Development and Characterization of Cellulose/Iron Acetate Nanofibers for Bone Tissue Engineering Applications
Abstract
:1. Introduction
2. Experimental
2.1. Nanofibers Mats Fabrication
2.2. Characterizations
2.3. Biomineralization Test
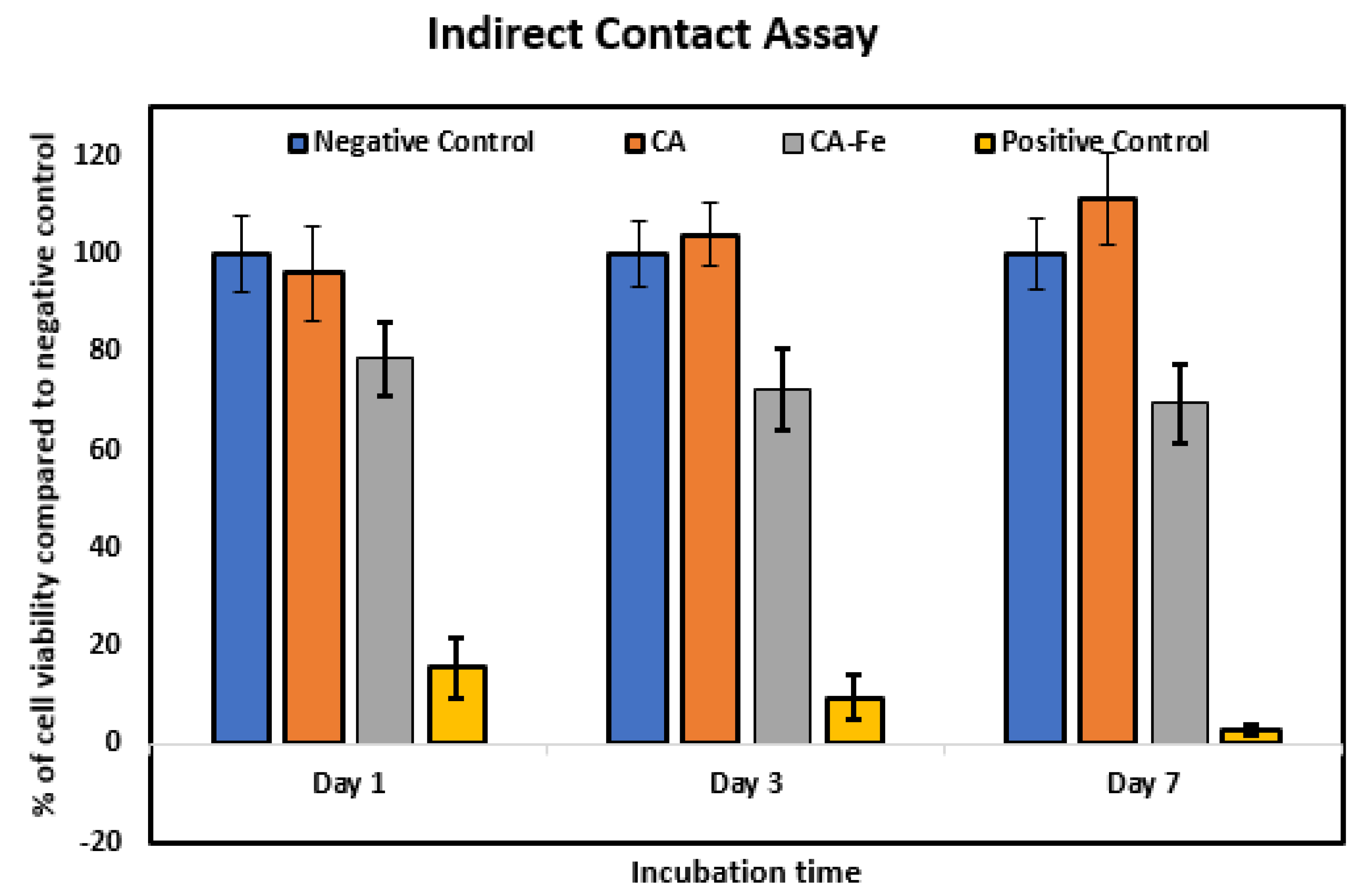
2.4. Indirect Cytotoxicity Assay
2.5. Mats Cell Attachment
2.6. Cell Proliferation on the Nanofiber Mats
3. Results and Discussions
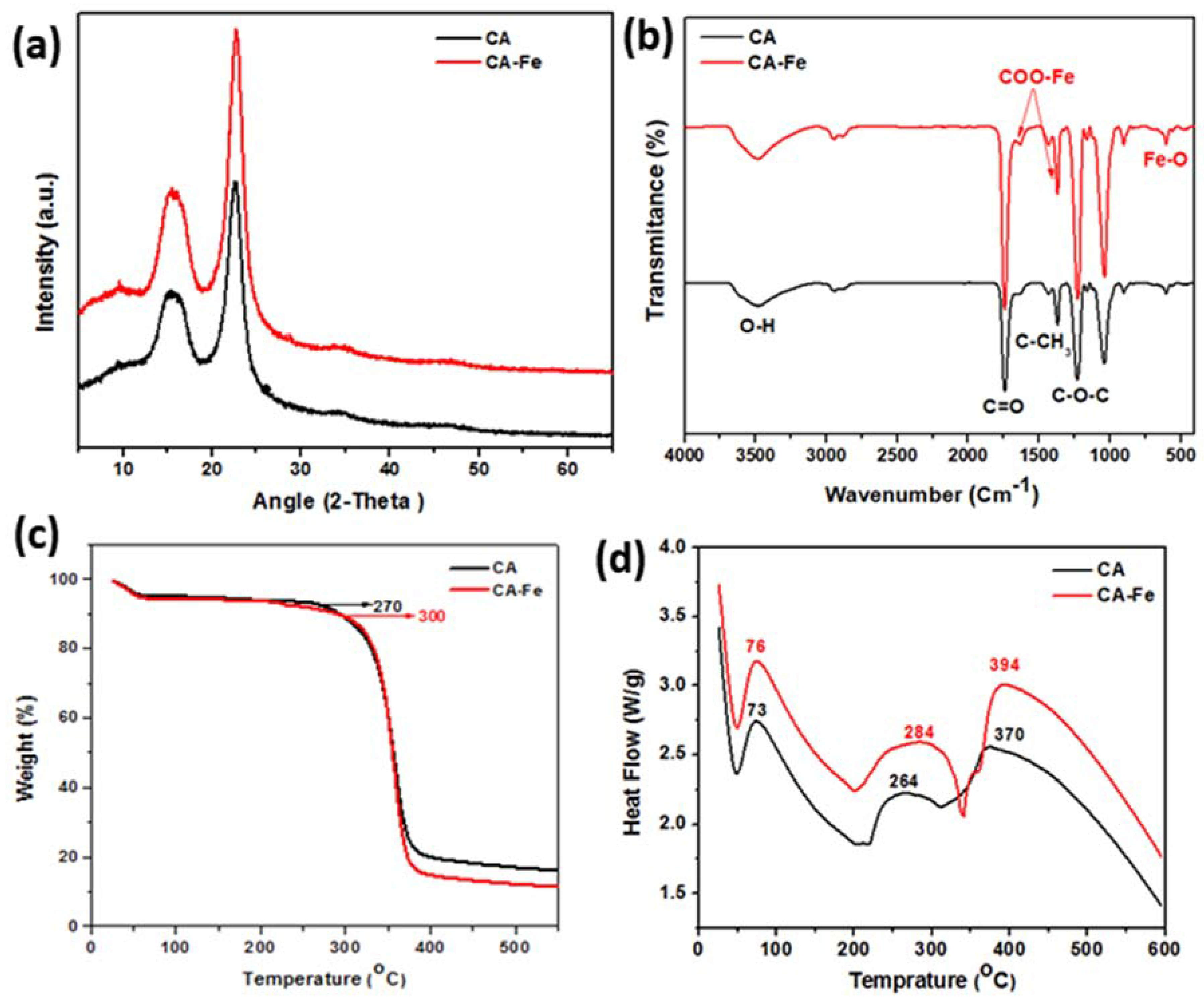
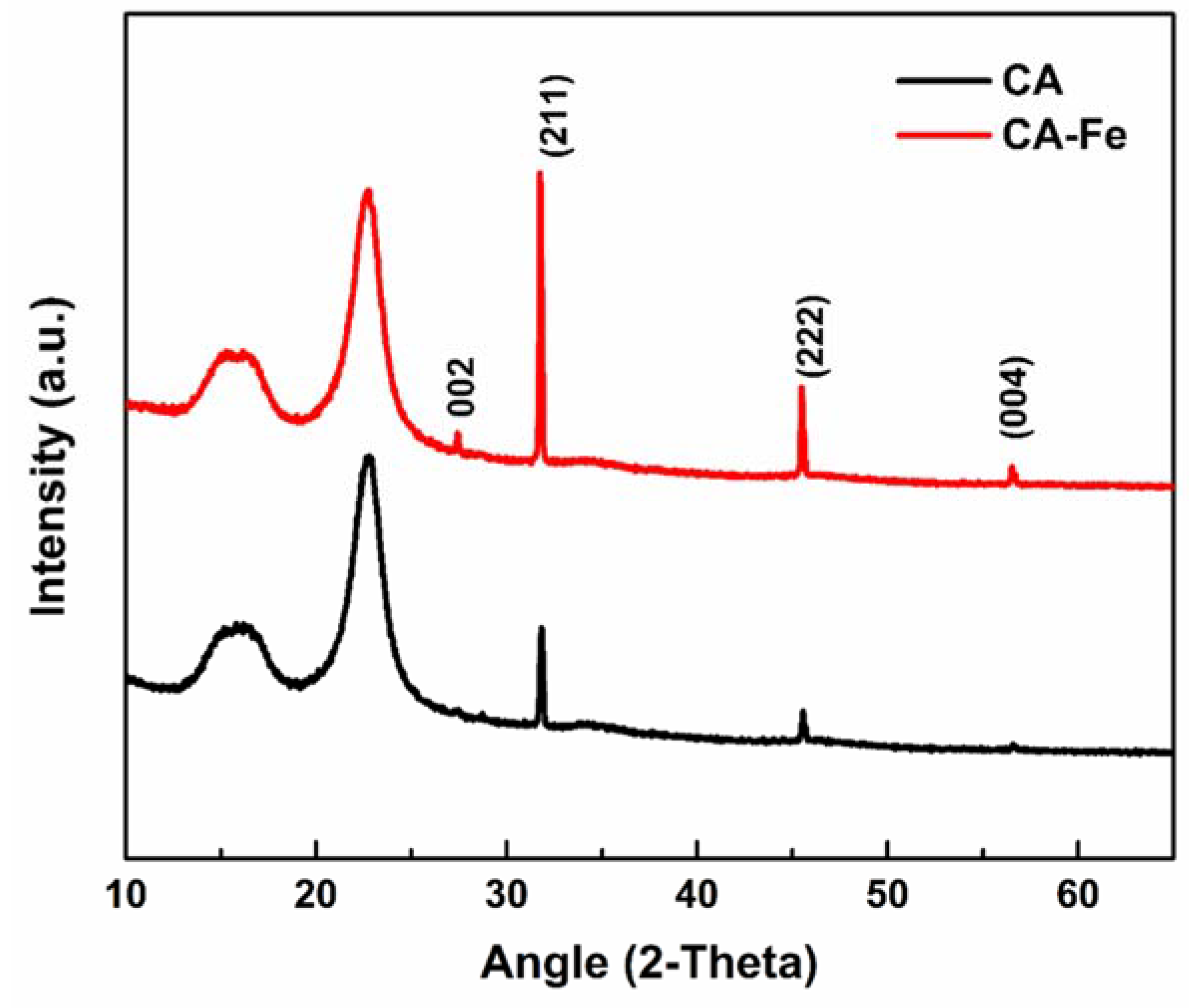
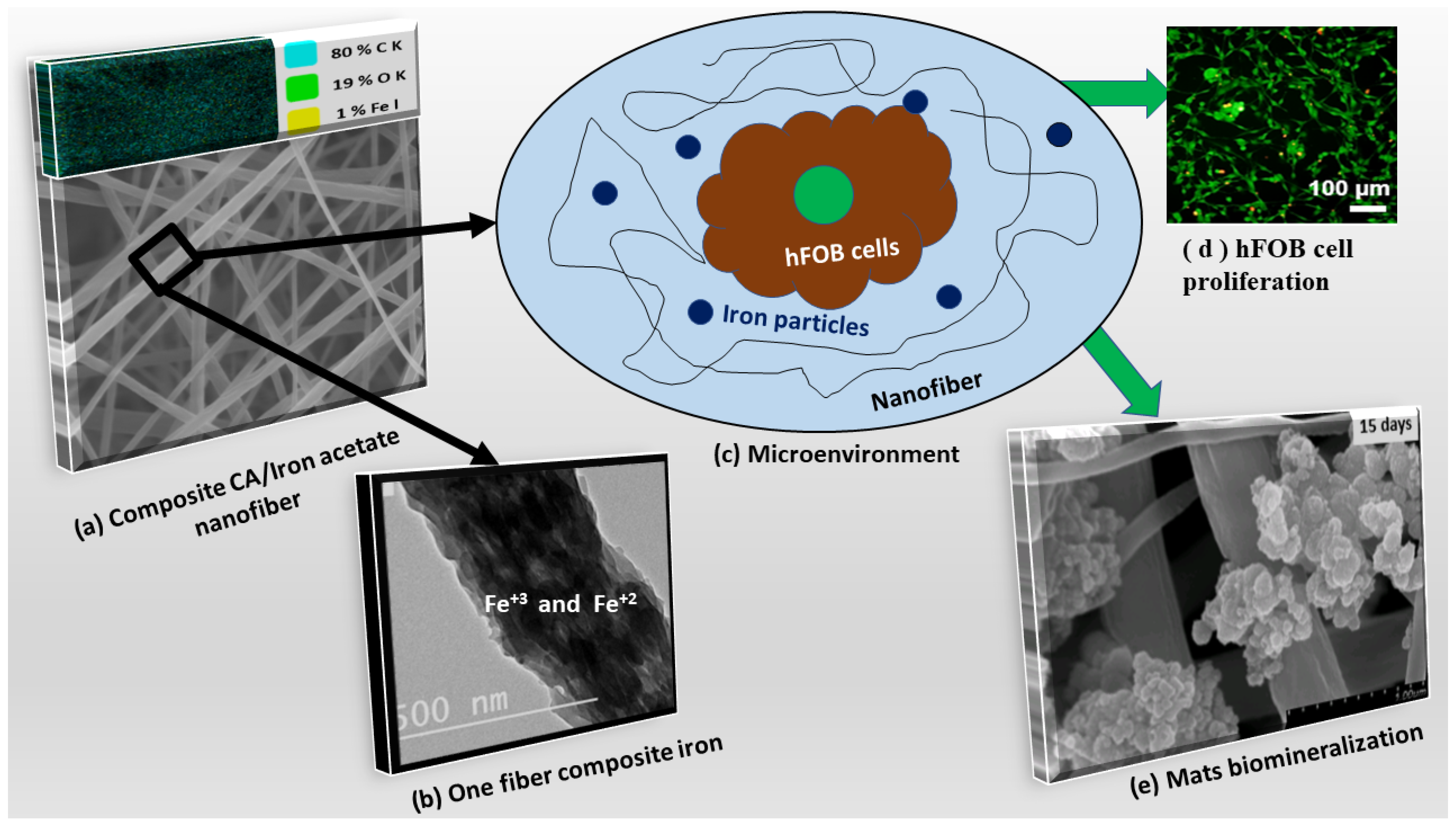
3.1. Characterization of the Developed Mats
3.2. Physiochemical Properties
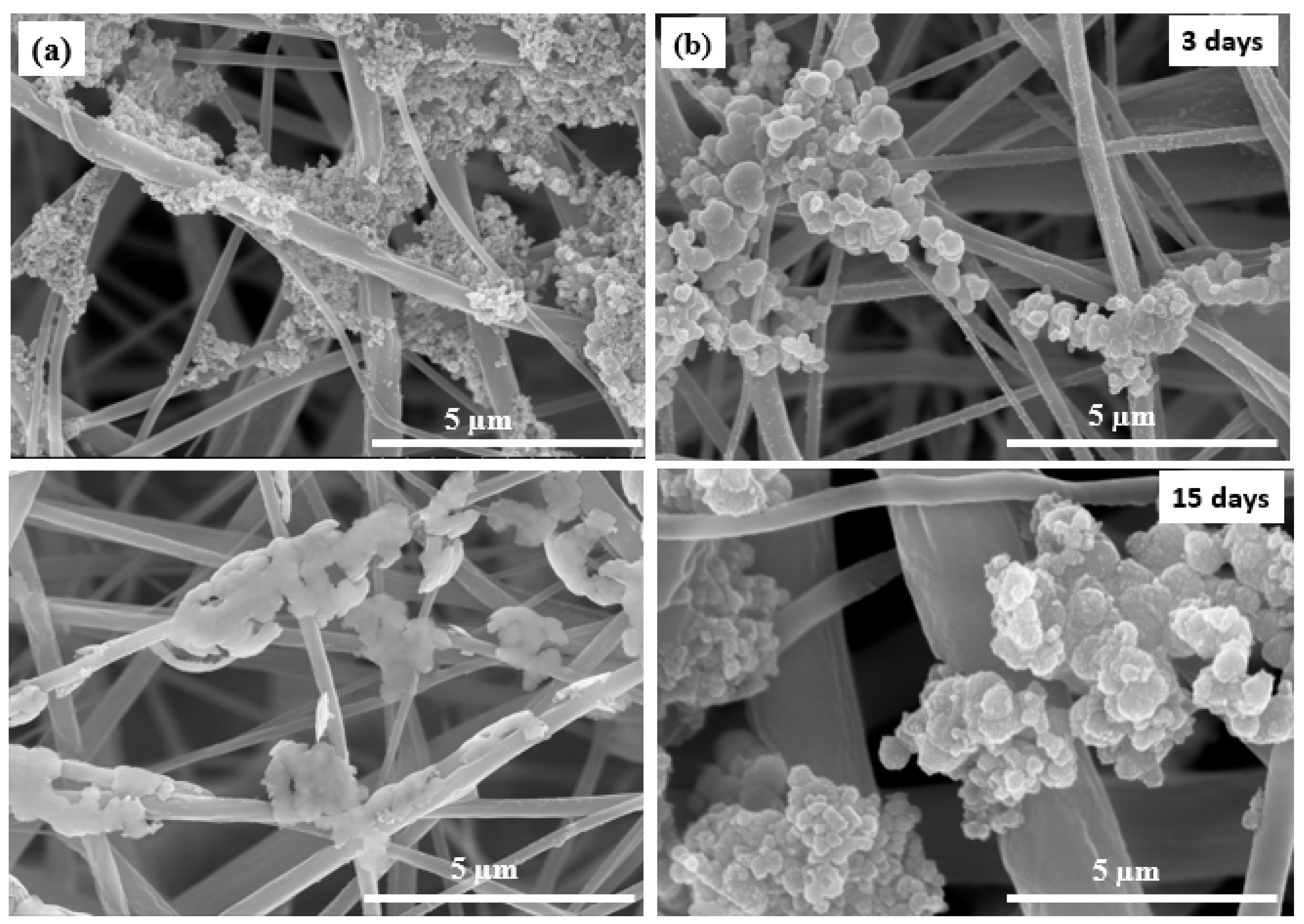
3.3. Biomimetic Mineralization
3.4. Biocompatibility
4. Conclusions and Future Prospective
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Langer, R.; Vacanti, J. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swetha, M.; Sahithi, K.; Moorthi, A.; Srinivasan, N.; Ramasamy, K.; Selvamurugan, N. Biocomposites containing natural polymers and hydroxyapatite for bone tissue engineering. Int. J. Biol. Macromol. 2010, 47, 1–4. [Google Scholar] [CrossRef]
- Healy, K.; Guldberg, R. Bone tissue engineering. J. Musculoskelet. Neuronal Interact. 2007, 7, 328. [Google Scholar]
- Sajesh, K.; Kiran, K.; Nair, S.V.; Jayakumar, R. Sequential layer-by-layer electrospinning of nano SrCO3/PRP loaded PHBV fibrous scaffold for bone tissue engineering. Compos. Part B Eng. 2016, 99, 445–452. [Google Scholar] [CrossRef]
- Abdal-hay, A.; Taha, M.; Mousa, H.M.; Bartnikowski, M.; Hassan, M.L.; Dewidar, M.; Ivanovski, S. Engineering of electrically-conductive poly(ε-caprolactone)/multi-walled carbon nanotubes composite nanofibers for tissue engineering applications. Ceram. Int. 2019, 45, 15736–15740. [Google Scholar] [CrossRef]
- Pietrucha, K. Physicochemical properties of 3D collagen-CS scaffolds for potential use in neural tissue engineering. Int. J. Biol. Macromol. 2015, 80, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Mousa, H.M.; Hussein, K.H.; Sayed, M.M.; El-Aassar, M.R.; Mohamed, I.M.A.; Kwak, H.-H.; Woo, H.-M.; Abdal-hay, A. Development of biocompatible tri-layered nanofibers patches with endothelial cells for cardiac tissue engineering. Eur. Polym. J. 2020, 129, 109630. [Google Scholar] [CrossRef]
- Lee, S.J.; Atala, A. Scaffold technologies for controlling cell behavior in tissue engineering. Biomed. Mater. 2013, 8, 010201. [Google Scholar] [CrossRef] [Green Version]
- Bhardwaj, N.; Nguyen, Q.T.; Chen, A.C.; Kaplan, D.L.; Sah, R.L.; Kundu, S.C. Potential of 3-D tissue constructs engineered from bovine chondrocytes/silk fibroin-chitosan for in vitro cartilage tissue engineering. Biomaterials 2011, 32, 5773–5781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Mousa, H.M.; Park, C.H.; Kim, C.S. Enhanced corrosion resistance and biocompatibility of AZ31 Mg alloy using PCL/ZnO NPs via electrospinning. Appl. Surf. Sci. 2017, 396, 249–258. [Google Scholar] [CrossRef]
- Shrestha, B.K.; Mousa, H.M.; Tiwari, A.P.; Ko, S.W.; Park, C.H.; Kim, C.S. Development of polyamide-6,6/chitosan electrospun hybrid nanofibrous scaffolds for tissue engineering application. Carbohydr. Polym. 2016, 148, 107–114. [Google Scholar] [CrossRef]
- Abdal-hay, A.; Mousa, H.M.; Khan, A.; Vanegas, P.; Lim, J.H. TiO2 nanorods coated onto nylon 6 nanofibers using hydrothermal treatment with improved mechanical properties. Colloids Surf. A Physicochem. Eng. Asp. 2014, 457, 275–281. [Google Scholar] [CrossRef]
- Ali, M.G.; Mousa, H.M.; Blaudez, F.; El-sadek, M.S.A.; Mohamed, M.A.; Abdel-Jaber, G.T.; Abdal-hay, A.; Ivanovski, S. Dual nanofiber scaffolds composed of polyurethane- gelatin/nylon 6- gelatin for bone tissue engineering. Colloids Surf. A Physicochem. Eng. Asp. 2020, 597, 124817. [Google Scholar] [CrossRef]
- Mousa, H.M.; Abdal-hay, A.; Bartnikowski, M.; Mohamed, I.M.A.; Yasin, A.S.; Ivanovski, S.; Park, C.H.; Kim, C.S. A Multifunctional Zinc Oxide/Poly(Lactic Acid) Nanocomposite Layer Coated on Magnesium Alloys for Controlled Degradation and Antibacterial Function. ACS Biomater. Sci. Eng. 2018, 4, 2169–2180. [Google Scholar] [CrossRef]
- Rezk, A.I.; Mousa, H.M.; Lee, J.; Park, C.H.; Kim, C.S. Composite PCL/HA/simvastatin electrospun nanofiber coating on biodegradable Mg alloy for orthopedic implant application. J. Coat. Technol. Res. 2019, 16, 477–489. [Google Scholar] [CrossRef]
- Sayed, M.M.; Mousa, H.M.; El-Aassar, M.R.; El-Deeb, N.M.; Ghazaly, N.M.; Dewidar, M.M.A. Abdal-hay, Enhancing mechanical and biodegradation properties of polyvinyl alcohol/silk fibroin nanofibers composite patches for Cardiac Tissue Engineering. Mater. Lett. 2019, 255, 126510. [Google Scholar] [CrossRef]
- Meyer, R.A.; Green, J.J. Biodegradable polymer iron oxide nanocomposites: The future of biocompatible magnetism. Nanomedicine 2015, 10, 3421–3425. [Google Scholar] [CrossRef] [Green Version]
- Mousa, H.M.; Tiwari, A.P.; Kim, J.; Adhikari, S.P.; Park, C.H.; Kim, C.S. A novel in situ deposition of hydroxyapatite nanoplates using anodization/hydrothermal process onto magnesium alloy surface towards third generation biomaterials. Mater. Lett. 2016, 164, 144–147. [Google Scholar] [CrossRef]
- Konwarh, R.; Karak, N.; Misra, M. Electrospun cellulose acetate nanofibers: The present status and gamut of biotechnological applications. Biotechnol. Adv. 2013, 31, 421–437. [Google Scholar] [CrossRef]
- Glasser, W.G. Prospects for future applications of cellulose acetate. In Macromolecular Symposia; Wiley Online Library: Hoboken, NJ, USA, 2004; pp. 371–394. [Google Scholar]
- De Taillac, L.B.; Porté-Durrieu, M.-C.; Labrugère, C.; Bareille, R.; Amédée, J.; Baquey, C. Grafting of RGD peptides to cellulose to enhance human osteoprogenitor cells adhesion and proliferation. Compos. Sci. Technol. 2004, 64, 827–837. [Google Scholar] [CrossRef]
- Gouma, P.; Xue, R.; Goldbeck, C.; Perrotta, P.; Balázsi, C. Nano-hydroxyapatite—Cellulose acetate composites for growing of bone cells. Mater. Sci. Eng. C 2012, 32, 607–612. [Google Scholar] [CrossRef]
- Salgado, A.; Coutinho, O.P.; Reis, R.L.; Davies, J. In vivo response to starch-based scaffolds designed for bone tissue engineering applications. J. Biomed. Mater. Res. Part A 2007, 80, 983–989. [Google Scholar] [CrossRef] [Green Version]
- Suchanek, W.; Yoshimura, M. Processing and properties of hydroxyapatite-based biomaterials for use as hard tissue replacement implants. J. Mater. Res. 1998, 13, 94–117. [Google Scholar] [CrossRef]
- Toxqui, L.; Vaquero, M.P. Chronic iron deficiency as an emerging risk factor for osteoporosis: A hypothesis. Nutrients 2015, 7, 2324–2344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Díaz-Castro, J.; López-Frías, M.R.; Campos, M.S.; López-Frías, M.; Alférez, M.J.; Nestares, T.; Ojeda, M.L.; López-Aliaga, I. Severe nutritional iron-deficiency anaemia has a negative effect on some bone turnover biomarkers in rats. Eur. J. Nutr. 2012, 51, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Dallman, P.R. Biochemical basis for the manifestations of iron deficiency. Annu. Rev. Nutr. 1986, 6, 13–40. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Peng, S.; Feng, P.; Shuai, C. Bone biomaterials and interactions with stem cells. Bone Res. 2017, 5, 17059. [Google Scholar] [CrossRef] [PubMed]
- Lang, S.B. Pyroelectric Effect in Bone and Tendon. Nature 1966, 212, 704–705. [Google Scholar] [CrossRef]
- Nomura, S.; Takano-Yamamoto, T. Molecular events caused by mechanical stress in bone. Matrix Biol. 2000, 19, 91–96. [Google Scholar] [CrossRef]
- Zhang, T.; Xu, Q.; Huang, T.; Ling, D.; Gao, J. New Insights into Biocompatible Iron Oxide Nanoparticles: A Potential Booster of Gene Delivery to Stem Cells. Small 2020, 16, 2001588. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhang, F.; Wang, Y.; Sun, X.; Choi, K.Y.; Liu, D.; Choi, J.-S.; Shin, T.-H.; Cheon, J.; Niu, G.; et al. Design Considerations of Iron-Based Nanoclusters for Noninvasive Tracking of Mesenchymal Stem Cell Homing. ACS Nano 2014, 8, 4403–4414. [Google Scholar] [CrossRef]
- Ullah, I.; Li, W.; Lei, S.; Zhang, Y.; Zhang, W.; Farooq, U.; Ullah, S.; Ullah, M.W.; Zhang, X. Simultaneous co-substitution of Sr2+/Fe3+ in hydroxyapatite nanoparticles for potential biomedical applications. Ceram. Int. 2018, 44, 21338–21348. [Google Scholar] [CrossRef]
- Ullah, I.; Zhang, W.; Yang, L.; Ullah, M.W.; Atta, O.M.; Khan, S.; Wu, B.; Wu, T.; Zhang, X. Impact of structural features of Sr/Fe co-doped HAp on the osteoblast proliferation and osteogenic differentiation for its application as a bone substitute. Mater. Sci. Eng. C 2020, 110, 110633. [Google Scholar] [CrossRef]
- Ullah, I.; Gloria, A.; Zhang, W.; Ullah, M.W.; Wu, B.; Li, W.; Domingos, M.; Zhang, X. Synthesis and Characterization of Sintered Sr/Fe-Modified Hydroxyapatite Bioceramics for Bone Tissue Engineering Applications. ACS Biomater. Sci. Eng. 2020, 6, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Pasandideh, Z.; Tajabadi, M.; Javadpour, J.; Mirkazemi, S.M. The effects of Fe3+ and Co2+ substitution in Ca10-x-yFexCoy(PO4)6(OH)2 hydroxyapatite nanoparticles: Magnetic, antibacterial, and improved drug release behavior. Ceram. Int. 2020, 46, 16104–16118. [Google Scholar] [CrossRef]
- Serra, I.; Fradique, R.; Vallejo, M.C.d.S.; Correia, T.R.; Miguel, S.; Correia, I. Production and characterization of chitosan/gelatin/β-TCP scaffolds for improved bone tissue regeneration. Mater. Sci. Eng. C 2015, 55, 592–604. [Google Scholar] [CrossRef]
- Lao, L.; Wang, Y.; Zhu, Y.; Zhang, Y.; Gao, C. Poly (lactide-co-glycolide)/hydroxyapatite nanofibrous scaffolds fabricated by electrospinning for bone tissue engineering. J. Mater. Sci. Mater. Med. 2011, 22, 1873–1884. [Google Scholar] [CrossRef] [PubMed]
- Mousa, H.M.; Hussein, K.H.; Raslan, A.A.; Lee, J.; Woo, H.M.; Park, C.H.; Kim, C.S. Amorphous apatite thin film formation on a biodegradable Mg alloy for bone regeneration: Strategy, characterization, biodegradation, and in vitro cell study. RSC Adv. 2016, 6, 22563–22574. [Google Scholar] [CrossRef]
- Adhikari, S.P.; Pant, H.R.; Mousa, H.M.; Lee, J.; Kim, H.J.; Park, C.H.; Kim, C.S. Synthesis of high porous electrospun hollow TiO2 nanofibers for bone tissue engineering application. J. Ind. Eng. Chem. 2016, 35, 75–82. [Google Scholar] [CrossRef]
- Hussein, K.H.; Park, K.-M.; Kang, K.-S.; Woo, H.-M. Biocompatibility evaluation of tissue-engineered decellularized scaffolds for biomedical application. Mater. Sci. Eng. C 2016, 67, 766–778. [Google Scholar] [CrossRef] [PubMed]
- Marras, S.I.; Kladi, K.P.; Tsivintzelis, I.; Zuburtikudis, I.; Panayiotou, C. Biodegradable polymer nanocomposites: The role of nanoclays on the thermomechanical characteristics and the electrospun fibrous structure. Acta Biomater. 2008, 4, 756–765. [Google Scholar] [CrossRef]
- Zong, X.; Kim, K.; Fang, D.; Ran, S.; Hsiao, B.S.; Chu, B. Structure and process relationship of electrospun bioabsorbable nanofiber membranes. Polymer 2002, 43, 4403–4412. [Google Scholar] [CrossRef]
- Gontijo, L.; Machado, R.; Miola, E.; Casteletti, L.; Nascente, P. Characterization of plasma-nitrided iron by XRD, SEM and XPS. Surf. Coat. Technol. 2004, 183, 10–17. [Google Scholar] [CrossRef]
- Miyamoto, T.; Takahashi, S.i.; Ito, H.; Inagaki, H.; Noishiki, Y. Tissue biocompatibility of cellulose and its derivatives. J. Biomed. Mater. Res. 1989, 23, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, K.; Renneckar, S.; Gatenholm, P. Biomimetic calcium phosphate crystal mineralization on electrospun cellulose-based scaffolds. ACS Appl. Mater. Interfaces 2011, 3, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Ahmadian-Fard-Fini, S.; Ghanbari, D.; Amiri, O.; Salavati-Niasari, M. Electro-spinning of cellulose acetate nanofibers/Fe/carbon dot as photoluminescence sensor for mercury (II) and lead (II) ions. Carbohydr. Polym. 2020, 229, 115428. [Google Scholar] [CrossRef]
- Matos, R.J.; Chaparro, C.I.; Silva, J.C.; Valente, M.A.; Borges, J.P.; Soares, P.I. Electrospun composite cellulose acetate/iron oxide nanoparticles non-woven membranes for magnetic hyperthermia applications. Carbohydr. Polym. 2018, 198, 9–16. [Google Scholar] [CrossRef]
- Filho, G.R.; Monteiro, D.S.; Meireles, C.d.; de Assunção, R.M.N.; Cerqueira, D.A.; Barud, H.S.; Ribeiro, S.J.; Messadeq, Y. Synthesis and characterization of cellulose acetate produced from recycled newspaper. Carbohydr. Polym. 2008, 73, 74–82. [Google Scholar] [CrossRef]
- Kostag, M.; Gericke, M.; Heinze, T.; El Seoud, O.A. Twenty-five years of cellulose chemistry: Innovations in the dissolution of the biopolymer and its transformation into esters and ethers. Cellulose 2019, 26, 139–184. [Google Scholar] [CrossRef]
- Pourmortazavi, S.M.; Farhadi, K.; Mirzajani, V.; Mirzajani, S.; Kohsari, I. Study on the catalytic effect of diaminoglyoxime on thermal behaviors, non-isothermal reaction kinetics and burning rate of homogeneous double-base propellant. J. Therm. Anal. Calorim. 2016, 125, 121–128. [Google Scholar] [CrossRef]
- Khan, S.B.; Alamry, K.A.; Bifari, E.N.; Asiri, A.M.; Yasir, M.; Gzara, L.; Ahmad, R.Z. Assessment of antibacterial cellulose nanocomposites for water permeability and salt rejection. J. Ind. Eng. Chem. 2015, 24, 266–275. [Google Scholar] [CrossRef]
- Wei, Y. Synthesis of Fe3O4 nanoparticles and their magnetic properties. arXiv 2020, arXiv:2001.06583. [Google Scholar] [CrossRef] [Green Version]
- Currey, J.D. The structure and mechanics of bone. J. Mater. Sci. 2012, 47, 41–54. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Nirmala, R.; Nam, K.T.; Navamathavan, R.; Park, S.J.; Kim, H.Y. Hydroxyapatite Mineralization on the Calcium Chloride Blended Polyurethane Nanofiber via Biomimetic Method. Nanoscale Res. Lett. 2010, 6, 2. [Google Scholar] [CrossRef] [Green Version]
- Hakimimehr, D.; Liu, D.-M.; Troczynski, T. In-situ preparation of poly (propylene fumarate)—Hydroxyapatite composite. Biomaterials 2005, 26, 7297–7303. [Google Scholar] [CrossRef] [PubMed]
- Bang, L.T.; Ramesh, S.; Purbolaksono, J.; Long, B.D.; Chandran, H.; Ramesh, S.; Othman, R. Development of a bone substitute material based on alpha-tricalcium phosphate scaffold coated with carbonate apatite/poly-epsilon-caprolactone. Biomed. Mater. 2015, 10, 045011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Z.; Yang, L.; Chen, K.-F.; Chen, G.-W.; Peng, Y.-P.; Chen, J.-K.; Suo, G.; Yu, J.; Wang, W.-C.; Lin, C.-H. Nano zerovalent iron particles induce pulmonary and cardiovascular toxicity in an in vitro human co-culture model. Nanotoxicology 2016, 10, 881–890. [Google Scholar] [CrossRef]
- Zhu, L.; Luo, D.; Liu, Y. Effect of the nano/microscale structure of biomaterial scaffolds on bone regeneration. Int. J. Oral Sci. 2020, 12, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yun, H.-M.; Ahn, S.-J.; Park, K.-R.; Kim, M.-J.; Kim, J.-J.; Jin, G.-Z.; Kim, H.-W.; Kim, E.-C. Magnetic nanocomposite scaffolds combined with static magnetic field in the stimulation of osteoblastic differentiation and bone formation. Biomaterials 2016, 85, 88–98. [Google Scholar] [CrossRef]
- Sapir, Y.; Cohen, S.; Friedman, G.; Polyak, B. The promotion of in vitro vessel-like organization of endothelial cells in magnetically responsive alginate scaffolds. Biomaterials 2012, 33, 4100–4109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, S.; Huang, N.; Xu, L.; Zhang, Y.; Liu, H.; Sun, H.; Leng, Y. Biocompatibility of pure iron: In vitro assessment of degradation kinetics and cytotoxicity on endothelial cells. Mater. Sci. Eng. C 2009, 29, 1589–1592. [Google Scholar] [CrossRef]
- Zhu, M.-T.; Wang, B.; Wang, Y.; Yuan, L.; Wang, H.-J.; Wang, M.; Ouyang, H.; Chai, Z.-F.; Feng, W.-Y.; Zhao, Y.-L. Endothelial dysfunction and inflammation induced by iron oxide nanoparticle exposure: Risk factors for early atherosclerosis. Toxicol. Lett. 2011, 203, 162–171. [Google Scholar] [CrossRef] [PubMed]

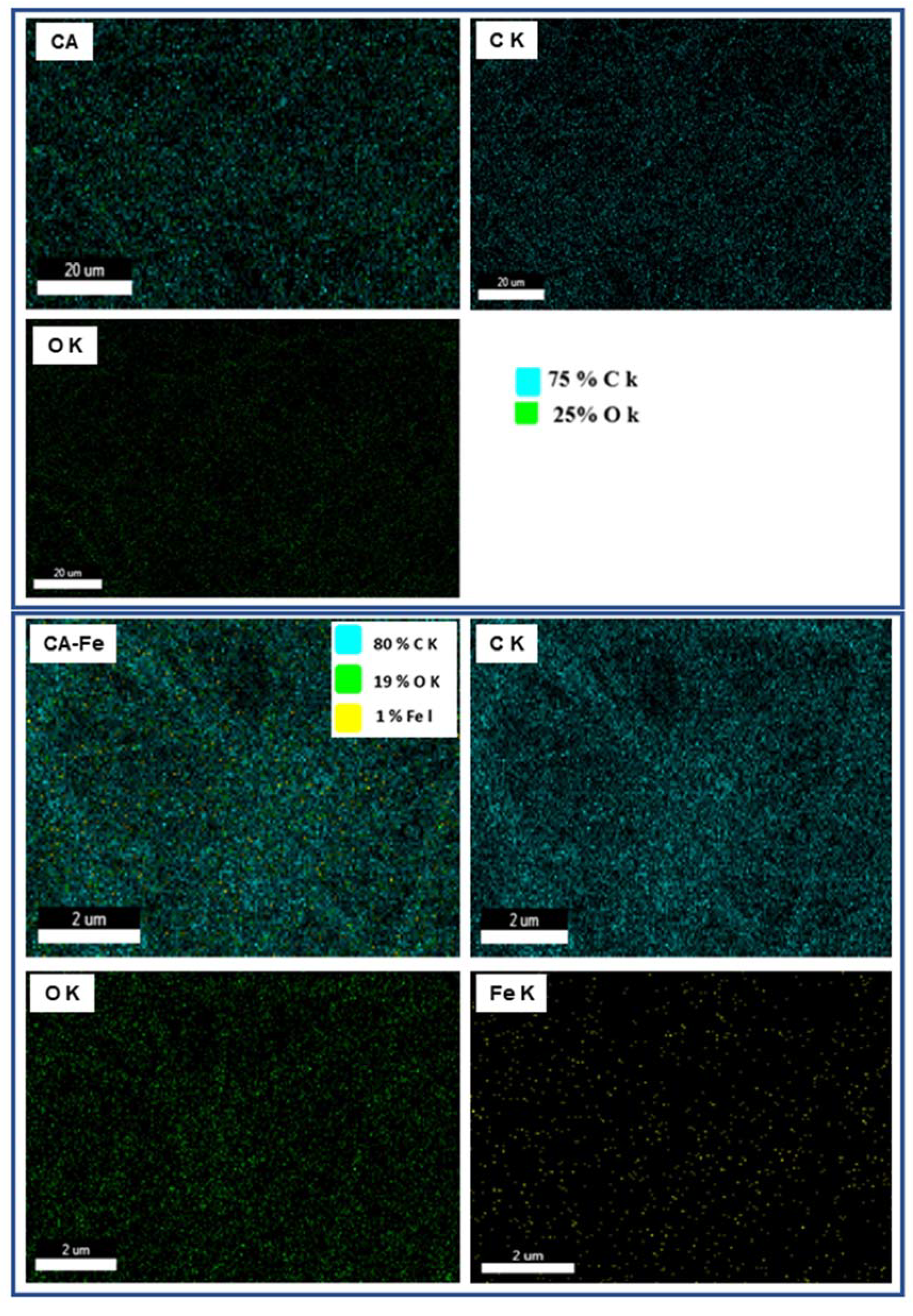



| Mat Name | Name | Start BE | Peak BE | End BE | Height CPS | FWHM eV | Area (P) CPS.eV | Area (N) KE^0.6 | at. % |
|---|---|---|---|---|---|---|---|---|---|
| CA | C1s | 297.98 | 285.83 | 279.18 | 5405.41 | 5.44 | 29,575.44 | 419.99 | 63.32 |
| O1s | 544.98 | 532.36 | 525.18 | 10,351.88 | 3.28 | 43,725.31 | 243.25 | 36.68 | |
| CA/Fe | C1s | 297.98 | 285.30 | 279.18 | 10,839.70 | 4.76 | 46,563.45 | 661.05 | 63.28 |
| O1s | 544.98 | 531.93 | 525.18 | 22,166.45 | 2.73 | 68,147.68 | 379.01 | 36.28 | |
| Fe2p | 739.98 | 707.71 | 700.18 | 412.90 | 0.10 | 4137.15 | 4.64 | 0.44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mousa, H.M.; Hussein, K.H.; Sayed, M.M.; Abd El-Rahman, M.K.; Woo, H.-M. Development and Characterization of Cellulose/Iron Acetate Nanofibers for Bone Tissue Engineering Applications. Polymers 2021, 13, 1339. https://doi.org/10.3390/polym13081339
Mousa HM, Hussein KH, Sayed MM, Abd El-Rahman MK, Woo H-M. Development and Characterization of Cellulose/Iron Acetate Nanofibers for Bone Tissue Engineering Applications. Polymers. 2021; 13(8):1339. https://doi.org/10.3390/polym13081339
Chicago/Turabian StyleMousa, Hamouda M., Kamal Hany Hussein, Mostafa M. Sayed, Mohamed K. Abd El-Rahman, and Heung-Myong Woo. 2021. "Development and Characterization of Cellulose/Iron Acetate Nanofibers for Bone Tissue Engineering Applications" Polymers 13, no. 8: 1339. https://doi.org/10.3390/polym13081339
APA StyleMousa, H. M., Hussein, K. H., Sayed, M. M., Abd El-Rahman, M. K., & Woo, H.-M. (2021). Development and Characterization of Cellulose/Iron Acetate Nanofibers for Bone Tissue Engineering Applications. Polymers, 13(8), 1339. https://doi.org/10.3390/polym13081339






