The conductive material used in electronics is usually copper. In addition, conductive elements of nickel, silver, gold, tin, or a zinc–lead alloy are applied to the surfaces of the boards. According to the above data, it was first decided to obtain the XRF qualitative spectrum of the tested computer pins. The obtained spectrum (
Figure 7a) confirmed the presence of some of these elements on the surface of the tested material, which was obtained from electro-scrap.
Figure 7b is, in turn, a comparative test, i.e., it is the spectrum of an empty vessel with a special X-ray film. After approximately estimating the area of the peaks obtained in spectrum 7a, which correspond to specific elements, it can be concluded that the samples contained large amounts of copper, nickel, and zinc.
To consider the above results and high copper(II) content, the tested pins had to be dissolved in nitric(V) acid first. Copper is a semi-precious metal, which means that it does not react with non-oxidizing acids (no signs of reaction with HCl). However, Cu reacts with oxidizing acids (e.g., HNO3).
Nitric acid and its salts are powerful oxidants—therefore, it reacts violently with metals that do not displace hydrogen (e.g., copper or silver). It also oxidizes certain non-metals, such as sulfur, carbon, and phosphorus. In addition, metals such as aluminum, chromium, and iron are passivated on contact with concentrated nitric(V) acid.
In the system studied here, it was observed that the nitric(V) acid dissolved the above metals, as well as the silver, leaving the remaining undissolved metals at the bottom of the vessel in the form of lumps or a brownish sludge. Gold that was not dissolved in HNO3 accumulated on the surface of the solution in the form of flakes. The gold flakes were separated with simple centrifugation methods.
The rest of the precipitate was dissolved in another solvent. For this purpose, a mixture of nitric(V) acid and hydrochloric acid (in a 1:3 ratio), which formed the so-called aqua regia, was used. Its powerful oxidizing properties were evidenced by the fact that it dissolved all precious metals. In addition to gold, metals such as platinum, palladium, hafnium, molybdenum, and zirconium also reacted. When hot, chromium, tungsten, tantalum, rhodium, and osmium also undergo this reaction. The freshly prepared mixture was colorless but began to take on an orange–amber color after only a few minutes. This happened through the formation of nitrosyl chloride according to the equation below:
3.2. Results of Recovery of Metal Ions on Polymer Films Containing Cyphos IL 101
Because previously published research results have shown that Cyphos IL 101 can be successfully used to recover gold from low metal concentrations [
28] and that it works well in an acidic environment [
34], we decided to use this particular ionic liquid to recover metal ions from the acidic solution obtained by leaching the computer pins with nitric acid and aqua regia. Additionally, ionic liquids also perfectly fit into the assumptions of green chemistry because they are a “green” alternative to the popular volatile, flammable, and often toxic organic solvents [
35]. Their use eliminates the emission of harmful substances into the natural environment because they are characterized by a practically immeasurable vapor pressure under moderate conditions [
36,
37].
The mechanism of the sorption process is related to the binding of the metal ions present in the solution to the Cyphos IL 101 molecule as a result of a complexation reaction. In the first stage, in an acidic solution, the proton detaches from the Cyphos IL 101 molecule, which allows the metal ion to attach to this site and form a complex compound. The prepared polymer films were immersed in solutions A and B. From this point onwards, samples were taken from each solution at specified intervals to assess the progress of the sorption process, i.e., to determine the content of metals not yet adsorbed on the polymer surface at any given time. The experiments of the sorption processes were repeated and standard deviations were calculated (
Table 2 and
Table 3).
The data in the above tables show that the best recovery occurred for the precious metals contained in the examined e-waste. In contrast, the recovery of non-ferrous metals (Co, Ni, Cu, Zn, Sn, Pb) occurred at 25–40%, as between 59 and 76% of these metals remained in the nitric(V) acid solution (A) and the aqua regia solution (B). This was most likely due to the selectivity of the metal ion binding agent found in the polymer films used, namely Cyphos IL 101.
This argument appears to be correct because when comparing the recovery of cobalt and gold, it is clear that Cyphos IL 101 performed more efficiently with the precious metal. Cobalt recovered from nitric(V) acid and aqua regia amounted only to 23 and 40%, respectively, while as much as 98.9% of the gold was recovered. The sorption process described could also be influenced by the composition of the solution that was studied. There are numerous examples in the literature of the use of Cyphos IL 101 for the recovery of various precious metal ions, e.g., palladium [
38], gold [
28], or platinum [
29]. The authors of this publication point out that the effectiveness of this compound is closely related to the composition of the medium from which the metal recovery is carried out.
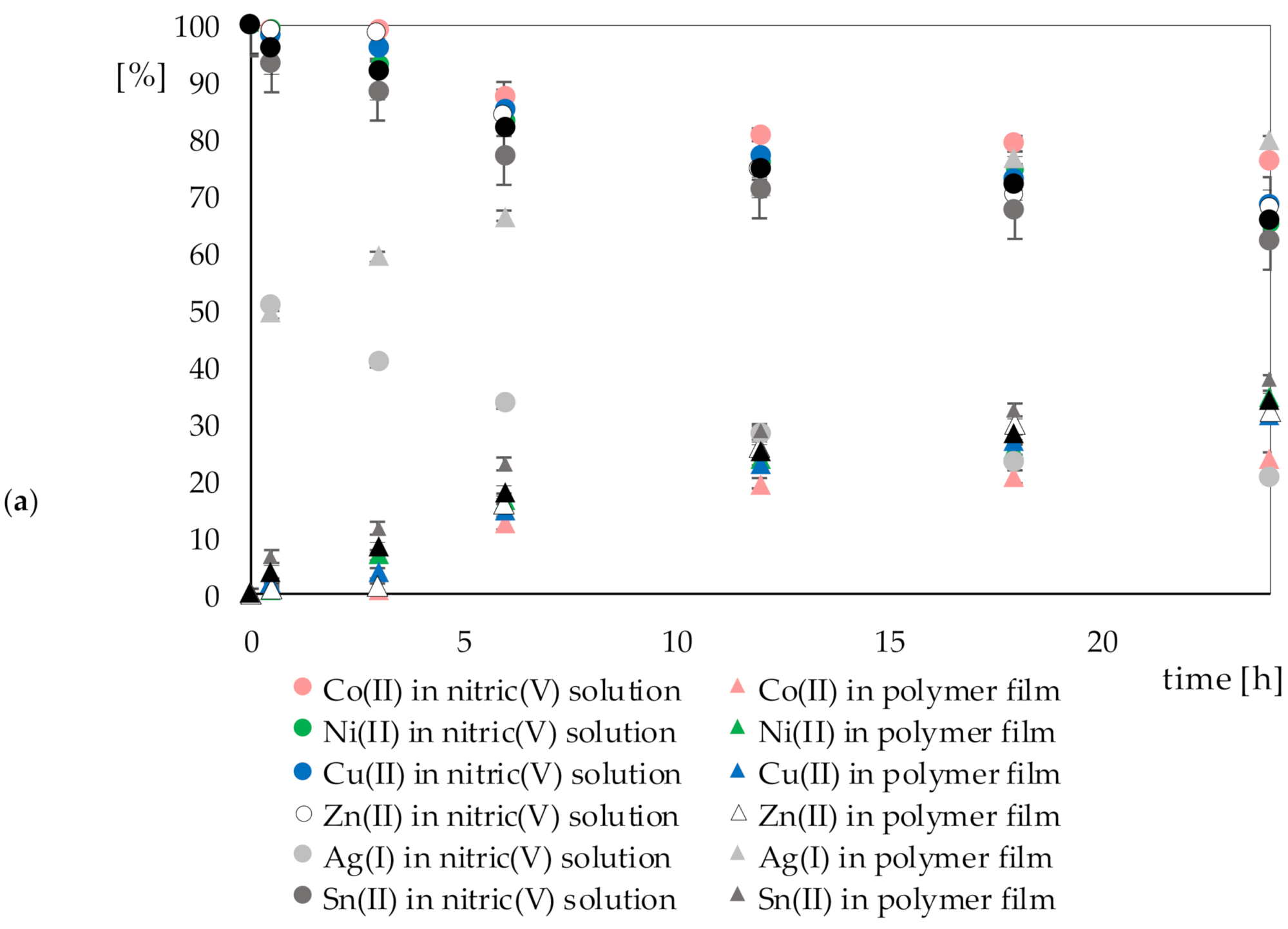
Figure 8a,b show the percentage change in metal ion concentrations during the sorption process in nitric(V) acid (
A) and aqua regia (
B), respectively, with the polymer film.
Only in the case of Au(III) ions can it be seen that it was possible to remove them almost completely from the solution. The amount of gold bound by the polymer films was proportional to the amount of gold lost in the solution. In the case of other metal ions, partial sorption was seen on the polymer films.
To describe the efficiency of removing metal ions from the aqueous solution using polymer films containing Cyphos IL 101, and based on the obtained results, the accumulation factor (AF) was calculated (Equation (8)). The mentioned parameter is described as follows:
where:
c0—the initial concentration of metal ions in the feed phase (mol/dm
3);
cmembrane—the concentration of metal ions in the membrane phase (mol/dm
3).
From the data shown in the graphs above (
Figure 9), it can be seen that the sorption process started immediately when the polymer films were dropped into the solutions. The sorption process could be quantified after only 0.5 h. For most metal ions, the accumulation factor after this time was still low, but in the case of sorption of silver, palladium, and gold ions, it was already 49.30%, 36.36%, and 20.84%, respectively. With time, all accumulation factors increased, and after 24 h, the non-precious metals did not exceed 40%. During the 24 h cycles of sorption, no decreases in accumulation factors were observed. This proved that Cyphos IL 101, which was used in the polymer films, bound the metal ions from the solution very strongly.
The sorption capacity of polymer films with 20 wt.% of Cyphos IL 101 was calculated using Equation (9):
where:
qt—the sorption capacity (mg/g),
V—the solution volume (dm
3),
c—the concentration of metal ions in the feed phase after time
t (mol/dm
3), and
m —the mass of the sorbent (g).
The results of the sorption capacity parameter after 24 h of sorption are presented in
Table 4.
The sorption capacity is a very important parameter of all sorbents. The sorption capacity of the studied polymer films for metal ions was higher in nitric(V) acid than in aqua regia. The highest and the lowest sorption capacities was obtained for Cu(II) and Pd(II); they were 4.91 and 3.05 × 10
−5 mg/g, respectively. The low values of sorption capacities for precious metal ions were caused by their small amounts in the studied WEEE. The received results can be compared with those found in the literature; e.g., the sorption capacities of materials that were obtained through impregnation of Cyphos IL101 on Florisil and Silica were 2.94 and 3.97 mg/g, respectively [
39]. The sorption capacity of Amberlite XAD-7 with immobilized Cyphos IL 101 was, in turn, 71 mg/g [
40], but the costs of preparing these resins were higher than those of the polymer films studied in this paper.
As a result of the sorption process on polymer films containing 20 wt.% of Cyphos IL 101, the following amounts of metals were recovered from 0.5 g of the computer pins studied (
Table 5).
Based on the obtained results, it was found that using a polymer film containing 20 wt.% of Cyphos IL 101 as an ion carrier can recover copper(II) (4.28454 mg) > nickel(II) (3.73884 mg) > gold(III) (0.36179 mg) > tin(II) (0.23698 mg) and trace amounts of Pb(II) > Co(II) > Ag(I) > Pd(II) from 0.5 g e-waste. This allowed the estimation of the number of metals in a tonne of e-waste (
Table 6).
The quantity of metals recovered with the polymer films appears to be small. However, an average Pole produces around 12 kg of e-waste per year, which amounts to almost 500,000 tonnes nationwide, which is around 77% of all waste of this type generated in the European Union. This number is so high because new sub-groups of e-waste that already exist in Western Europe are now beginning to emerge in Poland. The consequence of this phenomenon will be a continuous and dynamic increase in the amount of e-waste produced in our country. In contrast, the US produces the most electronic waste worldwide, according to a United Nations report. Western countries are also unable to recycle 100% of their e-waste, which is why they primarily transport it to African and Asian countries, as the cost of transport to these countries is twice as low as recycling on site [
41].
The recovery method proposed in this paper is a good alternative for recycling valuable metals from electronic waste. Furthermore, it should be noted that this method allows for very selective recovery of precious metals because the percentage that was successfully recovered in relation to other non-ferrous metals was high (98.9% Au(III), 79.3% Ag(I), and 63.6% Pd(II)).