Thermal Degradation Mechanism and Decomposition Kinetic Studies of Poly(Lactic Acid) and Its Copolymers with Poly(Hexylene Succinate)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of (PLA-b-PHSu) Copolymers
2.3. Characterization Methods
2.3.1. Tensile Mechanical Properties
2.3.2. Thermogravimetric Analysis (TGA)
2.3.3. Pyrolysis-Gas Chromatography/Mass Spectrometry (Py-GC/MS) Study
3. Results and Discussion
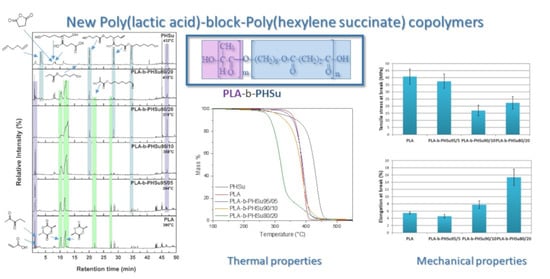
3.1. Tensile Mechanical Properties
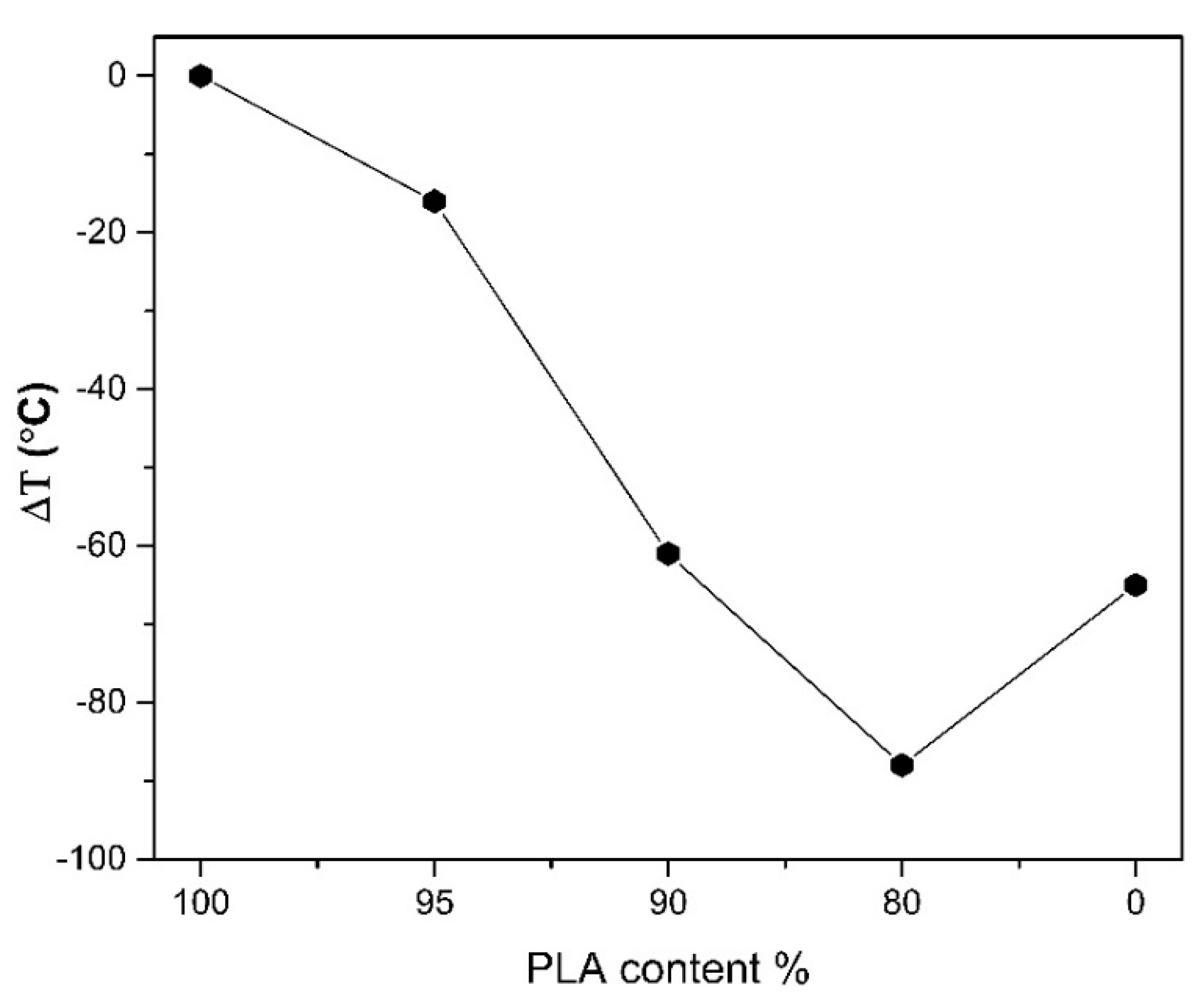
3.2. Thermogravimetric Analysis (TGA)
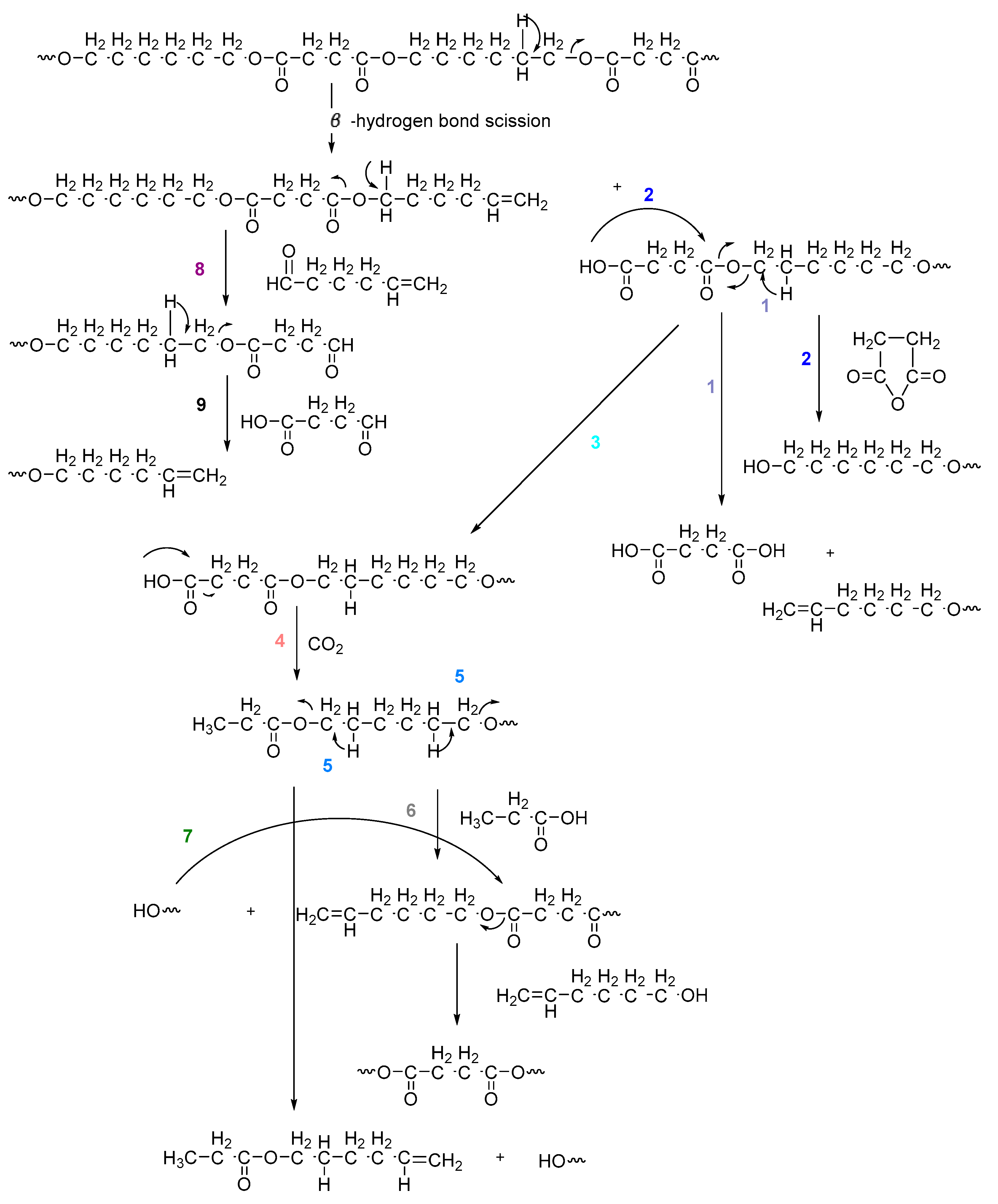
3.3. Pyrolysis-Gas Chromatography/Mass Spectrometry Study
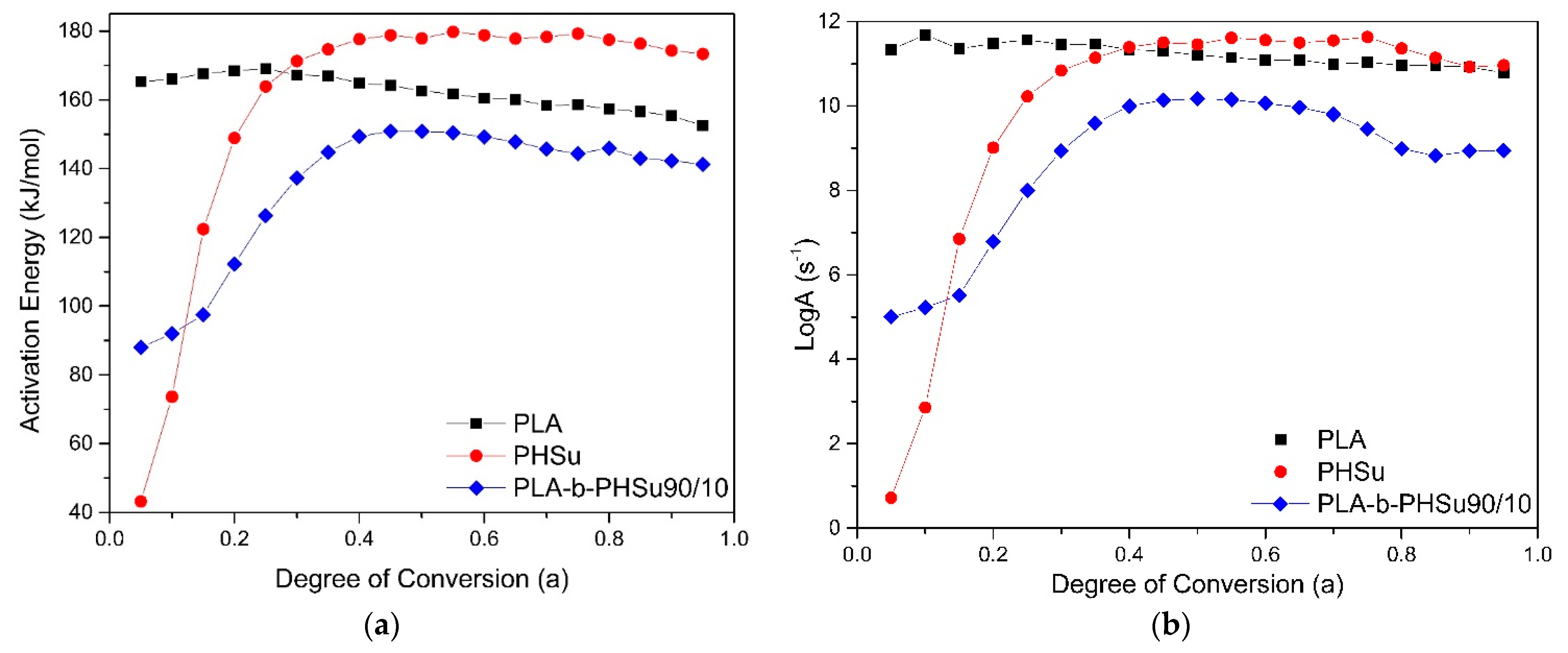
3.4. Kinetic Analysis Based on Thermogravimetric Data—Isoconversional Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pholharn, D.; Srithep, Y. Optimizationof poly(L-lactide)-polybutylene adipate terephthalate diblockcopolymer by ring opening polymerization. IOP Conf. Ser. Mater. Sci. Eng. 2019, 526, 1–4. [Google Scholar] [CrossRef]
- Kang, H.; Li, Y.; Gong, M.; Guo, Y.; Guo, Z.; Fang, Q.; Li, X. An environmentally sustainable plasticizer toughened polylactide. RSC Adv. 2018, 8, 11643–11651. [Google Scholar] [CrossRef]
- Muniyasamy, S.; Ofosu, O.; John, M.J.; Anandjiwala, R.D. Mineralization of poly(lactic acid) (PLA), Poly(3-hydroxybutyrate-co-valerate) (PHBV) and PLA/PHBV blend in compost and soil environments. J. Renew. Mater. 2016, 4, 133–145. [Google Scholar] [CrossRef]
- Dogan, S.K.; Boyacioglu, S.; Kodal, M.; Gokce, O.; Ozkoc, G. Thermally induced shape memory behavior, enzymatic degradation and biocompatibility of PLA/TPU blends: “Effects of compatibilization”. J. Mech. Behav. Biomed. Mater. 2017, 71, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Nanaki, S.; Viziridou, A.; Zamboulis, A.; Kostoglou, M.; Papageorgiou, G.Z.; Bikiaris, D.N. New Biodegradable Poly (l-lactide ) -Block- for Long-Acting Injectables of Naltrexone Drug. Polymers 2020, 12, 852. [Google Scholar] [CrossRef] [PubMed]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef]
- Middleton, J.C.; Tipton, A.J. Synthetic biodegradable polymers as orthopedic devices. Biomaterials 2000, 21, 2335–2346. [Google Scholar] [CrossRef]
- Panyam, J.; Labhasetwar, V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv. Drug Deliv. Rev. 2003, 55, 329–347. [Google Scholar] [CrossRef]
- Hamad, K.; Kaseem, M.; Yang, H.W.; Deri, F.; Ko, Y.G. Properties and medical applications of polylactic acid: A review. Express Polym. Lett. 2015, 9, 435–455. [Google Scholar] [CrossRef]
- Lv, S.; Zhang, Y.; Tan, H. Thermal and thermo-oxidative degradation kinetics and characteristics of poly (lactic acid) and its composites. Waste Manag. 2019, 87, 335–344. [Google Scholar] [CrossRef]
- Zou, H.; Yi, C.; Wang, L.; Liu, H.; Xu, W. Thermal degradation of poly(lactic acid) measured by thermogravimetry coupled to Fourier transform infrared spectroscopy. J. Therm. Anal. Calorim. 2009, 97, 929–935. [Google Scholar] [CrossRef]
- Wachsen, O.; Platkowski, K.; Reichert, K.H. Thermal degradation of poly-l -lactide -studies on kinetics, modelling and melt stabilisation. Polym. Degrad. Stab. 1997, 57, 87–94. [Google Scholar] [CrossRef]
- Khabbaz, F.; Karlsson, S.; Albertsson, A.C. Py-GC/MS an effective technique to characterizing of degradation mechanism of poly (L-lactide) in the different environment. J. Appl. Polym. Sci. 2000, 78, 2369–2378. [Google Scholar] [CrossRef]
- Chrissafis, K.; Roumeli, E.; Paraskevopoulos, K.M.; Nianias, N.; Bikiaris, D.N. Effect of different nanoparticles on thermal decomposition of poly (propylene sebacate )/ nanocomposites: Evaluation of mechanisms using TGA and TG—FTIR—GC/MS. J. Anal. Appl. Pyrolysis 2012, 96, 92–99. [Google Scholar] [CrossRef]
- Chrissafis, K. Detail kinetic analysis of the thermal decomposition of PLA with oxidized multi-walled carbon nanotubes. Thermochim. Acta 2010, 511, 163–167. [Google Scholar] [CrossRef]
- Monika, P.; Dhar, P.; Katiyar, V. Thermal degradation kinetics of polylactic acid/acid fabricated cellulose nanocrystal based bionanocomposites. Int. J. Biol. Macromol. 2017, 104, 827–836. [Google Scholar] [CrossRef]
- Yuzay, I.E.; Auras, R.; Soto-Valdez, H.; Selke, S. Effects of synthetic and natural zeolites on morphology and thermal degradation of poly(lactic acid) composites. Polym. Degrad. Stab. 2010, 95, 1769–1777. [Google Scholar] [CrossRef]
- Das, P.; Tiwari, P. Thermal degradation kinetics of plastics and model selection. Thermochim. Acta 2017, 654, 191–202. [Google Scholar] [CrossRef]
- Srithep, Y.; Pholharn, D. Plasticizer effect on melt blending of polylactide stereocomplex. E-Polymers 2017, 17, 409–416. [Google Scholar] [CrossRef]
- Madhavan Nampoothiri, K.; Nair, N.R.; John, R.P. An overview of the recent developments in polylactide (PLA) research. Bioresour. Technol. 2010, 101, 8493–8501. [Google Scholar] [CrossRef]
- Jain, R.A. The manufacturing techniques of various drug loaded biodegradable poly(lactide-co-glycolide) (PLGA) devices. Biomaterials 2000, 21, 2475–2490. [Google Scholar] [CrossRef]
- Ozdemir, E.; Hacaloglu, J. Characterizations of PLA-PEG blends involving organically modified montmorillonite. J. Anal. Appl. Pyrolysis 2017, 127, 343–349. [Google Scholar] [CrossRef]
- Abdelwahab, M.A.; Flynn, A.; Chiou, B.; Imam, S.; Orts, W.; Chiellini, E. Thermal, mechanical and morphological characterization of plasticized PLA e PHB blends. Polym. Degrad. Stab. 2012, 97, 1822–1828. [Google Scholar] [CrossRef]
- Song, H.; Lee, S.Y. Production of succinic acid by bacterial fermentation. Enzym. Microb. Technol. 2006, 39, 352–361. [Google Scholar] [CrossRef]
- Zheng, P.; Fang, L.; Xu, Y.; Dong, J.J.; Ni, Y.; Sun, Z.H. Succinic acid production from corn stover by simultaneous saccharification and fermentation using Actinobacillus succinogenes. Bioresour. Technol. 2010, 101, 7889–7894. [Google Scholar] [CrossRef] [PubMed]
- Mitra, T.; Sailakshmi, G.; Gnanamani, A.; Mandal, A.B. Studies on cross-linking of succinic acid with chitosan/collagen. Mater. Res. 2013, 16, 755–765. [Google Scholar] [CrossRef]
- Cheng, K.-K.; Zhao, X.-B.; Zeng, J.; Zhang, J.-A. Biotechnological production of succinic acid: Current state and perspectives. Biofuels. Bioprod. Biorefin. 2012, 6, 246–256. [Google Scholar] [CrossRef]
- Ye, H.M.; Tang, Y.R.; Song, Y.Y.; Xu, J.; Guo, B.H.; Zhou, Q. Aliphatic copolyester with isomorphism in limited composition range. Polymer 2014, 55, 5811–5820. [Google Scholar] [CrossRef]
- Tan, B.; Bi, S.; Emery, K.; Sobkowicz, M.J. Bio-based poly(butylene succinate-co-hexamethylene succinate) copolyesters with tunable thermal and mechanical properties. Eur. Polym. J. 2017, 86, 162–172. [Google Scholar] [CrossRef]
- Bai, Z.; Liu, Y.; Su, T.; Wang, Z. Effect of hydroxyl monomers on the Enzymatic degradation of poly(ethylene succinate), poly(butylene succinate), and poly(hexylene succinate). Polymers 2018, 10, 90. [Google Scholar] [CrossRef]
- Lostocco, M.R.; Huang, S.J. The hydrolysis of poly(lactic acid)/poly(hexamethylene succinate) blends. Polym. Degrad. Stab. 1998, 61, 225–230. [Google Scholar] [CrossRef]
- Lostocco, M.R.; Huang, S.J. Aliphatic polyester blends based upon poly(lactic acid) and oligomeric poly(hexamethylene succinate). J. Macromol. Sci. Pure Appl. Chem. 1997, 34, 2165–2175. [Google Scholar] [CrossRef]
- Chrysafi, I.; Pavlidou, E.; Christodoulou, E.; Vourlias, G.; Klonos, P.A.; Kyritsis, A.; Bikiaris, D.N. Effects of poly(hexylene succinate) amount on the crystallization and molecular mobility of poly(lactic acid) copolymers. Thermochim. Acta 2021, 698, 178883. [Google Scholar] [CrossRef]
- Oba, K.; Ishida, Y.; Ohtani, H.; Tsuge, S. Characterization of abnormal structures in thermally treated liquid crystalline aromatic polyesters by pyrolysis-gas chromatography in the presence of organic alkali. Polym. Degrad. Stab. 2002, 76, 85–94. [Google Scholar] [CrossRef]
- Navarro-Baena, I.; Sessini, V.; Dominici, F.; Torre, L.; Kenny, J.M.; Peponi, L. Design of biodegradable blends based on PLA and PCL: From morphological, thermal and mechanical studies to shape memory behavior. Polym. Degrad. Stab. 2016, 132, 97–108. [Google Scholar] [CrossRef]
- Mittal, V.; Akhtar, T.; Matsko, N. Mechanical, thermal, rheological and morphological properties of binary and ternary blends of PLA, TPS and PCL. Macromol. Mater. Eng. 2015, 300, 423–435. [Google Scholar] [CrossRef]
- Mohapatra, A.; Mohanty, S.; Nayak, S.K. Effect of PEG on PLA/PEG Blend and Its Nanocomposites: A Study of Thermo-Mechanical and Morphological Characterization. Polym. Compos. 2014, 35, 283–293. [Google Scholar] [CrossRef]
- Restrepo, I.; Medina, C.; Meruane, V.; Akbari-Fakhrabadi, A.; Flores, P.; Rodríguez-Llamazares, S. The effect of molecular weight and hydrolysis degree of poly(vinyl alcohol)(PVA) on the thermal and mechanical properties of poly(lactic acid)/PVA blends. Polimeros 2018, 28, 169–177. [Google Scholar] [CrossRef]
- Mamun, A.; Rahman, S.M.M.; Roland, S.; Mahmood, R. Impact of Molecular Weight on the Thermal Stability and the Miscibility of Poly(ε-caprolactone)/Polystyrene Binary Blends. J. Polym. Environ. 2018, 26, 3511–3519. [Google Scholar] [CrossRef]
- Chrissafis, K.; Paraskevopoulos, K.M.; Bikiaris, D.N. Effect of molecular weight on thermal degradation mechanism of the biodegradable polyester poly(ethylene succinate). Thermochim. Acta 2006, 440, 166–175. [Google Scholar] [CrossRef]
- Rizzarelli, P.; Carroccio, S. Thermo-oxidative processes in biodegradable poly(butylene succinate). Polym. Degrad. Stab. 2009, 94, 1825–1838. [Google Scholar] [CrossRef]
- Bikiaris, D.N.; Chrissafis, K.; Paraskevopoulos, K.M.; Triantafyllidis, K.S.; Antonakou, E.V. Investigation of thermal degradation mechanism of an aliphatic polyester using pyrolysis-gas chromatography-mass spectrometry and a kinetic study of the effect of the amount of polymerisation catalyst. Polym. Degrad. Stab. 2007, 92, 525–536. [Google Scholar] [CrossRef]
- Chrissafis, K.; Paraskevopoulos, K.M.; Bikiaris, D.N. Thermal degradation mechanism of poly (ethylene succinate) and poly ( butylene succinate ): Comparative study. Thermochim. Acta 2005, 435, 142–150. [Google Scholar] [CrossRef]
- Sato, H.; Furuhashi, M.; Yang, D.; Ohtani, H.; Tsuge, S.; Okada, M.; Tsunoda, K.; Aoi, K. A novel evaluation method for biodegradability of poly (butylene succinate-co-butylene adipate ) by pyrolysis-gas chromatography. Polym. Degrad. Stab. 2001, 73, 327–334. [Google Scholar] [CrossRef]
- Parcheta, P.; Koltsov, I.; Datta, J. Fully bio-based poly (propylene succinate ) synthesis and investigation of thermal degradation kinetics with released gases analysis. Polym. Degrad. Stab. 2018, 151, 90–99. [Google Scholar] [CrossRef]
- Aoyagi, Y.; Yamashita, K.; Doi, Y. Thermal degradation of poly [(R)-3-hydroxybutyrate], poly [e-caprolactone ], and poly [(S)-lactide ]. Polym. Degrad. Stab. 2002, 76, 53–59. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Parres, F.; López, J.; Jiménez, A. Development of a novel pyrolysis-gas chromatography/mass spectrometry method for the analysis of poly(lactic acid) thermal degradation products. J. Anal. Appl. Pyrolysis 2013, 101, 150–155. [Google Scholar] [CrossRef]
- Sun, C.; Li, C.; Tan, H.; Zhang, Y. Synergistic effects of wood fiber and polylactic acid during co-pyrolysis using TG-FTIR-MS and Py-GC/MS. Energy Convers. Manag. 2019, 202, 112212. [Google Scholar] [CrossRef]
- Kopinke, F.D.; Mackenzie, K. Mechanistic aspects of the thermal degradation of poly(lactic acid) and poly(β-hydroxybutyric acid). J. Anal. Appl. Pyrolysis 1997, 40–41, 43–53. [Google Scholar] [CrossRef]
- Tsanaktsis, V.; Vouvoudi, E.; Papageorgiou, G.Z.; Papageorgiou, D.G.; Chrissafis, K.; Bikiaris, D.N. Thermal degradation kinetics and decomposition mechanism of polyesters based on 2,5-furandicarboxylic acid and low molecular weight aliphatic diols. J. Anal. Appl. Pyrolysis 2015, 112, 369–378. [Google Scholar] [CrossRef]

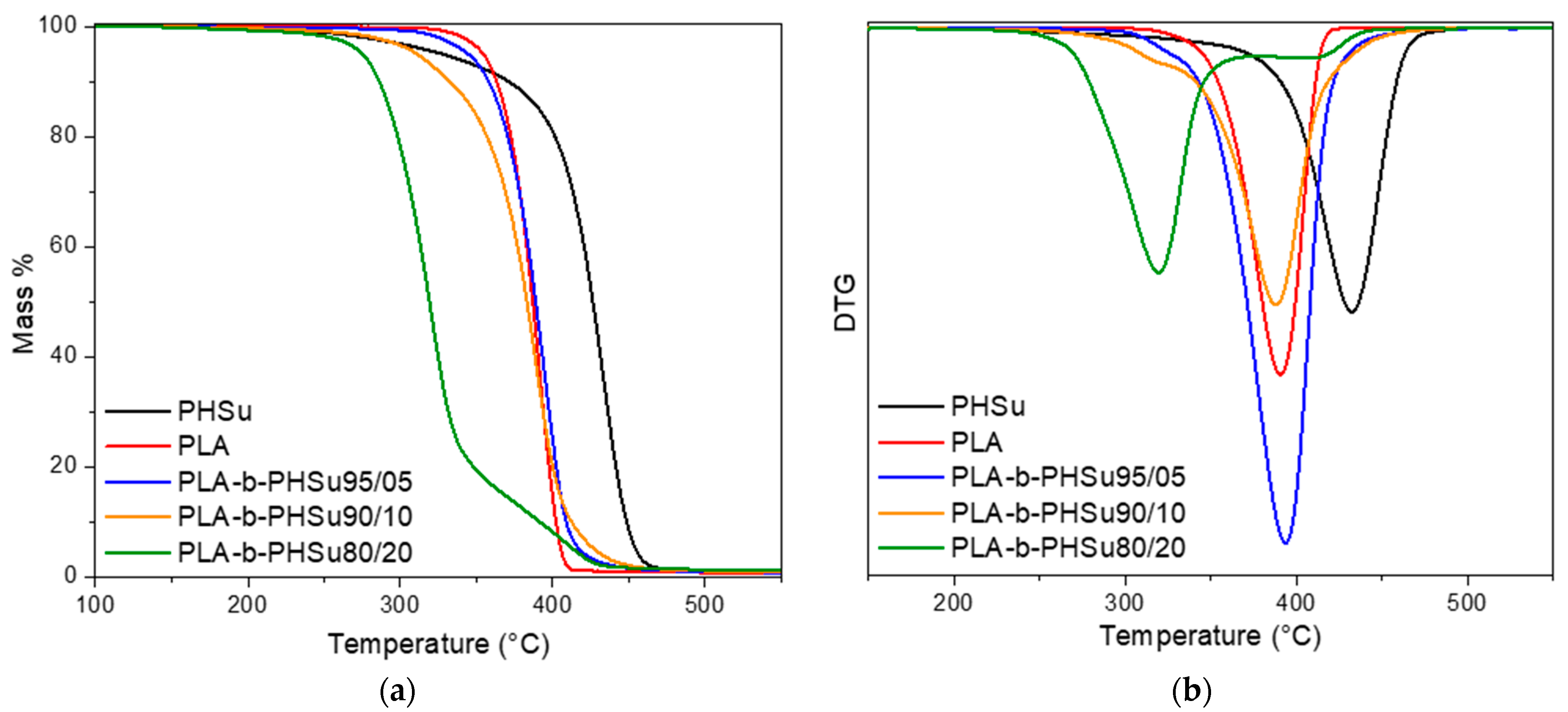
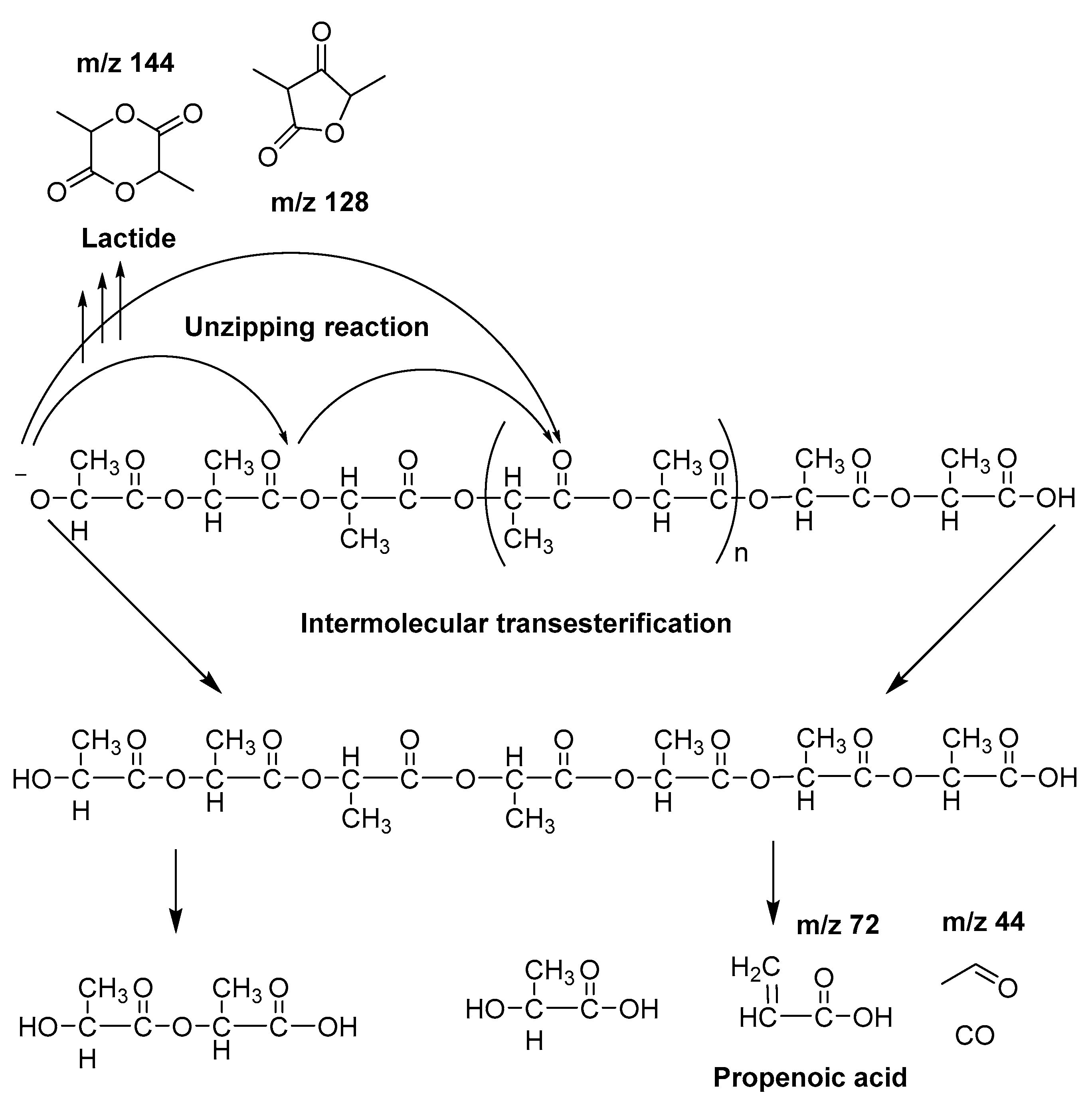
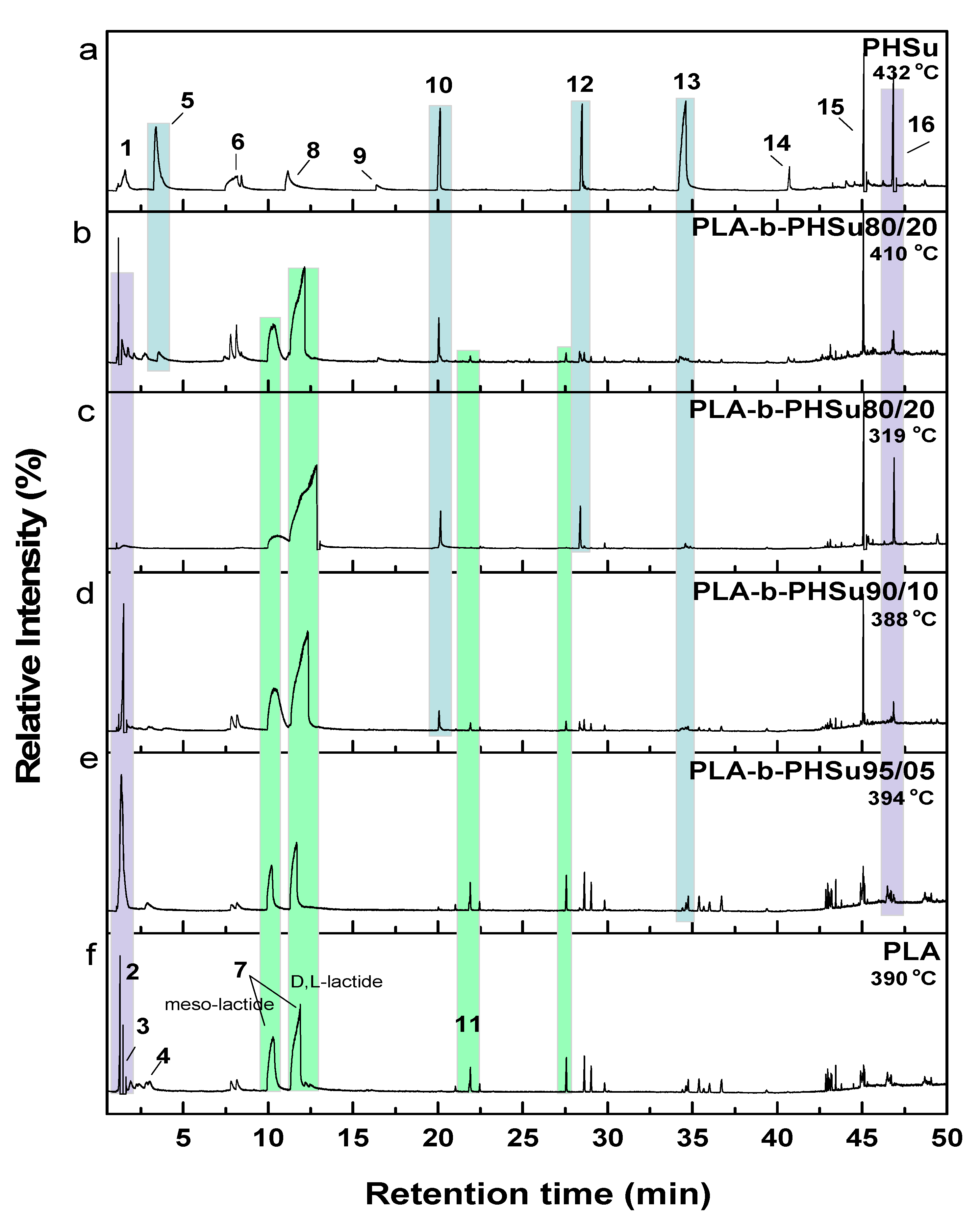

| Sample | Td,max (°C) |
|---|---|
| PHSu | 432.3 |
| PLA | 390.5 |
| PLA-b-PHSu95/05 | 331.9, 393.7 |
| PLA-b-PHSu90/10 | 324.8, 387.9 |
| PLA-b-PHSu80/20 | 319.4, 410.9 |
| Peak No. | PLA | PLA-b-PHSu 95/05 | PLA-b-PHSu 90/10 | PLA-b-PHSu 80/20 | PHSu | Mw (amu) | Possible Product | |
|---|---|---|---|---|---|---|---|---|
| Pyrolysis Temperature | ||||||||
| 390 °C | 394 °C | 388 °C | 319 °C | 410 °C | 432 °C | |||
| Rt (min) | ||||||||
| 1 | - | - | 1.19 | - | 1.19 | 1.18 | 44 | CO2 |
| 2 | 1.27 | 1.32 | - | - | 1.36 | - | 56 | acetaldehyde  |
| 3 | 1.90 | - | 1.88 | - | 1.82 | - | 100 | 2,3-pentanedione  |
| 4 | 2.85 | 2.86 | 2.90 | - | 2.74 | - | 72 | acrylic acid  |
| 5 | - | - | - | - | 3.44 | 3.38 | 82 | 1,5-hexadiene  |
| 6 | - | - | 7.82 | - | 7.80 | - | 100 | succinic anhydride 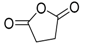 |
| 7 | - | 8.12 | 8.15 | - | 8.12 | 8.17 | 117 | 1,6-hexanediol  |
| 8 | - | - | - | - | 8.40 | 8.43 | 118 | succinic acid 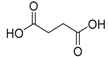 |
| 9 | 10.25 11.88 | 10.18 11.63 | 10.44 12.31 | 10.33 12.06 | 10.27 12.10 | - | 144 | meso-Lactide (3,6-dimethyl-1,4-dioxane-2,5-dione)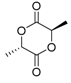 d,l-Lactide  |
| 10 | - | - | - | - | 16.39 | 16.41 | 146 | 4-hydroxybutyl propionate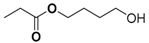 |
| 11 | - | 20.02 | 20.07 | 20.06 | 20.16 | 20.14 | 186 | hex-5-en-1-yl 4-hydroxybutanoate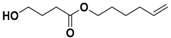 |
| 12 | 21.91 | 21.91 | 21.92 | - | 21.93 | - | 202 | PLA trimer or 7-oxoheptyl 2-hydroxypropanoate 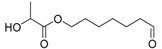 |
| 13 | - | - | 28.35 | 28.347 | 28.38 | 28.483 | 218 | 4-((6-hydroxyhexyl)oxy)-4-oxobutanoic acid |
| 14 | - | - | - | 34.35 | 34.33 | 34.60 | 272 | 4-((6-(acryloyloxy)hexyl)oxy)-4-oxobutanoic acid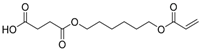 |
| 15 | - | - | - | - | 40.61 | 40.72 | 302 | 4-oxo-4-((6-((4-oxobutanoyl)oxy)hexyl)oxy)butanoic acid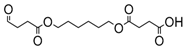 |
| 16 | - | - | 45.05 | 45.06 | 45.08 | 45.08 | 318 | bis(6-hydroxyhexyl) succinate 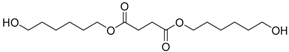 |
| 17 | - | 46.76 | 46.83 | 46.84 | 46.87 | 46.82 | 400 | 4-((6-((4-(hex-5-en-1-yloxy)-4-oxobutanoyl)oxy)hexyl)oxy)-4-oxobutanoic acid |
| Kinetic Model | Symbol | f(a) |
|---|---|---|
| n-order reactions | ||
| First order | F1 | |
| Second order | F2 | |
| nth order | Fn | |
| Diffusion | ||
| 1-D diffusion | D1 | |
| 2-D diffusion | D2 | |
| 3-D diffusion-Jander | D3 | |
| 3-D diffusion-Ginstling–Brounshtein | D4 | |
| Phase-boundary reactions | ||
| Contracting area | R2 | |
| Contracting volume | R3 | |
| Prout–Tompkins | B1 | |
| expanded Prout–Tomplins | Bn | |
| nth order with autocatalysis | C1 | |
| nth order with autocatalysis | Cn | |
| Nucleation and nuclei growth | ||
| Avrami–Erofeev | A2 | |
| Avrami–Erofeev | A3 | |
| Avrami–Erofeev | An | |
| 1st Step | ||||||
| Sample | Mechanism | Ea (kJ/mol) | logA (s−1) | logKcat | React. Order n | R2 |
| PHSu | Fn | 48 | 1.7 | - | 0.6 | 0.99994 |
| PLA | Cn | 161 | 10.6 | 0.8 | 1.0 | 0.99990 |
| PLA-b-PHSU90/10 | Cn | 91 | 5.6 | 0.01 | 0.8 | 0.99997 |
| 2nd Step | ||||||
| Sample | Mechanism | E(kJ/mol) | logA (s−) | logKcat | React. Order n | R2 |
| PHSu | Cn | 173 | 10.9 | 0.3 | 1.2 | 0.99994 |
| PLA-b-PHSU90/10 | Cn | 146 | 9.4 | 0.9 | 1.8 | 0.99997 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chrysafi, I.; Ainali, N.M.; Bikiaris, D.N. Thermal Degradation Mechanism and Decomposition Kinetic Studies of Poly(Lactic Acid) and Its Copolymers with Poly(Hexylene Succinate). Polymers 2021, 13, 1365. https://doi.org/10.3390/polym13091365
Chrysafi I, Ainali NM, Bikiaris DN. Thermal Degradation Mechanism and Decomposition Kinetic Studies of Poly(Lactic Acid) and Its Copolymers with Poly(Hexylene Succinate). Polymers. 2021; 13(9):1365. https://doi.org/10.3390/polym13091365
Chicago/Turabian StyleChrysafi, Iouliana, Nina Maria Ainali, and Dimitrios N. Bikiaris. 2021. "Thermal Degradation Mechanism and Decomposition Kinetic Studies of Poly(Lactic Acid) and Its Copolymers with Poly(Hexylene Succinate)" Polymers 13, no. 9: 1365. https://doi.org/10.3390/polym13091365
APA StyleChrysafi, I., Ainali, N. M., & Bikiaris, D. N. (2021). Thermal Degradation Mechanism and Decomposition Kinetic Studies of Poly(Lactic Acid) and Its Copolymers with Poly(Hexylene Succinate). Polymers, 13(9), 1365. https://doi.org/10.3390/polym13091365