Application of Polymer Drugs with Cerium Dioxide Nanomolecules and Mesenchymal Stem Cells for the Treatment of Skin Wounds in Aged Rats
Abstract
1. Introduction
2. Materials and Methods
- Control group—these are intact wounds without treatment.
- Group of mesenchymal stem cells (SC)—a culture of mesenchymal stem cells at a concentration of 100,000 SC per wound) was injected into the edges of the wounds of this group on the modeling day (0.2 mL: 0.1 mL of SC was injected into the lateral edge, and 0.1 mL of SC into the caudal edge). SC were isolated from the umbilical cord of a newborn after normal delivery at 38–40 weeks of gestation with the informed consent of a healthy mother and produced after the fourth passage by technology [25,26]. After the isolation process, the stem cells had a standard morphological structure of cells grown in a MEM culture medium (Lonsa), StemPro ™ MSC (Gibco, Grand Island, NY, USA), at a concentration of 0.5 × 106 per 1 mL.
- Smart polymeric nano-drug (SPN) group—citrate-stabilized cerium dioxide nanoparticles were integrated into a hydrogel polymer matrix. The cerium dioxide used in this work was synthesized at the Kurnakov Institute of General and Inorganic Chemistry of the Russian Academy of Sciences (Moscow) [27,28]. The hydrogel matrix contained natural and synthetic polymers of structure-forming polysaccharides (pectin, alginate, chitosan, agar–agar, and water-soluble cellulose derivatives, including carboxymethyl cellulose). To prepare the hydrogel base of the nano-drug in 975 mL of H2O, we added a dry sample of carboxymethylcellulose 20 g, pectin (Sigma Aldrich) 2 g, chitosan (Sigma Aldrich) 0.2 g, agar–agar (Roeper) 2 g, and sodium alginate (Sigma Aldrich) 1 g. Next, a colloidal sol with a cerium oxide concentration of 0.004 g/l was added dropwise to 25 mL of the prepared gel matrix with constant stirring. As a result, the concentration of substances in the gel base of the matrix was (in mass percent): carboxymethyl cellulose—1.0; pectin—0.1; chitosan—0.01; agar—0.1; alginate—0.05; cerium oxide in the form of a colloidal sol of nanoparticles—0.0002. A preliminary analysis of the developed agent, as well as the results of experiments, demonstrating its safety and biocompatibility, were presented earlier [24,25,29,30]. SPN was applied topically once a day on day 0, 1, 3, 5, and 7 by applying it over the wound with a needle-free syringe and filling its volume using 1 mL per cm2.
- Smart polymeric nano-drug + stem cells group (SPN + SC)—this differed from the SPN group only in that, in addition to cerium dioxide, mesenchymal stem cells of the human umbilical cord at a concentration 50,000 cells/cm2, produced using the same technology as for the SC group, were also added to the polymer matrix. The application of SPN + SC was carried out non-invasively by surface application of hydrogel days until the entire volume of the wound was filled (as in the SPN group for the same technology and at the same time).
3. Results
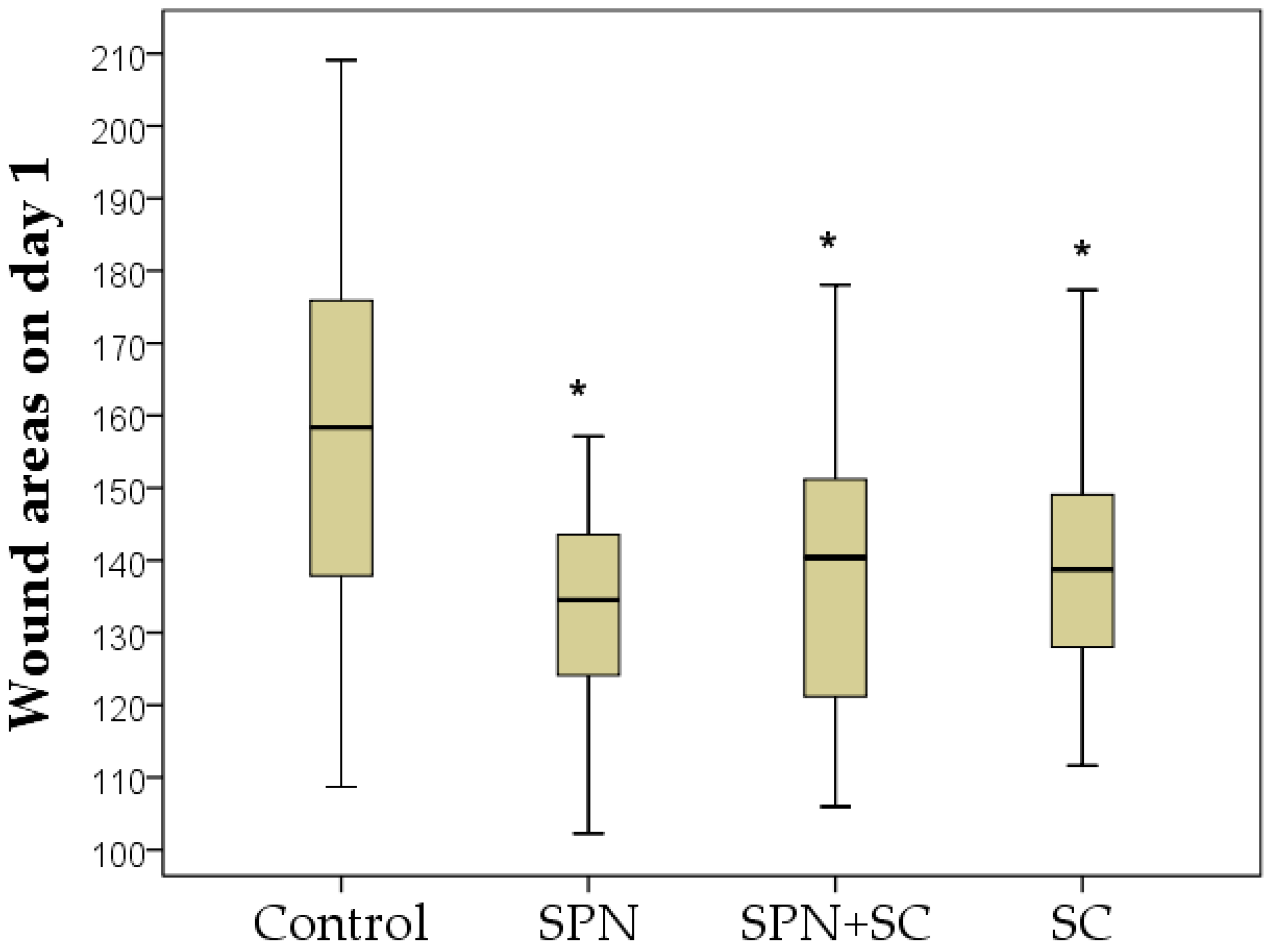
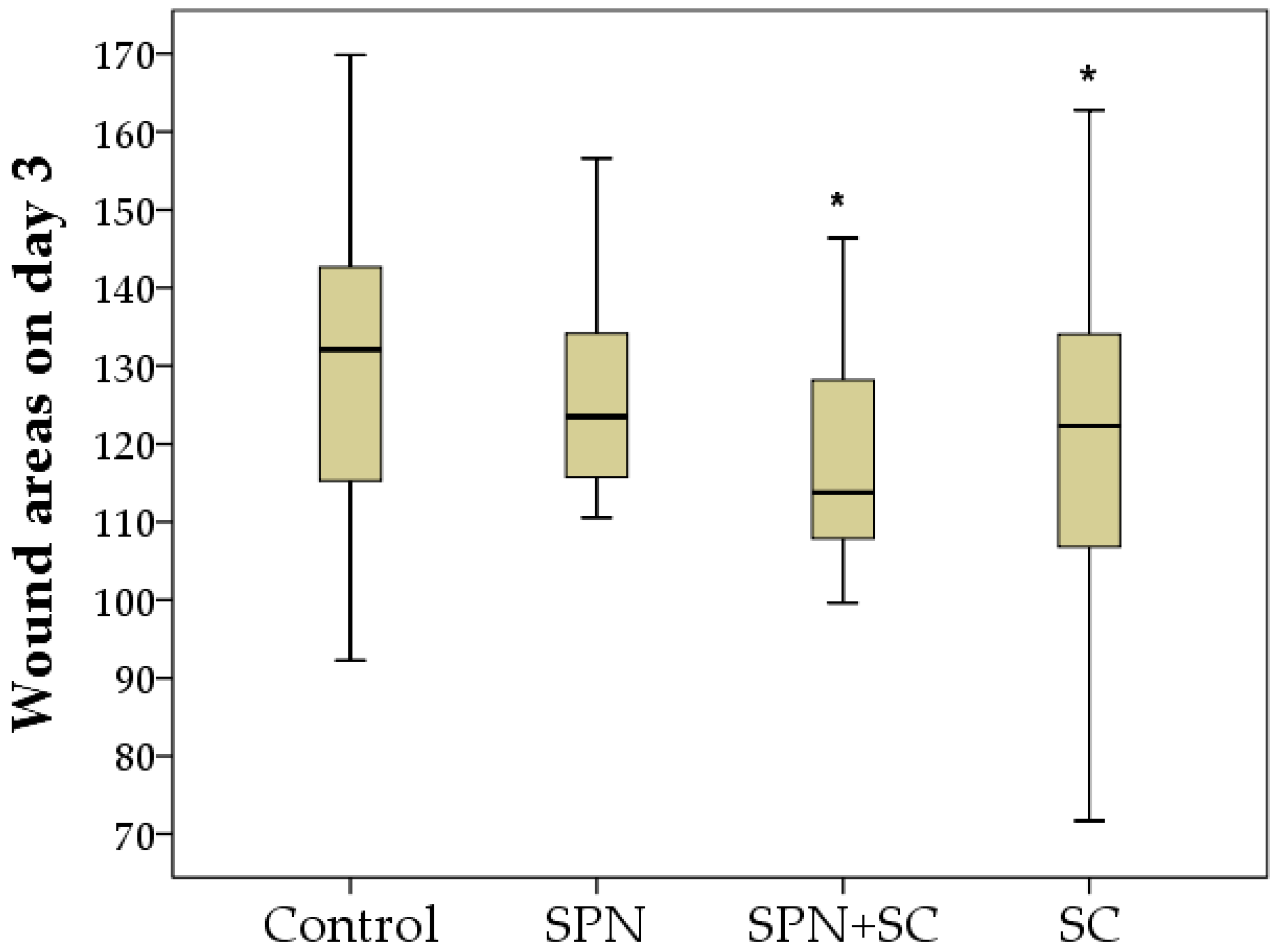
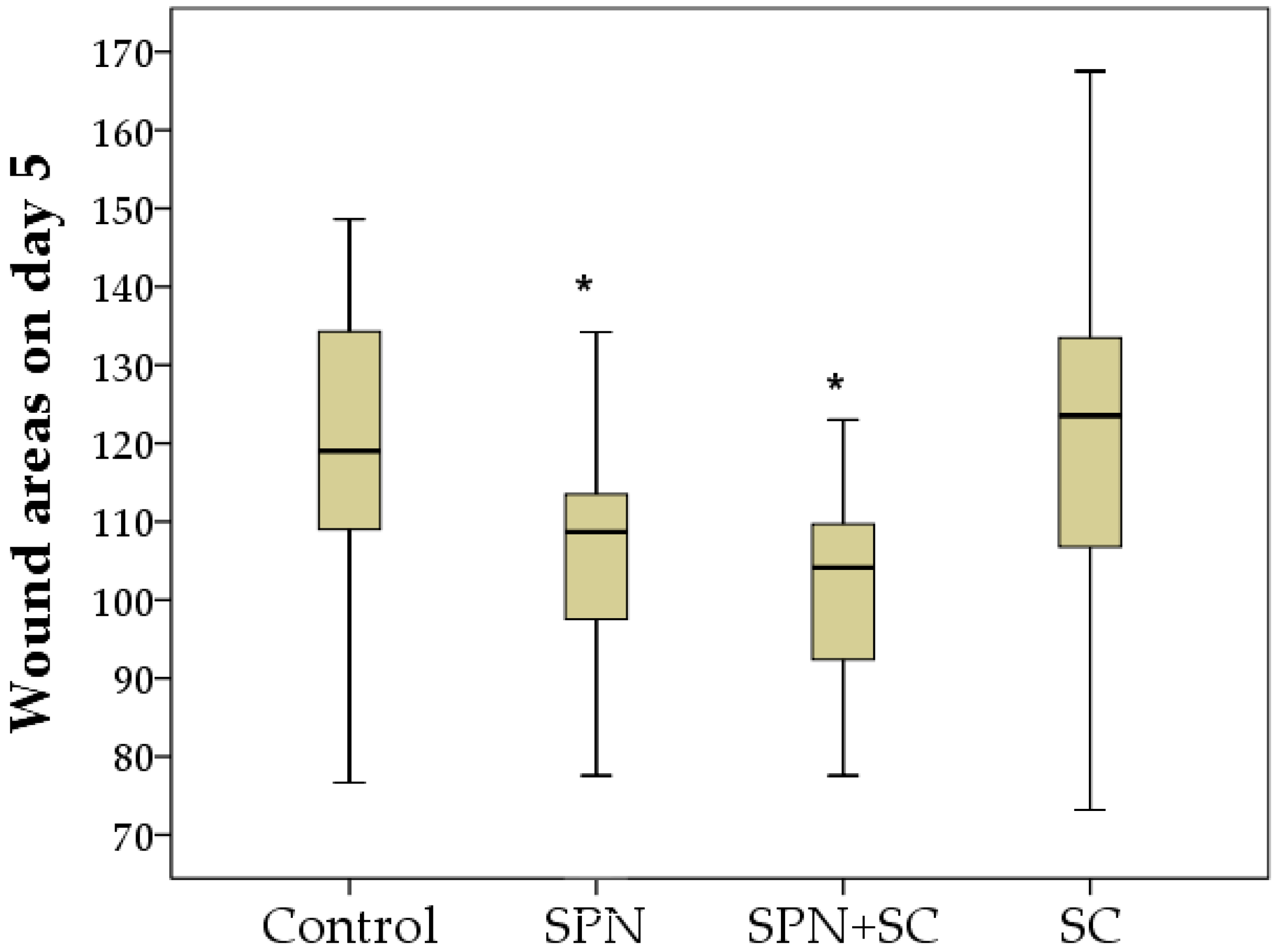
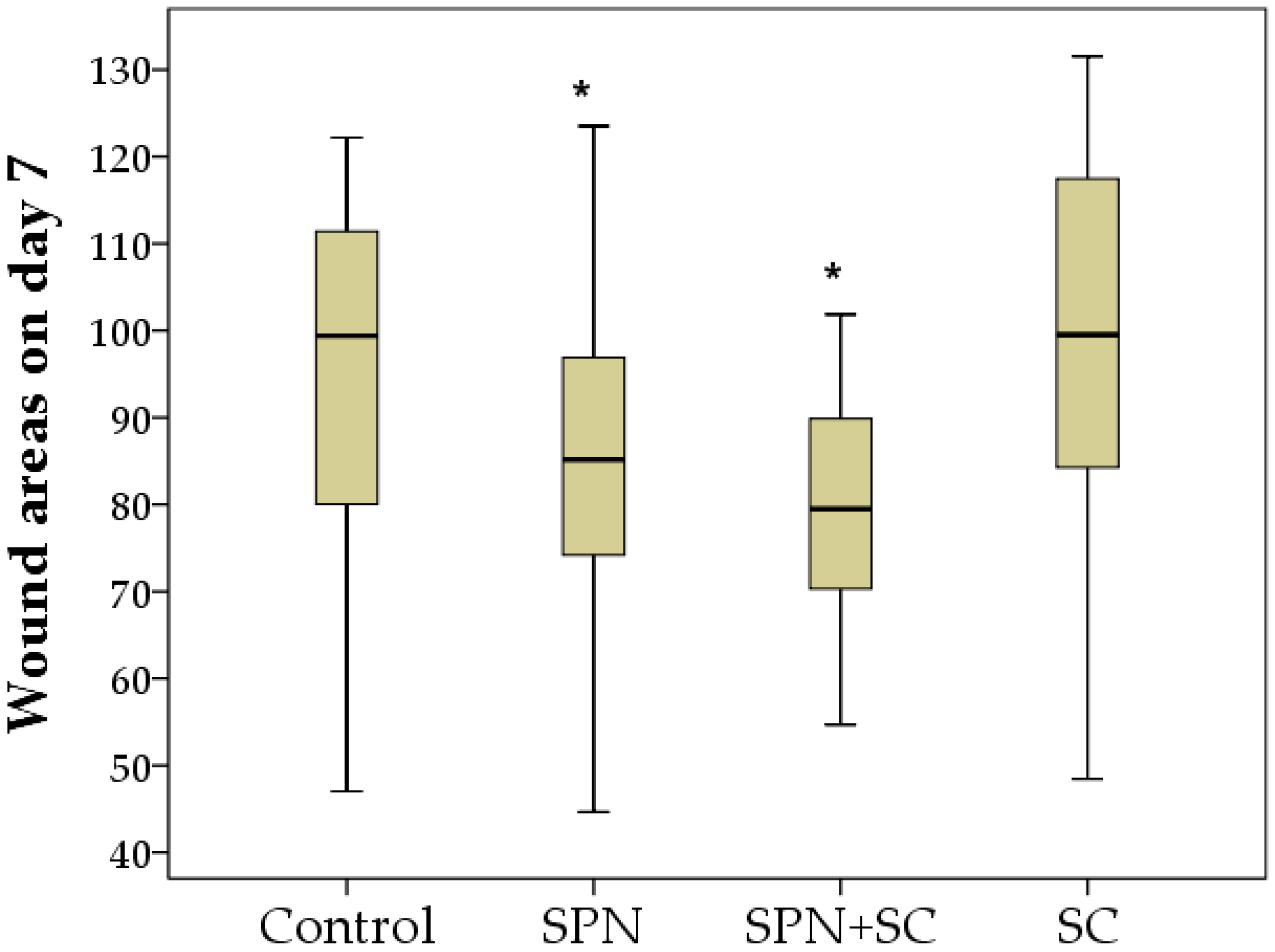
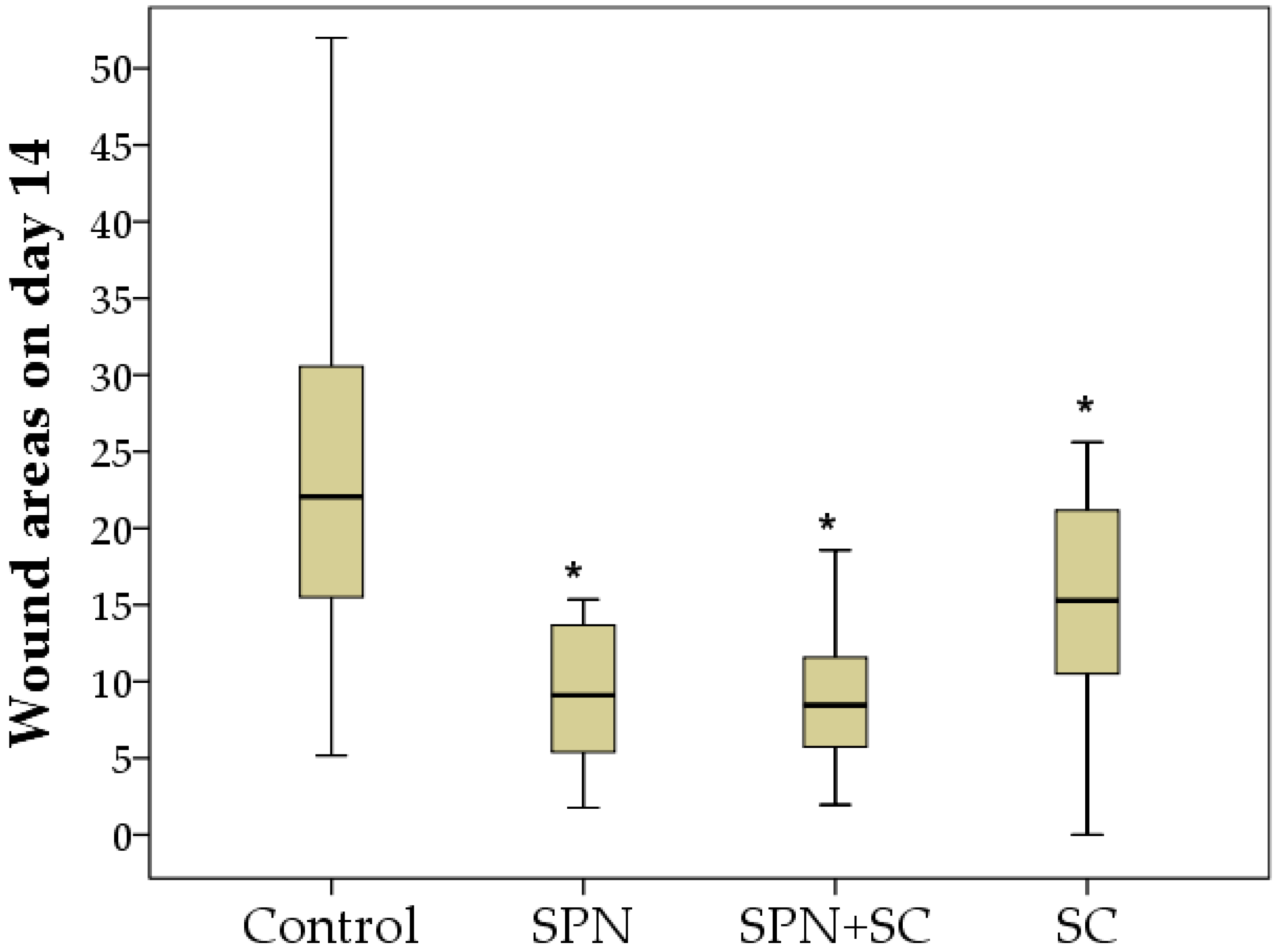
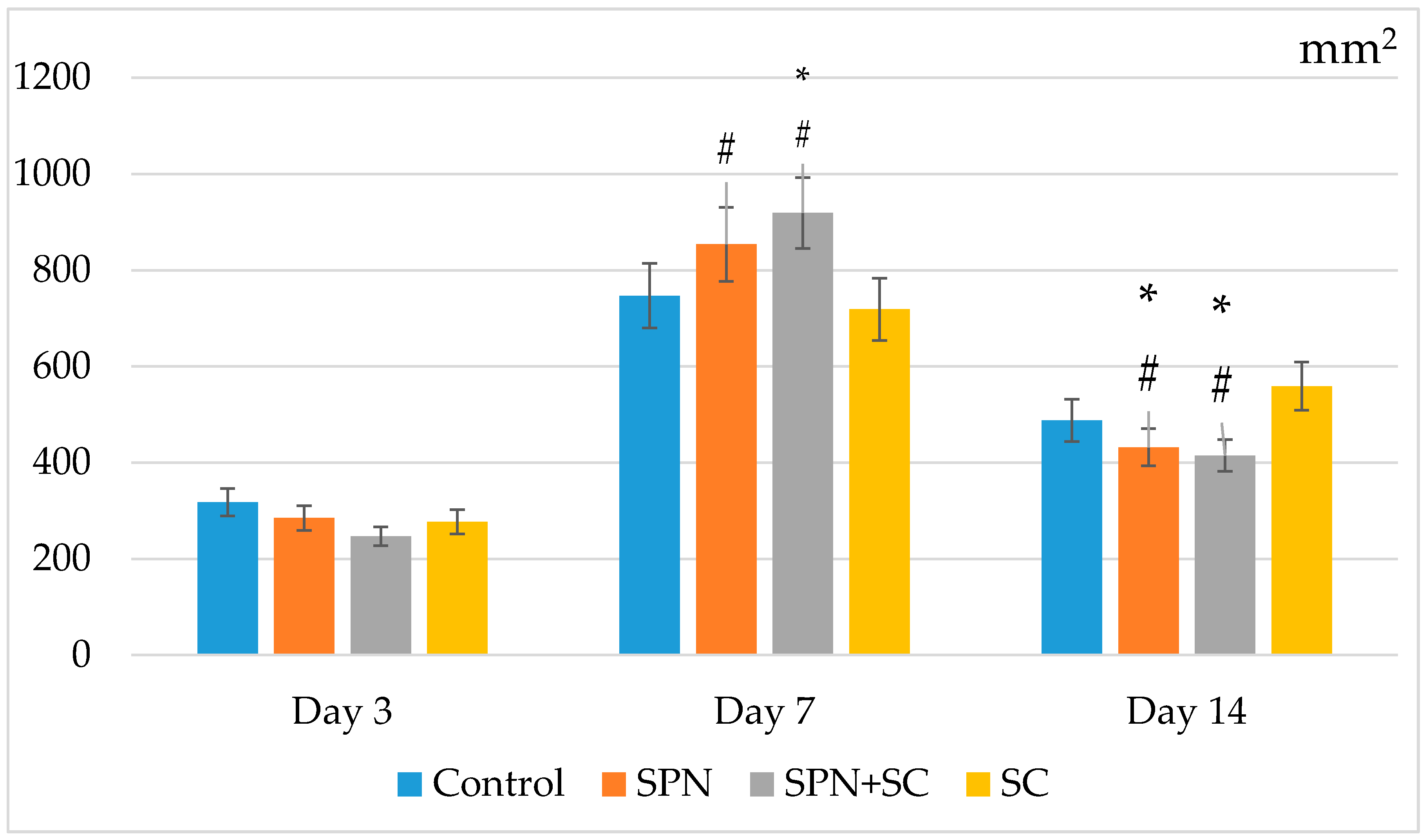
3.1. The Dynamics of Wound Areas
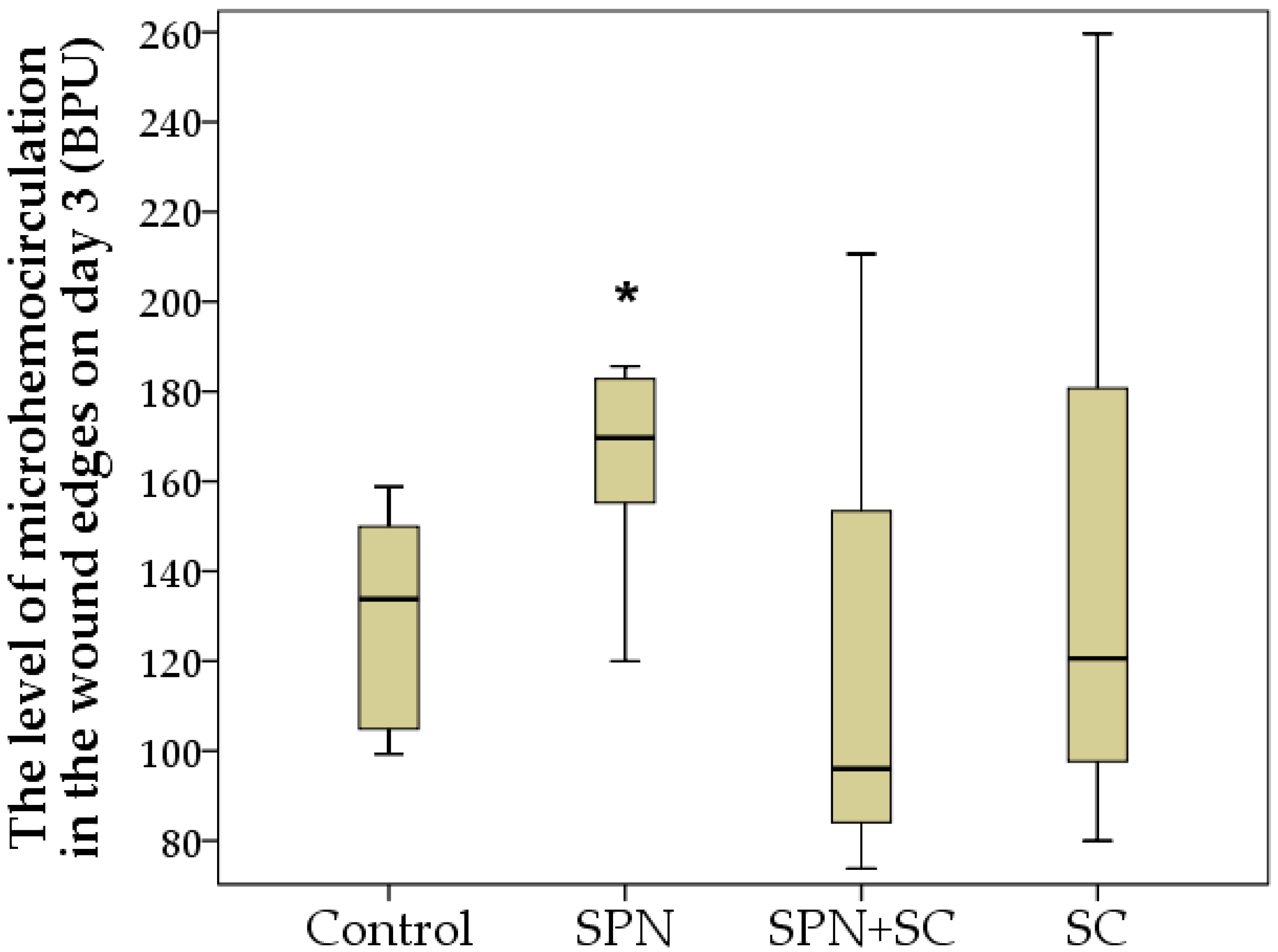
3.2. Microcirculation Dynamics
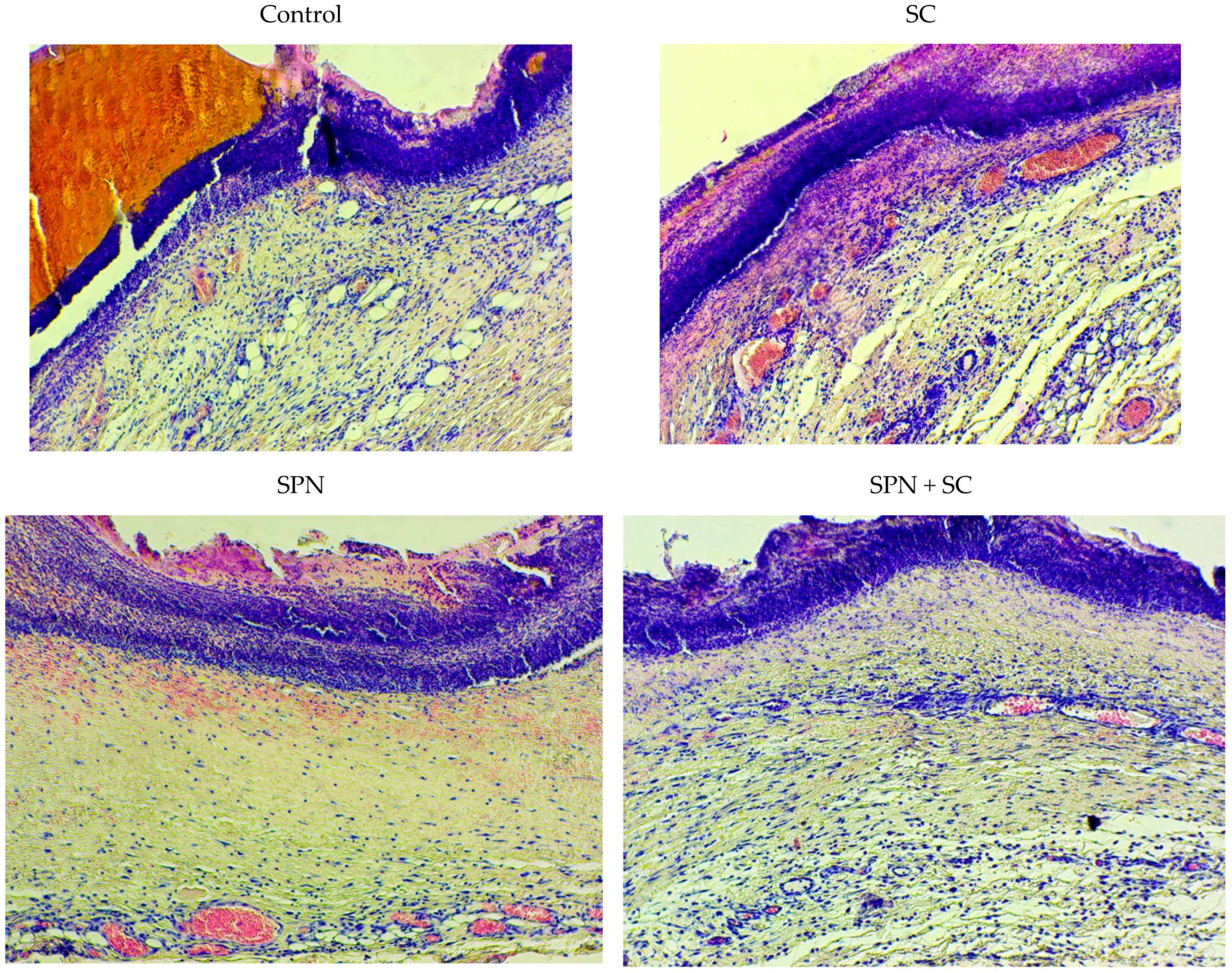
3.3. Morphometry and Microscopy of Wounds
4. Discussion
- The results obtained prove the high efficiency of drugs using nanomaterials integrated into a polymer matrix.
- The results of the use of smart polymeric drugs indicate the need or the possibility of creating fundamentally new, highly effective drugs.
- The drugs developed and studied in the manuscript can be used in clinical practice for the treatment of acute and long-term non-healing chronic wounds.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Han, G.; Ceilley, R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef]
- Stupin, V.A.; Gabitov, R.B.; Sinelnikova, T.G.; Silina, E.V. Biological Mechanisms of Chronic Wound and Diabetic Foot Healing: The Role of Collagen. Serbian J. Exp. Clin. Res. 2018, 19, 373–382. [Google Scholar] [CrossRef]
- Sun, X.; Ni, P.; Wu, M.; Huang, Y.; Ye, J.; Xie, T. A Clinicoepidemiological Profile of Chronic Wounds in Wound Healing Department in Shanghai. Int. J. Low. Extremity Wounds 2017, 16, 36–44. [Google Scholar] [CrossRef]
- Abasian, P.; Shakibi, S.; Maniati, M.S.; Khorasani, S.N.; Khalili, S. Targeted delivery, drug release strategies, and toxicity study of polymeric drug nanocarriers. Polym. Adv. Technol. 2021, 32, 931–944. [Google Scholar] [CrossRef]
- Alavi, S.F.; Abasian, P.; Eslami, H. Synthesis and characterization of polystyrene/poly(ethyl acrylate) mushroom-like Janus particles. Polym. Adv. Technol. 2021, 32, 1712–1726. [Google Scholar] [CrossRef]
- Jackson, W.M.; Nesti, L.J.; Tuan, R.S. Mesenchymal stem cell therapy for attenuation of scar formation during wound healing. Stem Cell Res. Ther. 2012, 3, 20. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Hu, H.; Gorecka, J.; Bai, H.; He, H.; Assi, R.; Isaji, T.; Wang, T.; Setia, O.; Lopes, L.; et al. Adipose-derived mesenchymal stem cells accelerate diabetic wound healing in a similar fashion as bone marrow-derived cells. Am. J. Physiol. Physiol. 2018, 315, C885–C896. [Google Scholar] [CrossRef]
- Kanji, S.; Das, H. Advances of Stem Cell Therapeutics in Cutaneous Wound Healing and Regeneration. Mediat. Inflamm. 2017, 2017, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Dehkordi, A.N.; Babaheydari, F.M.; Chehelgerdi, M.; Dehkordi, S.R. Skin tissue engineering: Wound healing based on stem-cell-based therapeutic strategies. Stem Cell Res. Ther. 2019, 10, 111. [Google Scholar] [CrossRef]
- Joseph, B.; George, A.; Gopi, S.; Kalarikkal, N.; Thomas, S. Polymer sutures for simultaneous wound healing and drug delivery—A review. Int. J. Pharm. 2017, 524, 454–466. [Google Scholar] [CrossRef]
- Hussain, Z.; Thu, H.E.; Shuid, A.N.; Katas, H.; Hussain, F. Recent Advances in Polymer-based Wound Dressings for the Treatment of Diabetic Foot Ulcer: An Overview of State-of-the-art. Curr. Drug Targets 2018, 19, 527–550. [Google Scholar] [CrossRef] [PubMed]
- Sahana, T.G.; Rekha, P.D. Biopolymers: Applications in wound healing and skin tissue engineering. Mol. Biol. Rep. 2018, 45, 2857–2867. [Google Scholar] [CrossRef]
- Kar, A.K.; Singh, A.; Dhiman, N.; Purohit, M.P.; Jagdale, P.; Kamthan, M.; Singh, D.; Kumar, M.; Ghosh, D.; Patnaik, S. Polymer-Assisted In Situ Synthesis of Silver Nanoparticles with Epigallocatechin Gallate (EGCG) Impregnated Wound Patch Potentiate Controlled Inflammatory Responses for Brisk Wound Healing. Int. J. Nanomed. 2019, 14, 9837–9854. [Google Scholar] [CrossRef]
- Matica, M.A.; Aachmann, F.L.; Tøndervik, A.; Sletta, H.; Ostafe, V. Chitosan as a Wound Dressing Starting Material: Antimicrobial Properties and Mode of Action. Int. J. Mol. Sci. 2019, 20, 5889. [Google Scholar] [CrossRef] [PubMed]
- Grace, J.L.; Schneider-Futschik, E.K.; Elliott, A.G.; Amado, M.; Truong, N.P.; Cooper, M.A.; Li, J.; Davis, T.P.; Quinn, J.F.; Velkov, T.; et al. Exploiting Macromolecular Design to Optimize the Antibacterial Activity of Alkylated Cationic Oligomers. Biomacromolecules 2018, 19, 4629–4640. [Google Scholar] [CrossRef] [PubMed]
- Nelson, B.C.; Johnson, M.E.; Walker, M.L.; Riley, K.R.; Sims, C.M. Antioxidant Cerium Oxide Nanoparticles in Biology and Medicine. Antioxidants 2016, 5, 15. [Google Scholar] [CrossRef]
- Dutta, D.; Mukherjee, R.; Patra, M.; Banik, M.; Dasgupta, R.; Mukherjee, M.; Basu, T. Green synthesized cerium oxide nanoparticle: A prospective drug against oxidative harm. Colloids Surfaces B Biointerfaces 2016, 147, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Rather, H.A.; Thakore, R.; Singh, R.; Jhala, D.; Singh, S.; Vasita, R. Antioxidative study of Cerium Oxide nanoparticle functionalised PCL-Gelatin electrospun fibers for wound healing application. Bioact. Mater. 2018, 3, 201–211. [Google Scholar] [CrossRef]
- Charbgoo, F.; Bin Ahmad, M.; Darroudi, M. Cerium oxide nanoparticles: Green synthesis and biological applications. Int. J. Nanomed. 2017, 12, 1401–1413. [Google Scholar] [CrossRef]
- Nethi, S.K.; Das, S.; Patra, C.R.; Mukherjee, S. Recent advances in inorganic nanomaterials for wound-healing applications. Biomater. Sci. 2019, 7, 2652–2674. [Google Scholar] [CrossRef]
- Ma, X.; Cheng, Y.; Jian, H.; Feng, Y.; Chang, Y.; Zheng, R.; Wu, X.; Wang, L.; Li, X.; Zhang, H. Hollow, Rough, and Nitric Oxide-Releasing Cerium Oxide Nanoparticles for Promoting Multiple Stages of Wound Healing. Adv. Healthc. Mater. 2019, 8, e1900256. [Google Scholar] [CrossRef]
- Huang, X.; Li, L.-D.; Lyu, G.-M.; Shen, B.-Y.; Han, Y.-F.; Shi, J.-L.; Teng, J.-L.; Feng, L.; Si, S.-Y.; Wu, J.-H.; et al. Chitosan-coated cerium oxide nanocubes accelerate cutaneous wound healing by curtailing persistent inflammation. Inorg. Chem. Front. 2017, 5, 386–393. [Google Scholar] [CrossRef]
- Zgheib, C.; Hilton, S.A.; Dewberry, L.C.; Hodges, M.M.; Ghatak, S.; Xu, J.; Singh, S.; Roy, S.; Sen, C.K.; Seal, S.; et al. Use of Cerium Oxide Nanoparticles Conjugated with MicroRNA-146a to Correct the Diabetic Wound Healing Impairment. J. Am. Coll. Surg. 2019, 228, 107–115. [Google Scholar] [CrossRef]
- Silina, E.V.; Manturova, N.E.; Vasin, V.I.; Artyushkova, E.B.; Khokhlov, N.V.; Ivanov, A.V.; Stupin, V.A. Efficacy of A Novel Smart Polymeric Nanodrug in the Treatment of Experimental Wounds in Rats. Polymers 2020, 12, 1126. [Google Scholar] [CrossRef] [PubMed]
- Silina, E.; Manturova, N.; Stupin, V. Mesenchymal Stem Cells Application in Wound Tissue Healing in Old Animals. Stem Cells Cloning Adv. Appl. 2020, 13, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Suzdaltseva, Y.; Zhidkih, S.; Kiselev, S.L.; Stupin, V. Locally Delivered Umbilical Cord Mesenchymal Stromal Cells Reduce Chronic Inflammation in Long-Term Nonhealing Wounds: A Randomized Study. Stem Cells Int. 2020. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, V.K.; Polezhaeva, O.S.; Shaporev, A.S.; Baranchikov, A.E.; Shcherbakov, A.B.; Usatenko, A.V. Synthesis and thermal stability of nanocrystalline ceria sols stabilized by citric and polyacrylic acids. Russ. J. Inorg. Chem. 2010, 55, 328–332. [Google Scholar] [CrossRef]
- Shcherbakov, A.B.; Zholobak, N.M.; Ivanov, V.K.; Ivanova, O.S.; Marchevsky, A.V.; Baranchikov, A.E.; Spivak, N.Y.; Tretyakov, Y.D. Synthesis and antioxidant activity of biocompatible maltodextrin-stabilized aqueous sols of nanocrystalline ceria. Russ. J. Inorg. Chem. 2012, 57, 1411–1418. [Google Scholar] [CrossRef]
- Zholobak, N.; Ivanov, V.; Shcherbakov, A.; Shaporev, A.; Polezhaeva, O.; Baranchikov, A.; Spivak, N.; Tretyakov, Y. UV-shielding property, photocatalytic activity and photocytotoxicity of ceria colloid solutions. J. Photochem. Photobiol. B Biol. 2011, 102, 32–38. [Google Scholar] [CrossRef]
- Ermakov, A.; Popov, A.; Ermakova, O.; Ivanova, O.; Baranchikov, A.; Kamenskikh, K.; Shekunova, T.; Shcherbakov, A.; Popova, N.; Ivanov, V. The first inorganic mitogens: Cerium oxide and cerium fluoride nanoparticles stimulate planarian regeneration via neoblastic activation. Mater. Sci. Eng. C 2019, 104, 109924. [Google Scholar] [CrossRef]
- Delfi, M.; Sartorius, R.; Ashrafizadeh, M.; Sharifi, E.; Zhang, Y.; De Berardinis, P.; Zarrabi, A.; Varma, R.S.; Tay, F.R.; Smith, B.R.; et al. Self-assembled peptide and protein nanostructures for anti-cancer therapy: Targeted delivery, stimuli-responsive devices and immunotherapy. Nano Today 2021, 38, 101119. [Google Scholar] [CrossRef]
- Makvandi, P.; Josic, U.; Delfi, M.; Pinelli, F.; Jahed, V.; Kaya, E.; Ashrafizadeh, M.; Zarepour, A.; Rossi, F.; Zarrabi, A.; et al. Drug Delivery (Nano)Platforms for Oral and Dental Applications: Tissue Regeneration, Infection Control, and Cancer Management. Adv. Sci. 2021, 8, 2004014. [Google Scholar] [CrossRef]
- Su, W.-H.; Wang, C.-J.; Fu, H.-C.; Sheng, C.-M.; Tsai, C.-C.; Cheng, J.-H.; Chuang, P.-C. Human Umbilical Cord Mesenchymal Stem Cells Extricate Bupivacaine-Impaired Skeletal Muscle Function via Mitigating Neutrophil-Mediated Acute Inflammation and Protecting against Fibrosis. Int. J. Mol. Sci. 2019, 20, 4312. [Google Scholar] [CrossRef]
- Wang, L.-T.; Ting, C.-H.; Yen, M.-L.; Liu, K.-J.; Sytwu, H.-K.; Wu, K.K.; Yen, B.L. Human mesenchymal stem cells (MSCs) for treatment towards immune- and inflammation-mediated diseases: Review of current clinical trials. J. Biomed. Sci. 2016, 23, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, D.; Liu, X.; Tang, S.; Wei, F. Human umbilical cord mesenchymal stem cells reduce systemic inflammation and attenuate LPS-induced acute lung injury in rats. J. Inflamm. 2012, 9, 33. [Google Scholar] [CrossRef] [PubMed]
- Prockop, D.J.; Oh, J.Y. Mesenchymal Stem/Stromal Cells (MSCs): Role as Guardians of Inflammation. Mol. Ther. 2012, 20, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Sah, S.K.; Lee, J.H.; Seo, K.-W.; Kang, K.-S.; Kim, T.-Y. Human umbilical cord blood-derived mesenchymal stem cells ameliorate psoriasis-like skin inflammation in mice. Biochem. Biophys. Rep. 2016, 9, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Takeo, M.; Lee, W.; Ito, M. Wound Healing and Skin Regeneration. Cold Spring Harb. Perspect. Med. 2015, 5, a023267. [Google Scholar] [CrossRef]
- Ojeh, N.; Pastar, I.; Tomic-Canic, M.; Stojadinovic, O. Stem Cells in Skin Regeneration, Wound Healing, and Their Clinical Applications. Int. J. Mol. Sci. 2015, 16, 25476–25501. [Google Scholar] [CrossRef]
- Iwata, Y.; Akamatsu, H.; Hasebe, Y.; Hasegawa, S.; Sugiura, K. Skin-resident stem cells and wound healing. Jpn. J. Clin. Immunol. 2017, 40, 1–11. [Google Scholar] [CrossRef][Green Version]
- Kosaric, N.; Kiwanuka, H.; Gurtner, G.C. Stem cell therapies for wound healing. Expert Opin. Biol. Ther. 2019, 19, 575–585. [Google Scholar] [CrossRef] [PubMed]





| Groups | Residents on Day 3 | Non-Residents on Day 3 | ||||
|---|---|---|---|---|---|---|
| Edge 1 | Center | Edge 2 | Edge 1 | Center | Edge 2 | |
| Control | 21.8 ± 4.6 | 35.6 ± 2.6 | 23.4 ± 6.4 | 78.2 ± 4.6 | 64.4 ± 2.6 | 76.6 ± 6.4 |
| SPN | 24.6 ± 5.2 | 37.0 ± 4.5 | 22.1 ± 6.1 | 75.4 ± 5.2 | 63.0 ± 4.5 | 77.9 ± 6.1 |
| SPN + SC | 35.8 ± 10.1 | 37.9 ± 4.7 | 21.4 ± 9.9 | 64.2 ± 10.1 | 62.1 ± 4.7 | 78.6 ± 9.9 |
| Stem Cells | 26.3 ± 5.1 | 37.7 ± 4.9 | 22.5 ± 6.4 | 73.7 ± 5.1 | 62.3 ± 4.9 | 77.5 ± 6.4 |
| Residents on Day 7 | Non-Residents on Day 7 | |||||
|---|---|---|---|---|---|---|
| Groups | Edge 1 | Center | Edge 2 | Edge 1 | Center | Edge 2 |
| Control | 76.1 ± 3.9 | 56.4 ± 5.9 | 72.2 ± 7.1 | 23.9 ± 3.9 | 43.6 ± 5.9 | 27.8 ± 7.1 |
| SPN | 77.4 ± 4.0 | 62.0 ± 3.4 * | 72.3 ± 6.5 | 22.6 ± 4.0 | 38.0 ± 3.4 * | 27.7 ± 6.5 |
| SPN + SC | 79.0 ± 4.4 | 63.7 ± 2.3 * | 72.5 ± 6.1 | 21.0 ± 4.4 | 36.3 ± 2.3 * | 27.5 ± 6.1 |
| Stem Cells | 75.7 ± 3.0 | 60.3 ± 3.0 | 71.5 ± 4.4 | 24.3 ± 3.0 | 39.7 ± 3.0 | 28.5 ± 4.4 |
| Groups | Residents on Day 14 | Non-Residents on Day 14 | ||||
|---|---|---|---|---|---|---|
| Edge 1 | Center | Edge 2 | Edge 1 | Center | Edge 2 | |
| Control | 72.8 ± 5.4 | 72.6 ± 6.0 | 80.4 ± 4.9 | 27.2 ± 5.4 | 27.4 ± 6.0 | 19.6 ± 6.0 |
| SPN | 76.3 ± 3.0 | 71.5 ± 4.9 | 77.2 ± 4.3 | 23.7 ± 3.0 | 28.5 ± 4.9 | 22.8 ± 4.3 |
| SPN + SC | 77.1 ± 2.8 | 69.4 ± 5.4 * | 76.7 ± 5.5 | 22.9 ± 2.8 | 30.6 ± 5.4 * | 23.3 ± 5.5 |
| Stem Cells | 75.6 ± 3.1 | 75.2 ± 4.1 | 78.0 ± 3.1 | 24.4 ± 3.1 | 24.8 ± 4.1 | 22.0 ± 3.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silina, E.V.; Stupin, V.A.; Suzdaltseva, Y.G.; Aliev, S.R.; Abramov, I.S.; Khokhlov, N.V. Application of Polymer Drugs with Cerium Dioxide Nanomolecules and Mesenchymal Stem Cells for the Treatment of Skin Wounds in Aged Rats. Polymers 2021, 13, 1467. https://doi.org/10.3390/polym13091467
Silina EV, Stupin VA, Suzdaltseva YG, Aliev SR, Abramov IS, Khokhlov NV. Application of Polymer Drugs with Cerium Dioxide Nanomolecules and Mesenchymal Stem Cells for the Treatment of Skin Wounds in Aged Rats. Polymers. 2021; 13(9):1467. https://doi.org/10.3390/polym13091467
Chicago/Turabian StyleSilina, Ekaterina Vladimirovna, Victor Aleksandrovich Stupin, Yulia Gennadievna Suzdaltseva, Salekh Rovshanovich Aliev, Igor Sergeevich Abramov, and Nikolay Valerievich Khokhlov. 2021. "Application of Polymer Drugs with Cerium Dioxide Nanomolecules and Mesenchymal Stem Cells for the Treatment of Skin Wounds in Aged Rats" Polymers 13, no. 9: 1467. https://doi.org/10.3390/polym13091467
APA StyleSilina, E. V., Stupin, V. A., Suzdaltseva, Y. G., Aliev, S. R., Abramov, I. S., & Khokhlov, N. V. (2021). Application of Polymer Drugs with Cerium Dioxide Nanomolecules and Mesenchymal Stem Cells for the Treatment of Skin Wounds in Aged Rats. Polymers, 13(9), 1467. https://doi.org/10.3390/polym13091467






