weng, L.; Xie, J. Smart Electrospun Nanofibers for Controlled Drug Release: Recent Advances and New Perspectives. Curr. Pharm. Des. 2015, 21, 1944–1959. [Google Scholar] [CrossRef] [Green Version]
- Hassiba, A.J.; El Zowalaty, M.E.; Webster, T.J.; Abdullah, A.M.; Nasrallah, G.K.; Khalil, K.A.; Luyt, A.S.; Elzatahry, A.A. Synthesis, characterization, and antimicrobial properties of novel double layer nanocomposite electrospun fibers for wound dressing applications. Int. J. Nanomed. 2017, 12, 2205–2213. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Chen, J.; Shi, M.; Zhang, H.; Ma, P.X.; Guo, B. Electroactive anti-oxidant polyurethane elastomers with shape memory property as non-adherent wound dressing to enhance wound healing. Chem. Eng. J. 2019, 375, 121999. [Google Scholar] [CrossRef]
- Zhang, S.; Hou, J.; Yuan, Q.; Xin, P.; Cheng, H.; Gu, Z.; Wu, J. Arginine derivatives assist dopamine-hyaluronic acid hybrid hydrogels to have enhanced antioxidant activity for wound healing. Chem. Eng. J. 2020, 392, 123775. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, H.; Guo, B.; Dong, R.; Qiu, Y.; Ma, P.X. Antibacterial anti-oxidant electroactive injectable hydrogel as self-healing wound dressing with hemostasis and adhesiveness for cutaneous wound healing. Biomaterials 2017, 122, 34–47. [Google Scholar] [CrossRef]
- Aduba, D.C.; Yang, H. Polysaccharide fabrication platforms and biocompatibility assessment as candidate wound dressing materials. Bioengineering 2017, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Tang, X.; Jia, Y.; Ho, C.T.; Huang, Q. Applications and delivery mechanisms of hyaluronic acid used for topical/transdermal delivery—A review. Int. J. Pharm. 2020, 578, 119127. [Google Scholar] [CrossRef]
- El-Aassar, M.R.; Ibrahim, O.M.; Fouda, M.M.G.; El-Beheri, N.G.; Agwa, M.M. Wound healing of nanofiber comprising Polygalacturonic/Hyaluronic acid embedded silver nanoparticles: In-vitro and in-vivo studies. Carbohydr. Polym. 2020, 238, 116175. [Google Scholar] [CrossRef]
- Brenner, E.K.; Schiffman, J.D.; Toth, L.J.; Szewczyk, J.C.; Schauer, C.L. Phosphate salts facilitate the electrospinning of hyaluronic acid fiber mats. J. Mater. Sci. 2013, 48, 7805–7811. [Google Scholar] [CrossRef] [Green Version]
- Ahire, J.J.; Robertson, D.D.; van Reenen, A.J.; Dicks, L.M.T. Polyethylene oxide (PEO)-hyaluronic acid (HA) nanofibers with kanamycin inhibits the growth of Listeria monocytogenes. Biomed. Pharmacother. 2017, 86, 143–148. [Google Scholar] [CrossRef]
- Sarkar, S.; Chaudhary, A.; Saha, T.K.; Das, A.K.; Chatterjee, J. Modulation of collagen population under honey assisted wound healing in diabetic rat model. Wound Med. 2018, 20, 7–17. [Google Scholar] [CrossRef]
- Rückriemen, J.; Klemm, O.; Henle, T. Manuka honey (Leptospermum scoparium) inhibits jack bean urease activity due to methylglyoxal and dihydroxyacetone. Food Chem. 2017, 230, 540–546. [Google Scholar] [CrossRef]
- Yang, X.; Fan, L.; Ma, L.; Wang, Y.; Lin, S.; Yu, F.; Pan, X.; Luo, G.; Zhang, D.; Wang, H. Green electrospun Manuka honey/silk fibroin fibrous matrices as potential wound dressing. Mater. Des. 2017, 119, 76–84. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, S.; Gao, Y.; Zhai, Y. Electrospun nanofibers as a wound dressing for treating diabetic foot ulcer. Asian J. Pharm. Sci. 2019, 14, 130–143. [Google Scholar] [CrossRef]
- Patil, S.; Desai, N.; Mahadik, K.; Paradkar, A. Can green synthesized propolis loaded silver nanoparticulate gel enhance wound healing caused by burns? Eur. J. Integr. Med. 2015, 7, 243–250. [Google Scholar] [CrossRef]
- Debats, I.B.J.G.; Wolfs, T.G.A.M.; Gotoh, T.; Cleutjens, J.P.M.; Peutz-Kootstra, C.J.; van der Hulst, R.R.W.J. Role of arginine in superficial wound healing in man. Nitric OxideBiol. Chem. 2009, 21, 175–183. [Google Scholar] [CrossRef]
- Iacob, A.T.; Drăgan, M.; Gheţu, N.; Pieptu, D.; Vasile, C.; Buron, F.; Routier, S.; Giusca, S.E.; Caruntu, I.D.; Profire, L. Preparation, characterization and wound healing effects of new membranes based on chitosan, hyaluronic acid and arginine derivatives. Polymers 2018, 8, 607. [Google Scholar] [CrossRef] [Green Version]
- Bennacef-Heffar, N.; Laraba-Djebari, F. Beneficial effects of Heparin and L Arginine on dermonecrosis effect induced by Vipera lebetina venom: Involvement of NO in skin regeneration. Acta Trop. 2017, 171, 226–232. [Google Scholar] [CrossRef]
- Rieger, K.A.; Birch, N.P.; Schiffman, J.D. Electrospinning chitosan/poly(ethylene oxide) solutions with essential oils: Correlating solution rheology to nanofiber formation. Carbohydr. Polym. 2016, 139, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Oryan, A.; Alemzadeh, E. Effects of insulin on wound healing: A review of animal and human evidences. Life Sci. 2017, 174, 59–67. [Google Scholar] [CrossRef]
- Emanuelli, T.; Burgeiro, A.; Carvalho, E. Effects of insulin on the skin: Possible healing benefits for diabetic foot ulcers. Arch. Dermatol. Res. 2016, 308, 677–694. [Google Scholar] [CrossRef]
- Agatonovic-Kustrin, S.; Babazadeh Ortakand, D.; Morton, D.W.; Yusof, A.P. Rapid evaluation and comparison of natural products and antioxidant activity in calendula, feverfew, and German chamomile extracts. J. Chromatogr. A 2015, 1385, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Nicolaus, C.; Junghanns, S.; Hartmann, A.; Murillo, R.; Ganzera, M.; Merfort, I. In vitro studies to evaluate the wound healing properties of Calendula officinalis extracts. J. Ethnopharmacol. 2017, 196, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Rad, Z.P.; Mokhtari, J.; Abbasi, M. Calendula officinalis extract/PCL/Zein/Gum arabic nanofibrous bio-composite scaffolds via suspension, two-nozzle and multilayer electrospinning for skin tissue engineering. Int. J. Biol. Macromol. 2019, 135, 530–543. [Google Scholar]
- Khodabakhshi, D.; Eskandarinia, A.; Kefayat, A.; Rafienia, M.; Navid, S.; Karbasi, S.; Moshtaghian, J. In vitro and in vivo performance of a propolis-coated polyurethane wound dressing with high porosity and antibacterial efficacy. Colloids Surf. B Biointerfaces 2019, 178, 177–184. [Google Scholar] [CrossRef]
- Hussein, Y.; El-Fakharany, E.M.; Kamoun, E.A.; Loutfy, S.A.; Amin, R.; Taha, T.H.; Salim, S.A.; Amer, M. Electrospun PVA/hyaluronic acid/L-arginine nanofibers for wound healing applications: Nanofibers optimization and in vitro bioevaluation. Int. J. Biol. Macromol. 2020, 164, 667–676. [Google Scholar] [CrossRef]
- Adeli, H.; Khorasani, M.T.; Parvazinia, M. Wound dressing based on electrospun PVA/chitosan/starch nanofibrous mats: Fabrication, antibacterial and cytocompatibility evaluation and in vitro healing assay. Int. J. Biol. Macromol. 2019, 122, 238–254. [Google Scholar] [CrossRef]
- Tomadoni, B.; Ponce, A.; Pereda, M.; Ansorena, M.R. Vanillin as a natural cross-linking agent in chitosan-based films: Optimizing formulation by response surface methodology. Polym. Test. 2019, 78, 105935. [Google Scholar] [CrossRef]
- Sadiq, A.; Mahmood, F.; Ullah, F.; Ayaz, M.; Ahmad, S.; Haq, F.U.; Khan, G.; Jan, M.S. Synthesis, anticholinesterase and antioxidant potentials of ketoesters derivatives of succinimides: A possible role in the management of alzheimer’s. Chem. Cent. J. 2015, 9, 31. [Google Scholar] [CrossRef] [Green Version]
- Xue, M.; Wang, Z.; Sun, S.F.; Huang, Z.S.; Zhang, X.X.; Ma, J.; Dong, X.L. Mechanism investigation on the formation of high valent iron intermediate in Fe(VI) oxidation using ABTS as a probe: Effect of excess Fe(VI). Chem. Eng. J. 2020, 387, 124123. [Google Scholar] [CrossRef]
- Orhan, I.E.; Senol, F.S.; Yılmaz, B.S.; Altun, M.L.; Özbilgin, S.; Yazgan, A.N.; Yüksel, E.; İşcan, G.S. Neuroprotective potential of Viburnum orientale Pallas through enzyme inhibition and antioxidant activity assays. S.Afr. J. Bot. 2018, 114, 126–131. [Google Scholar] [CrossRef]
- Ajmal, G.; Bonde, G.V.; Mittal, P.; Khan, G.; Pandey, V.K.; Bakade, B.V.; Mishra, B. Biomimetic PCL-gelatin based nanofibers loaded with ciprofloxacin hydrochloride and quercetin: A potential antibacterial and anti-oxidant dressing material for accelerated healing of a full thickness wound. Int. J. Pharm. 2019, 567, 118480. [Google Scholar] [CrossRef]
- ISO. ISO 10993-5:2009(E): Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity; ISO: Geneva, Switzerland, 2009. [Google Scholar]
- ISO. ISO 10993-4. Biological Evaluation of Medical Devices—Part 4: Selection of Tests for Interactions with Blood; ISO: Geneva, Switzerland, 2017. [Google Scholar]
- Brenner, E.K.; Schiffman, J.D.; Thompson, E.A.; Toth, L.J.; Schauer, C.L. Electrospinning of hyaluronic acid nanofibers from aqueous ammonium solutions. Carbohydr. Polym. 2012, 87, 926–929. [Google Scholar] [CrossRef]
- Séon-Lutz, M.; Couffin, A.C.; Vignoud, S.; Schlatter, G.; Hébraud, A. Electrospinning in water and in situ crosslinking of hyaluronic acid/cyclodextrin nanofibers: Towards wound dressing with controlled drug release. Carbohydr. Polym. 2019, 207, 276–287. [Google Scholar] [CrossRef]
- El-Aassar, M.R.; El-Beheri, N.G.; Agwa, M.M.; Eltaher, H.M.; Alseqely, M.; Sadik, W.S.; El-Khordagui, L. Antibiotic-free combinational hyaluronic acid blend nanofibers for wound healing enhancement. Int. J. Biol. Macromol. 2021, 167, 1552–1563. [Google Scholar] [CrossRef]
- De Oliveira, S.A.; da Silva, B.C.; Riegel-Vidotti, I.C.; Urbano, A.; de Sousa Faria-Tischer, P.C.; Tischer, C.A. Production and characterization of bacterial cellulose membranes with hyaluronic acid from chicken comb. Int. J. Biol. Macromol. 2017, 97, 642–653. [Google Scholar] [CrossRef]
- Pucić, I.; Jurkin, T. FTIR assessment of poly(ethylene oxide) irradiated in solid state, melt and aqeuous solution. Radiat. Phys. Chem. 2012, 81, 1426–1429. [Google Scholar] [CrossRef]
- Sarmento, B.; Ferreira, D.; Veiga, F.; Ribeiro, A. Characterization of insulin-loaded alginate nanoparticles produced by ionotropic pre-gelation through DSC and FTIR studies. Carbohydr. Polym. 2006, 66, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Hossen, M.S.; Ali, M.Y.; Jahurul, M.H.A.; Abdel-Daim, M.M.; Gan, S.H.; Khalil, M.I. Beneficial roles of honey polyphenols against some human degenerative diseases: A review. Pharmacol. Rep. 2017, 69, 1194–1205. [Google Scholar] [CrossRef]
- Bolanos De La Torre, A.A.S.; Henderson, T.; Nigam, P.S.; Owusu-Apenten, R.K. A universally calibrated microplate ferric reducing antioxidant power (FRAP) assay for foods and applications to Manuka honey. Food Chem. 2015, 174, 119–123. [Google Scholar] [CrossRef]
- Weber, M.; Steinle, H.; Golombek, S.; Hann, L.; Schlensak, C.; Wendel, H.P.; Avci-Adali, M. Blood-Contacting Biomaterials: In Vitro Evaluation of the Hemocompatibility. Front. Bioeng. Biotechnol. 2018, 6, 99. [Google Scholar] [CrossRef]
- Ullah, A.; Ullah, S.; Khan, M.Q.; Hashmi, M.; Nam, P.D.; Kato, Y.; Tamada, Y.; Kim, I.S. Manuka honey incorporated cellulose acetate nanofibrous mats: Fabrication and in vitro evaluation as a potential wound dressing. Int. J. Biol. Macromol. 2020, 155, 479–489. [Google Scholar] [CrossRef]
- Oryan, A.; Alemzadeh, E.; Moshiri, A. Potential role of propolis in wound healing: Biological properties and therapeutic activities. Biomed. Pharmacother. 2018, 98, 469–483. [Google Scholar] [CrossRef]
- Rathinamoorthy, R.; Sasikala, L. In vivo—Wound healing studies of Leptospermum scoparium honey loaded chitosan bioactive wound dressing. Wound Med. 2019, 26, 100162. [Google Scholar] [CrossRef]
- Ebrahimifard, F.; Nooraei, N.; Fathi, M.; Dehghani, M. Effect of Insulin on Healing of Pressure Sore. Arch. Crit. Care Med. 2015, 1, e527. [Google Scholar] [CrossRef]
- Abou Zekry, S.S.; Abdellatif, A.; Azzazy, H.M.E. Fabrication of pomegranate/honey nanofibers for use as antibacterial wound dressings. Wound Med. 2020, 28, 100181. [Google Scholar] [CrossRef]
- Fayemi, O.E.; Ekennia, A.C.; Katata-Seru, L.; Ebokaiwe, A.P.; Ijomone, O.M.; Onwudiwe, D.C.; Ebenso, E.E. Antimicrobial and Wound Healing Properties of Polyacrylonitrile-Moringa Extract Nanofibers. ACS Omega 2018, 3, 4791–4797. [Google Scholar] [CrossRef]
- Motealleh, B.; Zahedi, P.; Rezaeian, I.; Moghimi, M.; Abdolghaffari, A.H.; Zarandi, M.A. Morphology, drug release, antibacterial, cell proliferation, and histology studies of chamomile-loaded wound dressing mats based on electrospun nanofibrous poly(ε-caprolactone)/polystyrene blends. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 977–987. [Google Scholar] [CrossRef]
- Hrynyk, M.; Martins-Green, M.; Barron, A.E.; Neufeld, R.J. Sustained prolonged topical delivery of bioactive human insulin for potential treatment of cutaneous wounds. Int. J. Pharm. 2010, 398, 146–154. [Google Scholar] [CrossRef]
- Unnithan, A.R.; Gnanasekaran, G.; Sathishkumar, Y.; Lee, Y.S.; Kim, C.S. Electrospun antibacterial polyurethane-cellulose acetate-zein composite mats for wound dressing. Carbohydr. Polym. 2014, 102, 884–892. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, V.; Singh, V.K.; Sharma, P.K.; Singh, V. Essential oil-based nanostructures for inflammation and rheumatoid arthritis. J. Drug Deliv. Sci. Technol. 2020, 60, 101983. [Google Scholar] [CrossRef]







| HA-PEO-NFs | Solvent | ACs |
|---|---|---|
| HA_PEO@P | normal saline | P: 7% (wt/V) 1 |
| HA_PEO@IP | normal saline | I: 100 IU/mL P: 7% (wt/V) |
| HA_PEO@PC | Calendula officinalis infusion | P: 7% (wt/V) |
| HA_PEO@ML | normal saline | M: 7% (wt/V) 1 L: 7%(wt/V) 1 |
| HA-PEO-ACs | Ostwald I Model | Carreau–Yassuda Model Zero-Shear Viscosity, η0 (Pa∙s) | Apparent Viscosity, at 100 s−1 (ηa,100, Pa∙s) | |
|---|---|---|---|---|
| Flow Behavior Index, a (K) | Consistency Coefficient, b (n) | |||
| HA_PEO@IP | 2.5 | 0.56 | 1.5 | 0.4 |
| HA_PEO@ML | 3.8 | 0.59 | 1.7 | 1.5 |
| HA_PEO@P | 2.3 | 0.57 | 1.2 | 0.4 |
| HA_PEO@PC | 1.9 | 0.58 | 0.9 | 0.3 |
| Sample | Diameter of Inhibition Area (mm) | |||
|---|---|---|---|---|
| S. aureus ATCC 25923 | E. coli ATCC 25922 | P. aeruginosa ATCC 27853 | C. albicans ATCC 10231 | |
| HA_PEO@P | 11 | 0 | 0 | 0 |
| HA_PEO@IP | 11 | 0 | 0 | 0 |
| HA_PEO@PC | 17 | 16 | 14 | 0 |
| HA_PEO@ML | 12 | 15 | 10 | 0 |
| HA_PEO | 0 | 0 | 0 | 0 |
| CIP (5 µg/disc) | 26 | 28 | 30 | Nt |
| VRC (1 µg/disc) | nt | nt | nt | 27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ionescu, O.M.; Mignon, A.; Iacob, A.T.; Simionescu, N.; Confederat, L.G.; Tuchilus, C.; Profire, L. New Hyaluronic Acid/Polyethylene Oxide-Based Electrospun Nanofibers: Design, Characterization and In Vitro Biological Evaluation. Polymers 2021, 13, 1291. https://doi.org/10.3390/polym13081291
Ionescu OM, Mignon A, Iacob AT, Simionescu N, Confederat LG, Tuchilus C, Profire L. New Hyaluronic Acid/Polyethylene Oxide-Based Electrospun Nanofibers: Design, Characterization and In Vitro Biological Evaluation. Polymers. 2021; 13(8):1291. https://doi.org/10.3390/polym13081291
Chicago/Turabian StyleIonescu, Oana Maria, Arn Mignon, Andreea Teodora Iacob, Natalia Simionescu, Luminita Georgeta Confederat, Cristina Tuchilus, and Lenuța Profire. 2021. "New Hyaluronic Acid/Polyethylene Oxide-Based Electrospun Nanofibers: Design, Characterization and In Vitro Biological Evaluation" Polymers 13, no. 8: 1291. https://doi.org/10.3390/polym13081291
APA StyleIonescu, O. M., Mignon, A., Iacob, A. T., Simionescu, N., Confederat, L. G., Tuchilus, C., & Profire, L. (2021). New Hyaluronic Acid/Polyethylene Oxide-Based Electrospun Nanofibers: Design, Characterization and In Vitro Biological Evaluation. Polymers, 13(8), 1291. https://doi.org/10.3390/polym13081291