Molecular Dynamics Investigation of the Thermo-Mechanical Properties of the Moisture Invaded and Cross-Linked Epoxy System
Abstract
:1. Introduction
2. MD Simulation Methodology
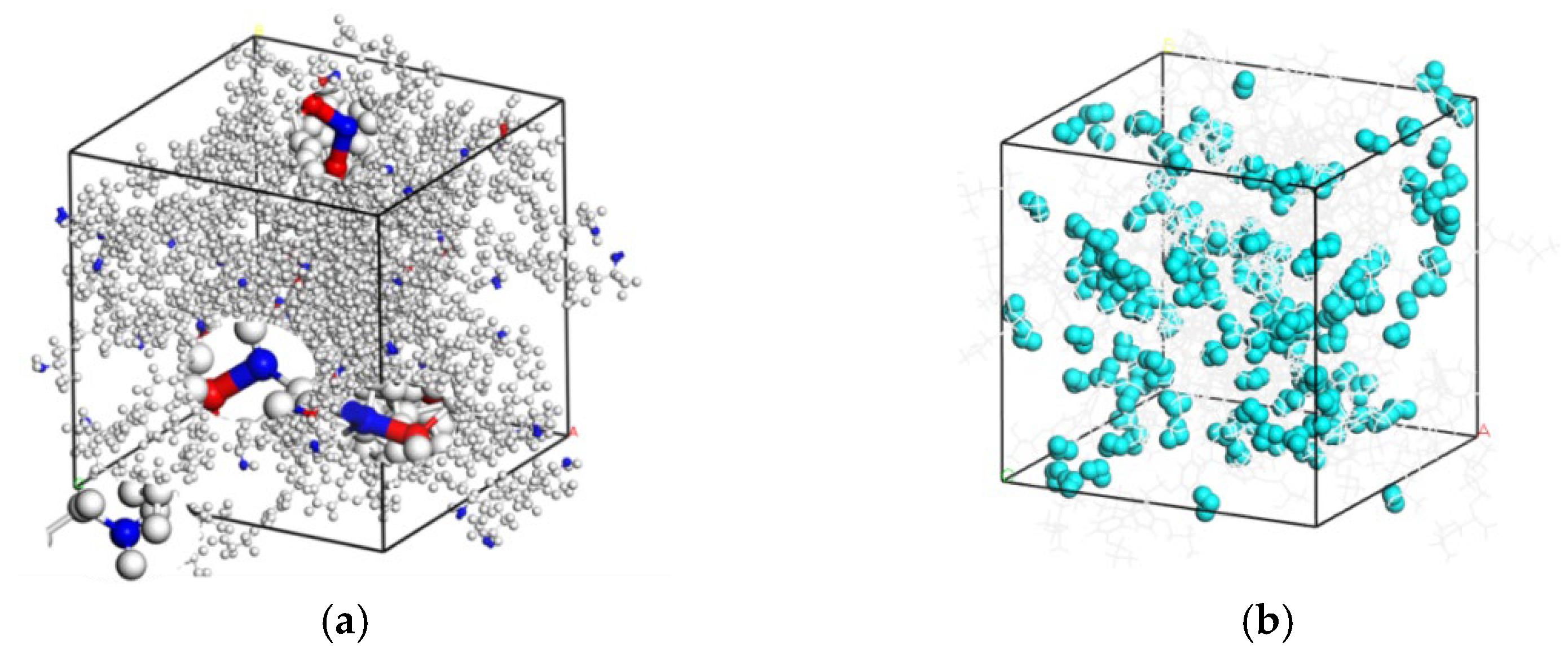
2.1. Composition of Simulation Cell
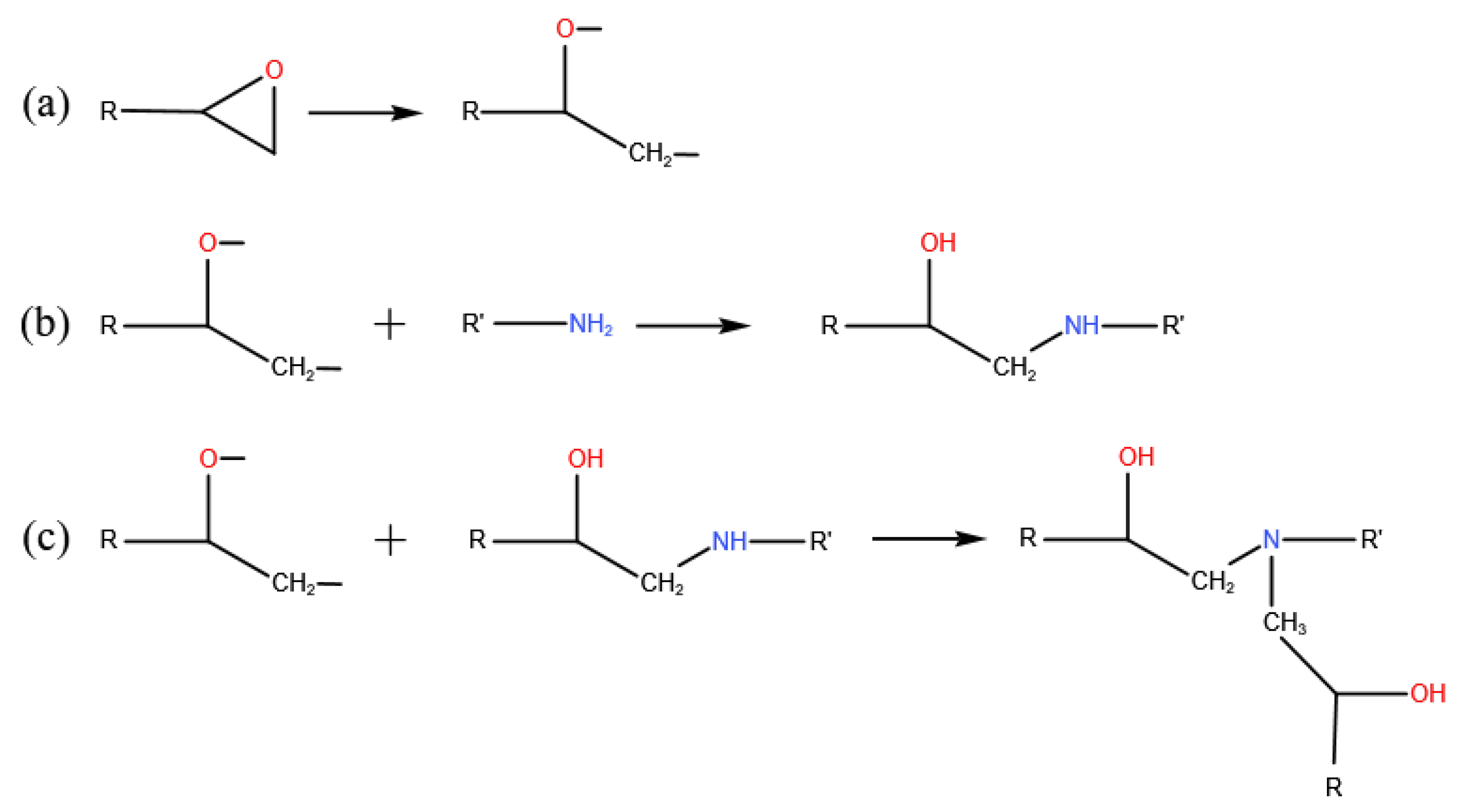
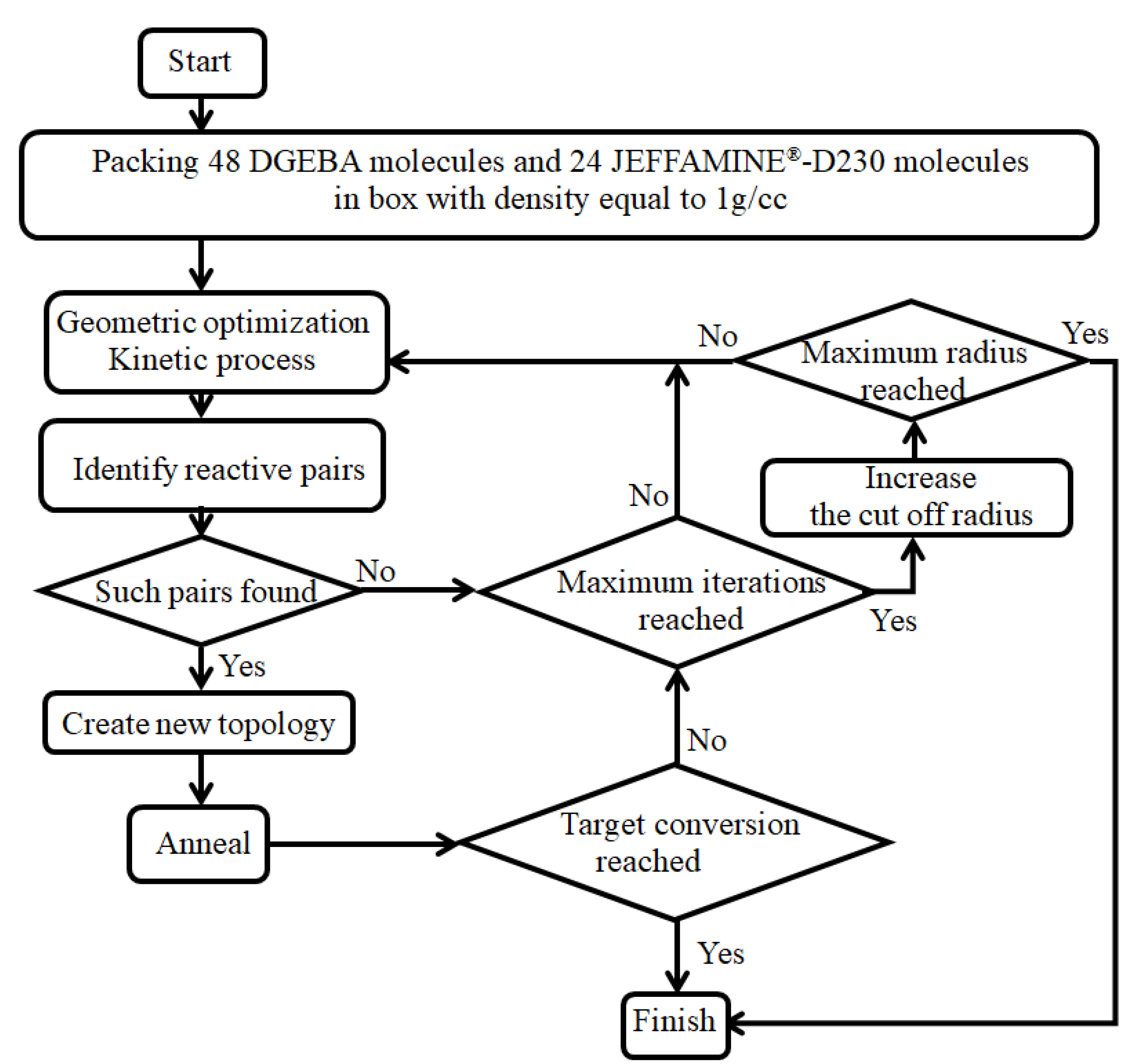
2.2. Cross-Linking Algorithm
2.3. Force Field and Simulation Details
3. Results and Discussion
3.1. Effects of Moisture on the Structure of Epoxy Polymer
3.1.1. Analysis for the Hydrogen Bond
3.1.2. Free Volume
3.1.3. Mean Square Displacement
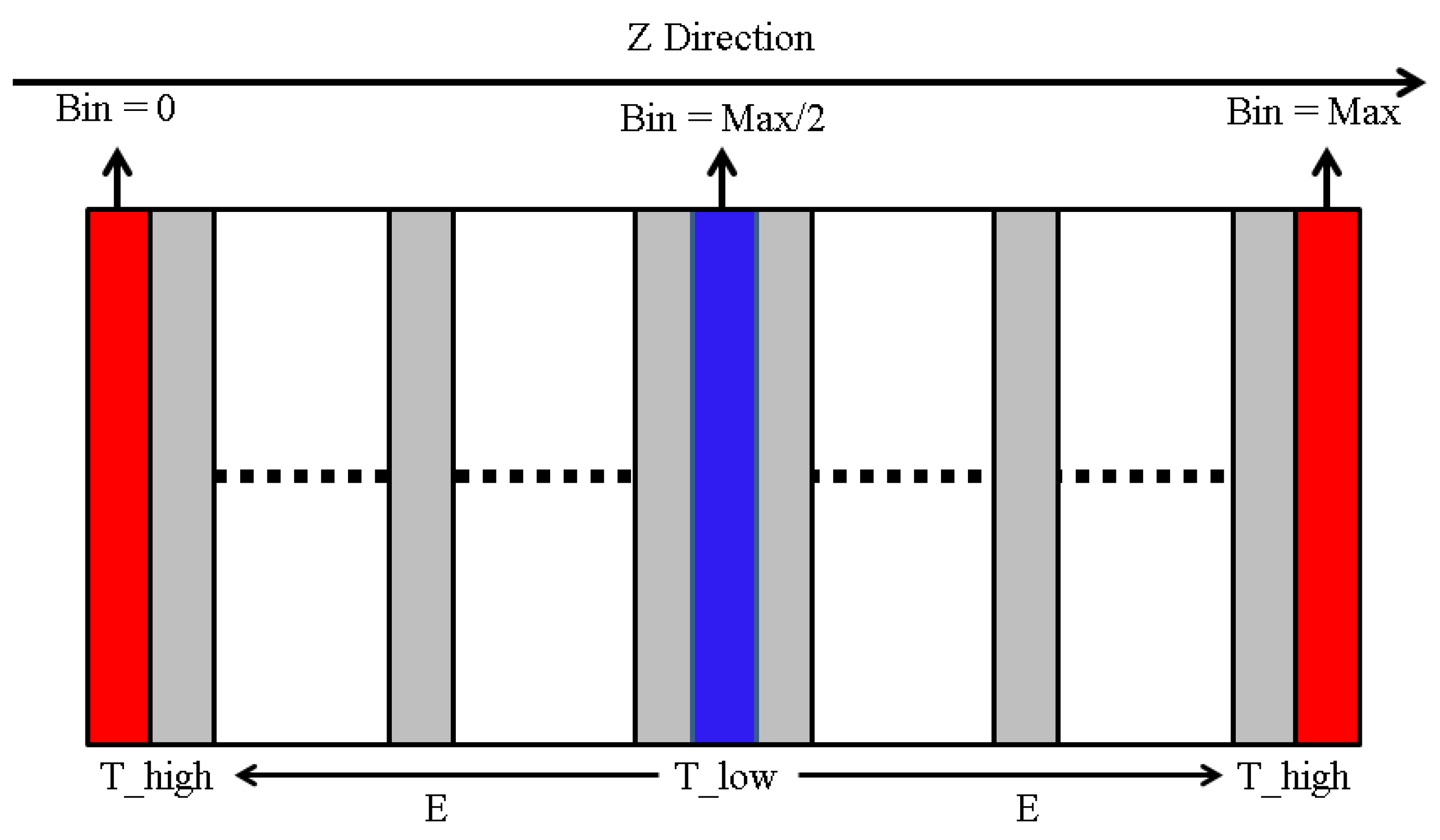
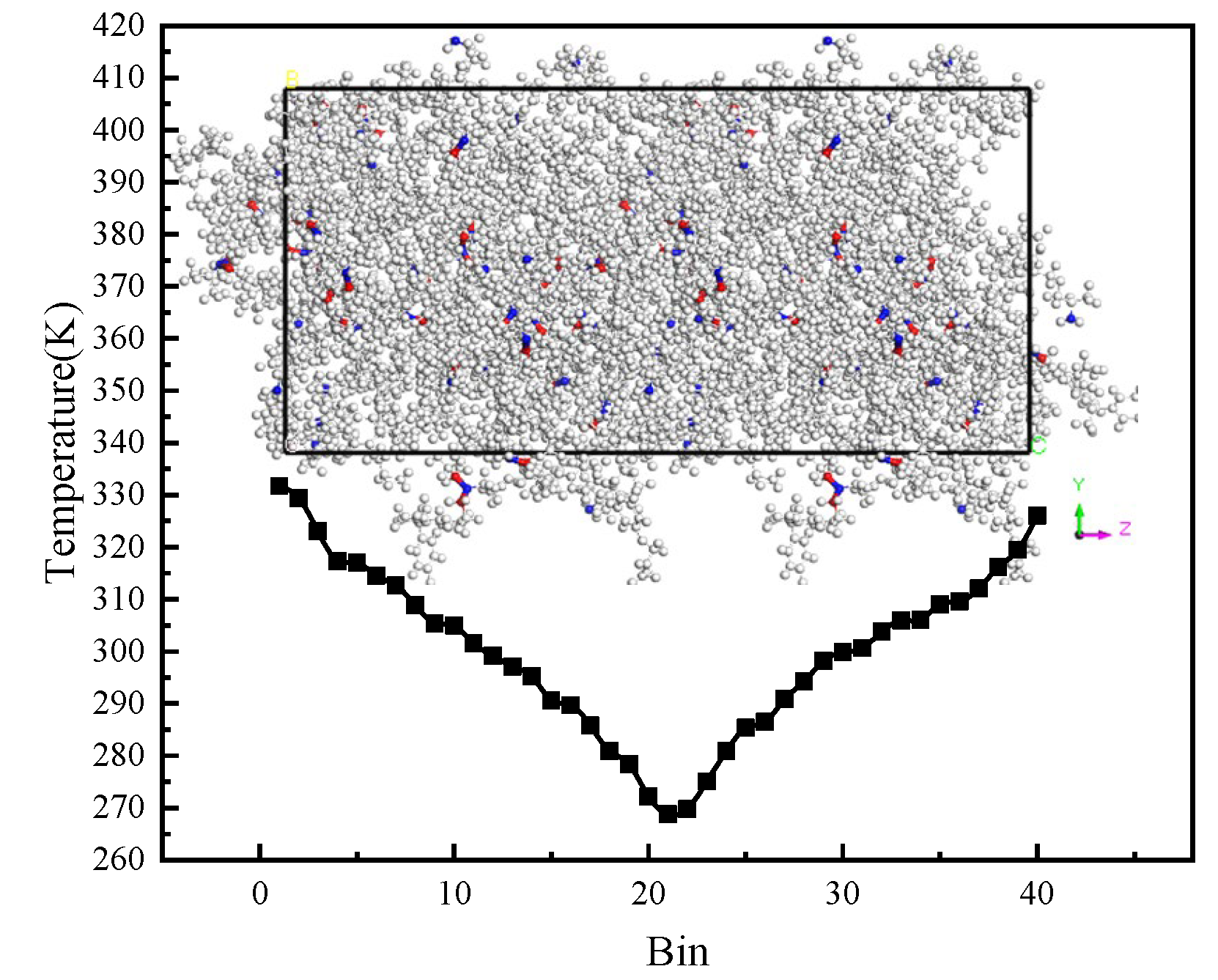
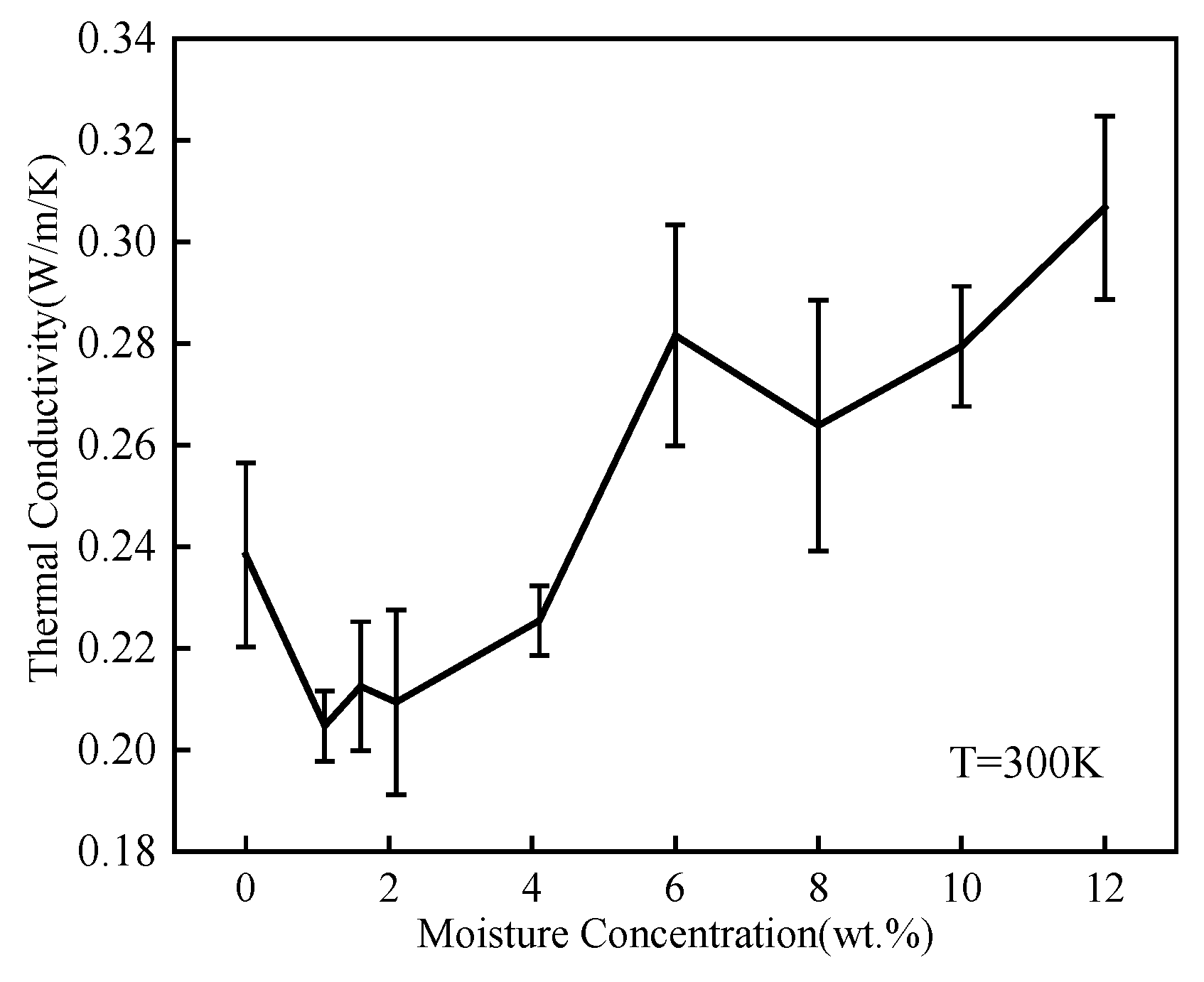
3.2. Thermal Conductivity
3.3. Mechanical Response to Uniaxial Tension
3.3.1. Role of Conversion
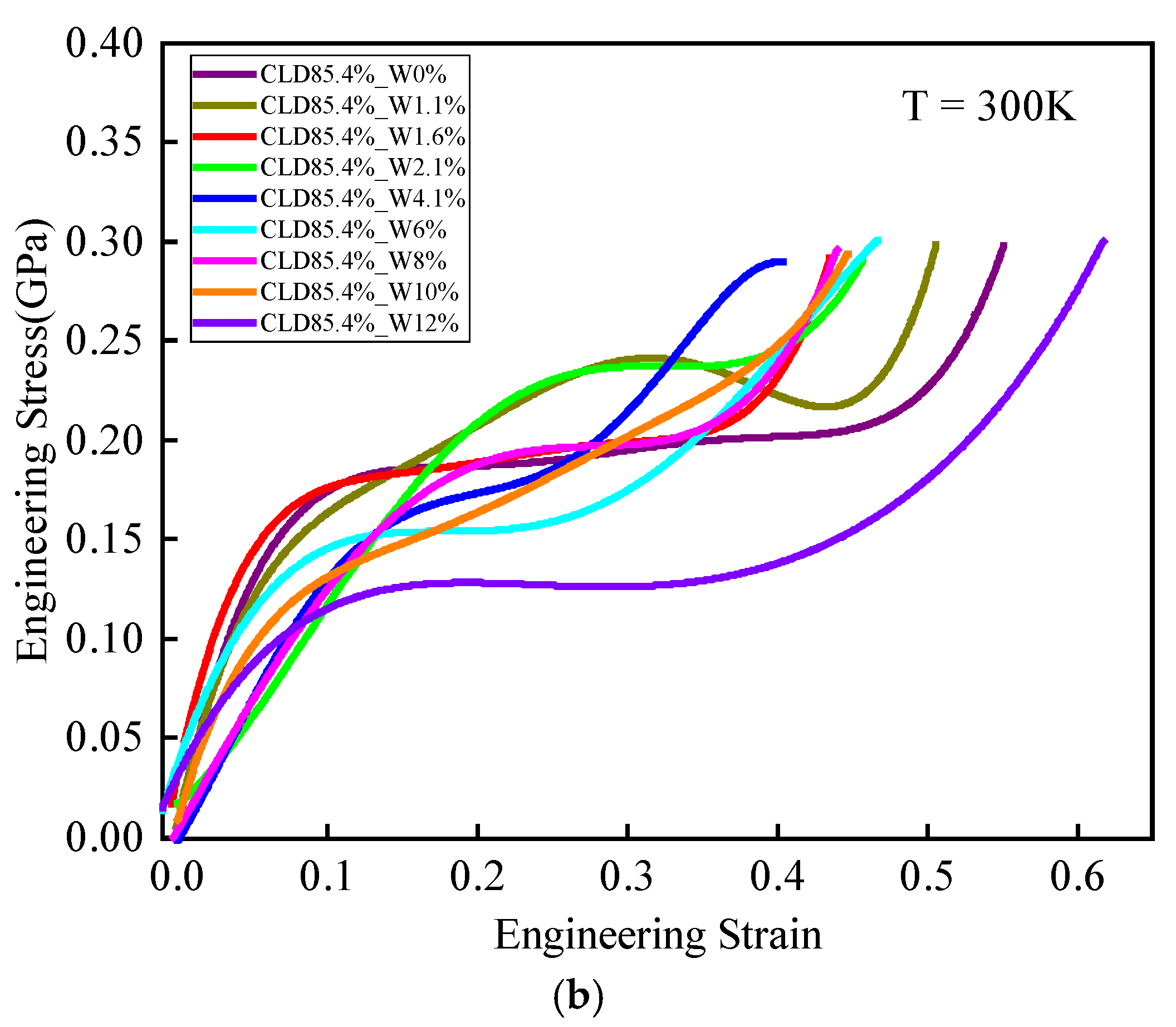
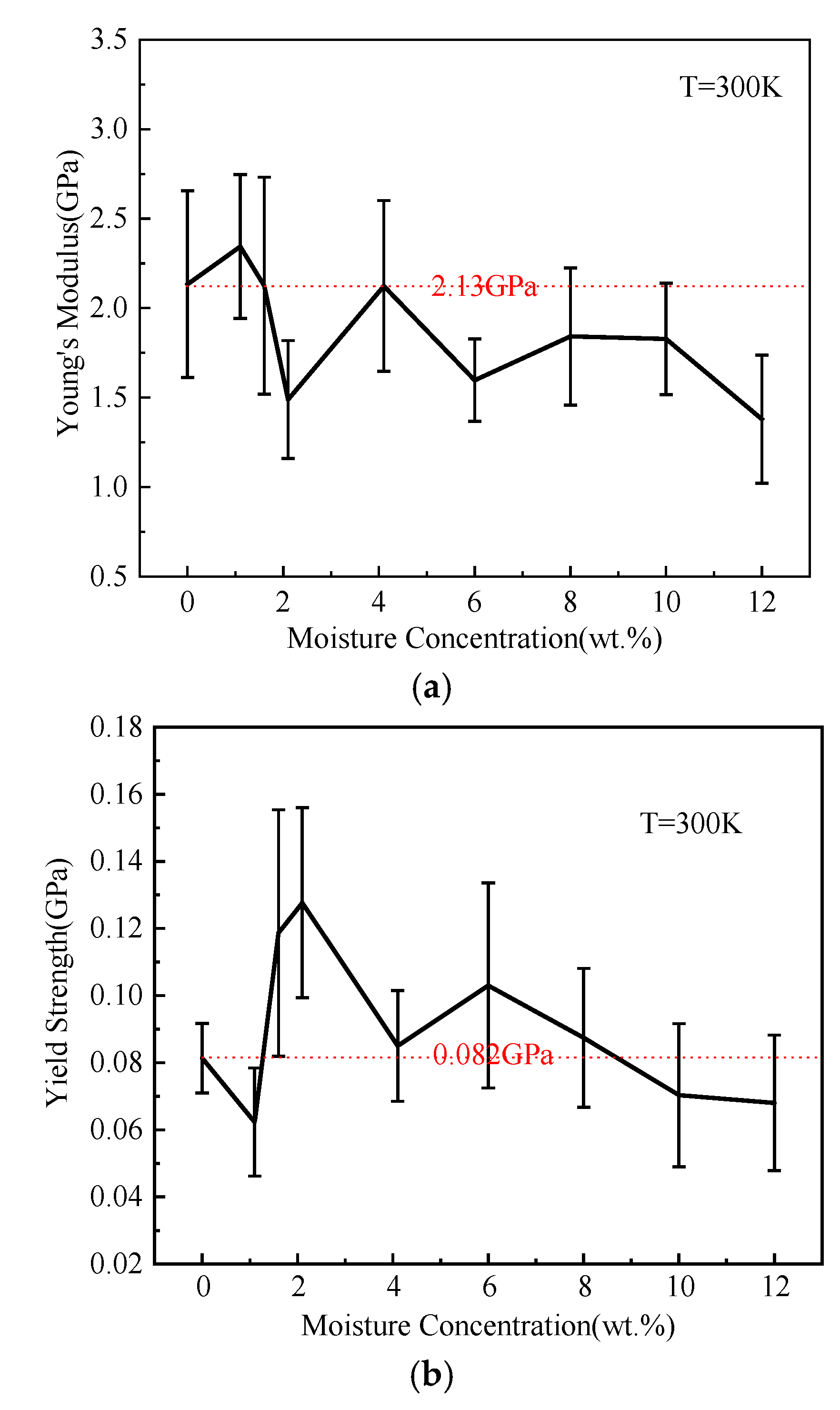
3.3.2. Role of Moisture Concentration
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, S.; Sun, Y.; Sheng, C.; Feng, Z.; Li, R.; Xue, L.; Liu, J.; Liu, S. Warpage Analysis and Prediction of the Advanced Fan-out Technology Based on Process Mechanics. IEEE Trans. Compon. Packag. Manuf. Technol. 2021, 11, 2201–2213. [Google Scholar] [CrossRef]
- Topal, E.; Gluch, J.; Clausner, A.; Cardoso, A.; Zschech, E. Determination of the Filler Distribution in an Epoxy Molding Compound Using High-Resolution X-Ray Computed Tomography. IEEE Trans. Compon. Packag. Manuf. Technol. 2021, 11, 504–509. [Google Scholar] [CrossRef]
- Jin, F.-L.; Li, X.; Park, S.-J. Synthesis and application of epoxy resins: A review. J. Ind. Eng. Chem. 2015, 29, 1–11. [Google Scholar] [CrossRef]
- Kanitkar, A.; Chernobryvko, M.; Rossi, M.; Ndip, I.; Braun, T.; Muller, F.; Lang, K.D.; Wieland, M.; Goetze, C.; Bin Halim, S.; et al. Fork-Coupled Resonators for Characterization of Mold Material for 5G Applications. In Proceedings of the 23rd International Microwave and Radar Conference (MIKON), Warsaw, Poland, 5–8 October 2020. [Google Scholar] [CrossRef]
- Andriani, Y.; Wang, X.; Seng, H.L.D.; Teo, S.L.; Liu, S.; Lau, B.L.; Zhang, X. Effect of Boron Nitride Nanosheets on Properties of a Commercial Epoxy Molding Compound Used in Fan-Out Wafer-Level Packaging. IEEE Trans. Compon. Packag. Manuf. Technol. 2020, 10, 990–999. [Google Scholar] [CrossRef]
- Wu, J.-Y.; Chen, T.-W.; Chiu, T.-C.; Chen, D.-L.; Chen, T.-Y.; Shih, M.-K. Coupled Hygro-Thermo-Mechanical Analysis of Moisture Induced Interfacial Stresses in Fan-Out Package. In Proceedings of the 14th International Microsystems, Packaging, Assembly and Circuits Technology Conference, Taipei, Taiwan, 23–25 October 2019; pp. 76–79. [Google Scholar] [CrossRef]
- Iwai, M.; Ukawa, K.; Ichizawa, G.; Mori, T. Latest Technologies of Epoxy Molding Compound (EMC) for FO-WLP. Int. Symp. Microelectron. 2020, 2020, 000051–000056. [Google Scholar] [CrossRef]
- Park, J.; Lee, H.; Lee, S.; Kyung, Y.; Kim, J.H.; Lee, K.; Paik, K.-W. Fabrication and Characterization of Epoxy Molding Films (EMFs) for Wafer-Level and Panel-Level Fan Out Packages. In Proceedings of the IEEE 68th Electronic Components and Technology Conference, San Diego, CA, USA, 29 May–1 June 2018; pp. 712–717. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, X.; Lim, S.P.S.; Lim, S.S.B.; Lau, B.L.; Han, Y.; Jong, M.C.; Liu, S.; Wang, X.; Andriani, Y. Package Level Warpage Simulation of Fan-out Wafer Level Package (FOWLP) Considering Viscoelastic Material Properties. In Proceedings of the IEEE 20th Electronics Packaging Technology Conference, Singapore, 4–7 December 2018; pp. 836–842. [Google Scholar] [CrossRef]
- Liu, S.; Liu, Y. Modeling and Simulation for Microelectronic Packaging Assembly: Manufacturing, Reliability and Testing, 1st ed.; Chemical Industry: Singapore, 2011. [Google Scholar] [CrossRef]
- Huang, Y.E.; Hagen, D.; Dody, G.; Burnette, T. Effect of solder reflow temperature profile on plastic package delamination. In Proceedings of the 23rd IEEE/CPMT International Electronics Manufacturing Technology Symposium, Austin, TX, USA, 21 October 1998; pp. 105–111. [Google Scholar] [CrossRef]
- Mei, Y.H.; Liu, S.; Suhir, E. Parametric study of a VLSI plastic package subjected to encapsulation, moisture absorption and solder reflow process. Am. Soc. Mech. Eng. EEP Conf. Pap. 1995, 12, 159–174. [Google Scholar] [CrossRef]
- Liu, S.; Mei, Y. Behavior of delaminated plastic IC packages subjected to encapsulation cooling, moisture absorption, and wave soldering. IEEE Trans. Compon. Packag. Manuf. Technol. Part A 1995, 18, 634–645. [Google Scholar] [CrossRef]
- Chiu, T.-C.; Wu, J.-Y.; Liu, W.-T.; Liu, C.-W.; Chen, D.-L.; Shih, M.; Tarng, D. A Mechanics Model for the Moisture Induced Delamination in Fan-Out Wafer-Level Package. In Proceedings of the IEEE 70th Electronic Components Technology Conference, Lake Buena Vista, FL, USA, 26–29 May 2020; pp. 1205–1211. [Google Scholar] [CrossRef]
- Fan, X.; Zhao, J.-H. Moisture diffusion and integrated stress analysis in encapsulated microelectronics devices. In Proceedings of the 12th International Conference on Thermal, Mechanical Multi-Physics Simulation Experiments in Microelectronics Microsystems, Linz, Austria, 18–20 April 2011; pp. 1–8. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, J.; Chu, H.-W.; Zhang, G.; Fan, X. A Review on Water Vapor Pressure Model for Moisture Permeable Materials Subjected to Rapid Heating. Appl. Mech. Rev. 2018, 70, 020803. [Google Scholar] [CrossRef]
- Moy, P.; Karasz, F.E. Epoxy-water interactions. Polym. Eng. Sci. 1980, 20, 315–319. [Google Scholar] [CrossRef]
- Luo, S.; Leisen, J.; Wong, C. Study on mobility of water and polymer chain in epoxy for microelectronic applications. In Proceedings of the Components and Technology Conference, Lake Buena Vista, FL, USA, 31 May–3 June 2011; pp. 149–154. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, P.; Yuan, B.; Ji, W.; Cheng, Z.; Qiu, X. Real-Space Identification of Intermolecular Bonding with Atomic Force Microscopy. Science 2013, 342, 611–614. [Google Scholar] [CrossRef] [Green Version]
- Starkova, O.; Gaidukovs, S.; Platnieks, O.; Barkane, A.; Garkusina, K.; Palitis, E.; Grase, L. Water absorption and hydrothermal ageing of epoxy adhesives reinforced with amino-functionalized graphene oxide nanoparticles. Polym. Degrad. Stab. 2021, 191, 109670. [Google Scholar] [CrossRef]
- Zhang, D.; Li, K.; Li, Y.; Sun, H.; Cheng, J.; Zhang, J. Characteristics of water absorption in amine-cured epoxy networks: A molecular simulation and experimental study. Soft Matter 2018, 14, 8740–8749. [Google Scholar] [CrossRef]
- Masoumi, S.; Arab, B.; Valipour, H. A study of thermo-mechanical properties of the cross-linked epoxy: An atomistic simulation. Polymer 2015, 70, 351–360. [Google Scholar] [CrossRef]
- Yarovsky, I.; Evans, E. Computer simulation of structure and properties of crosslinked polymers: Application to epoxy resins. Polymer 2002, 43, 963–969. [Google Scholar] [CrossRef]
- Sun, Y.; Guo, Y.; Yang, H. A molecular dynamics study of crosslinked epoxy networks: Construction of atomistic models. Mol. Simul. 2019, 46, 121–127. [Google Scholar] [CrossRef]
- Maicas, R.; Yungerman, I.; Weber, Y.; Srebnik, S. United-Atom Molecular Dynamics Study of the Mechanical and Thermomechanical Properties of an Industrial Epoxy. Polymers 2021, 13, 3443. [Google Scholar] [CrossRef]
- Masoumi, S.; Valipour, H. Effects of moisture exposure on the crosslinked epoxy system: An atomistic study. Model. Simul. Mater. Sci. Eng. 2016, 24, 035011. [Google Scholar] [CrossRef]
- Nogueira, P.; Torres, A.; Abad, M.-J.; Cano, J.; Barral, L. Effect of water sorption on the structure and mechanical properties of an epoxy resin system. J. Appl. Polym. Sci. 2001, 80, 71–80. [Google Scholar] [CrossRef]
- Papanicolaou, G.C.; Kosmidou, T.; Vatalis, A.S.; Delides, C.G. Water absorption mechanism and some anomalous effects on the mechanical and viscoelastic behavior of an epoxy system. J. Appl. Polym. Sci. 2005, 99, 1328–1339. [Google Scholar] [CrossRef]
- Wu, C.; Xu, W. Atomistic simulation study of absorbed water influence on structure and properties of crosslinked epoxy resin. Polymer 2007, 48, 5440–5448. [Google Scholar] [CrossRef]
- Littell, J.D.; Ruggeri, C.; Goldberg, R.; Roberts, G.D.; Arnold, W.; Binienda, W. Measurement of epoxy resin tension, com-pression, and shear stress-strain curves over a wide range of strain rates using small test specimens. J. Aerosp. Eng. 2008, 21, 162–173. [Google Scholar] [CrossRef]
- Tam, L.-H.; Wu, C. Molecular Mechanics of the Moisture Effect on Epoxy/Carbon Nanotube Nanocomposites. Nanomaterials 2017, 7, 324. [Google Scholar] [CrossRef] [Green Version]
- Guha, R.D.; Idolor, O.; Grace, L. An atomistic simulation study investigating the effect of varying network structure and polarity in a moisture contaminated epoxy network. Comput. Mater. Sci. 2020, 179, 109683. [Google Scholar] [CrossRef]
- Jang, C.; Sirk, T.W.; Andzelm, J.W.; Abrams, C.F. Comparison of Crosslinking Algorithms in Molecular Dynamics Simulation of Thermosetting Polymers. Macromol. Theory Simul. 2015, 24, 260–270. [Google Scholar] [CrossRef]
- Fan, J.; Anastassiou, A.; Macosko, C.W.; Tadmor, E.B. Molecular dynamics predictions of thermomechanical properties of an epoxy thermosetting polymer. Polymer 2020, 196, 122477. [Google Scholar] [CrossRef]
- Dassault Systemes Biovia Corp. BIOVIA Materials Studio 2017; Dassault Systemes Biovia Corp.: San Diego, CA, USA, 2017. [Google Scholar]
- Sun, H.; Mumby, S.J.; Maple, J.R.; Hagler, A.T. Ab Initio Calculations on Small Molecule Analogs of Polycarbonates. J. Phys. Chem. 1995, 99, 5873–5882. [Google Scholar] [CrossRef]
- Mayo, S.L.; Olafson, B.D.; Goddard, W.A. DREIDING: A generic force field for molecular simulations. J. Phys. Chem. 1990, 94, 8897–8909. [Google Scholar] [CrossRef]
- Souza, I.; Martins, J. Metric tensor as the dynamical variable for variable-cell-shape molecular dynamics. Phys. Rev. B 1997, 55, 8733–8742. [Google Scholar] [CrossRef] [Green Version]
- Berendsen, H.J.C.; Postma, J.P.M.; Van Gunsteren, W.F.; DiNola, A.; Haak, J.R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef] [Green Version]
- Nosé, S. Constant Temperature Molecular Dynamics Methods. Prog. Theor. Phys. Suppl. 1991, 103, 1–46. [Google Scholar] [CrossRef] [Green Version]
- Forcite Guide Materials Studio 2017; Dassault Systemes Biovia Corp.: San Diego, CA, USA, 2017. Available online: https://globex.coe.pku.edu.cn/file/upload/201807/16/1259524417.pdf (accessed on 24 September 2021).
- McDonald, I.K.; Thornton, J. Satisfying Hydrogen Bonding Potential in Proteins. J. Mol. Biol. 1994, 238, 777–793. [Google Scholar] [CrossRef]
- Connolly, M.L. Solvent-Accessible Surfaces of Proteins and Nucleic Acids. Science 1983, 221, 709–713. [Google Scholar] [CrossRef] [Green Version]
- Müller-Plathe, F. A simple nonequilibrium molecular dynamics method for calculating the thermal conductivity. J. Chem. Phys. 1997, 106, 6082–6085. [Google Scholar] [CrossRef]
- Jelinski, L.W.; Dumais, J.J.; Cholli, A.L.; Ellis, T.S.; Karasz, F.E. Nature of the water-epoxy interaction. Macromolecules 1985, 18, 1091–1095. [Google Scholar] [CrossRef]
- Hirschfelder, J.; Stevenson, D.; Eyring, H. A Theory of Liquid Structure. J. Chem. Phys. 1937, 5, 896. [Google Scholar] [CrossRef]
- Fox, T.; Flory, P. The glass temperature and related properties of polystyrene. Influence of molecular weight. J. Polym. Sci. Part B: Polym. Phys. 1954, 14, 315–319. [Google Scholar] [CrossRef]
- Hussain, A.R.J.; Alahyari, A.A.; Eastman, S.A.; Thibaud-Erkey, C.; Johnston, S.; Sobkowicz, M. Review of polymers for heat exchanger applications: Factors concerning thermal conductivity. Appl. Therm. Eng. 2017, 113, 1118–1127. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Strachan, A. Molecular dynamics predictions of thermal and mechanical properties of thermoset polymer EPON862/DETDA. Polymer 2011, 52, 2920–2928. [Google Scholar] [CrossRef]
- Yang, S.; Qu, J. Computing thermomechanical properties of crosslinked epoxy by molecular dynamic simulations. Polymer 2012, 53, 4806–4817. [Google Scholar] [CrossRef]
- Xin, D.; Han, Q. A molecular dynamics investigation on the compression of cross-linked epoxy resins. Mol. Simul. 2015, 41, 1–6. [Google Scholar] [CrossRef]
- Sirk, T.W.; Khare, K.; Karim, M.; Lenhart, J.L.; Andzelm, J.W.; McKenna, G.B.; Khare, R. High strain rate mechanical properties of a cross-linked epoxy across the glass transition. Polymer 2013, 54, 7048–7057. [Google Scholar] [CrossRef]
- Ma, J.; Mo, M.-S.; Du, X.-S.; Rosso, P.; Friedrich, K.; Kuan, H. Effect of inorganic nanoparticles on mechanical property, fracture toughness and toughening mechanism of two epoxy systems. Polymer 2008, 49, 3510–3523. [Google Scholar] [CrossRef]
- Amaral, C.; Rodriguez, R.S.; Garcia, F.G.; Junior, L.; Carvalho, E. Impact of aliphatic amine comonomers on DGEBA epoxy network properties. Polym. Eng. Sci. 2013, 54, 2132–2138. [Google Scholar] [CrossRef]
- Shan, L.; Verghese, K.; Robertson, C.G.; Reifsnider, K. Effect of network structure of epoxy DGE-BA-poly(oxypropylene)diamines on tensile behavior. J. Polym. Sci. Part B-Polym. Phys. 1999, 37, 2815–2819. [Google Scholar] [CrossRef]
- Soni, N.J.; Lin, P.; Khare, R. Effect of cross-linker length on the thermal and volumetric properties of cross-linked epoxy network. Polymer 2012, 53, 1015–1019. [Google Scholar] [CrossRef]
- Lee, A.; McKenna, G.B. Effect of crosslink density on physical ageing of epoxy networks. Polymer 1988, 29, 1812–1817. [Google Scholar] [CrossRef]
- Zhang, W.; Qing, Y.; Zhong, W.; Sui, G.; Yang, X. Mechanism of modulus improvement for epoxy resin matrices: A molecular dynamics simulation. React. Funct. Polym. 2017, 111, 60–67. [Google Scholar] [CrossRef]
- Nolte, A.; Treat, N.D.; Cohen, R.; Rubner, M. Effect of relative humidity on the Young's modulus of polyelectrolyte multilayer films and related nonionic polymers. Macromolecules 2008, 41, 5793–5798. [Google Scholar] [CrossRef]
- Goglio, L.; Rezaei, M. Variations in mechanical properties of an epoxy adhesive on exposure to warm moisture. J. Adhes. Sci. Technol. 2012, 28, 1394–1404. [Google Scholar] [CrossRef]
- Tam, L.-H.; Lau, D. Moisture effect on the mechanical and interfacial properties of epoxy-bonded material system: An atomistic and experimental investigation. Polymer 2015, 57, 132–142. [Google Scholar] [CrossRef]
- Cui, T.; Verberne, P.; Meguid, S. Characterization and atomistic modeling of the effect of water absorption on the me-chanical properties of thermoset polymers. Acta Mech. 2018, 229, 745–761. [Google Scholar] [CrossRef]
- Asasaari, S.F.M.; Wong, K.J.; Tamin, M.N.; Johar, M. Moisture absorption effects on the mechanical properties of carbon/epoxy composites. Int. J. Struct. Integr. 2020, 11, 605–614. [Google Scholar] [CrossRef]


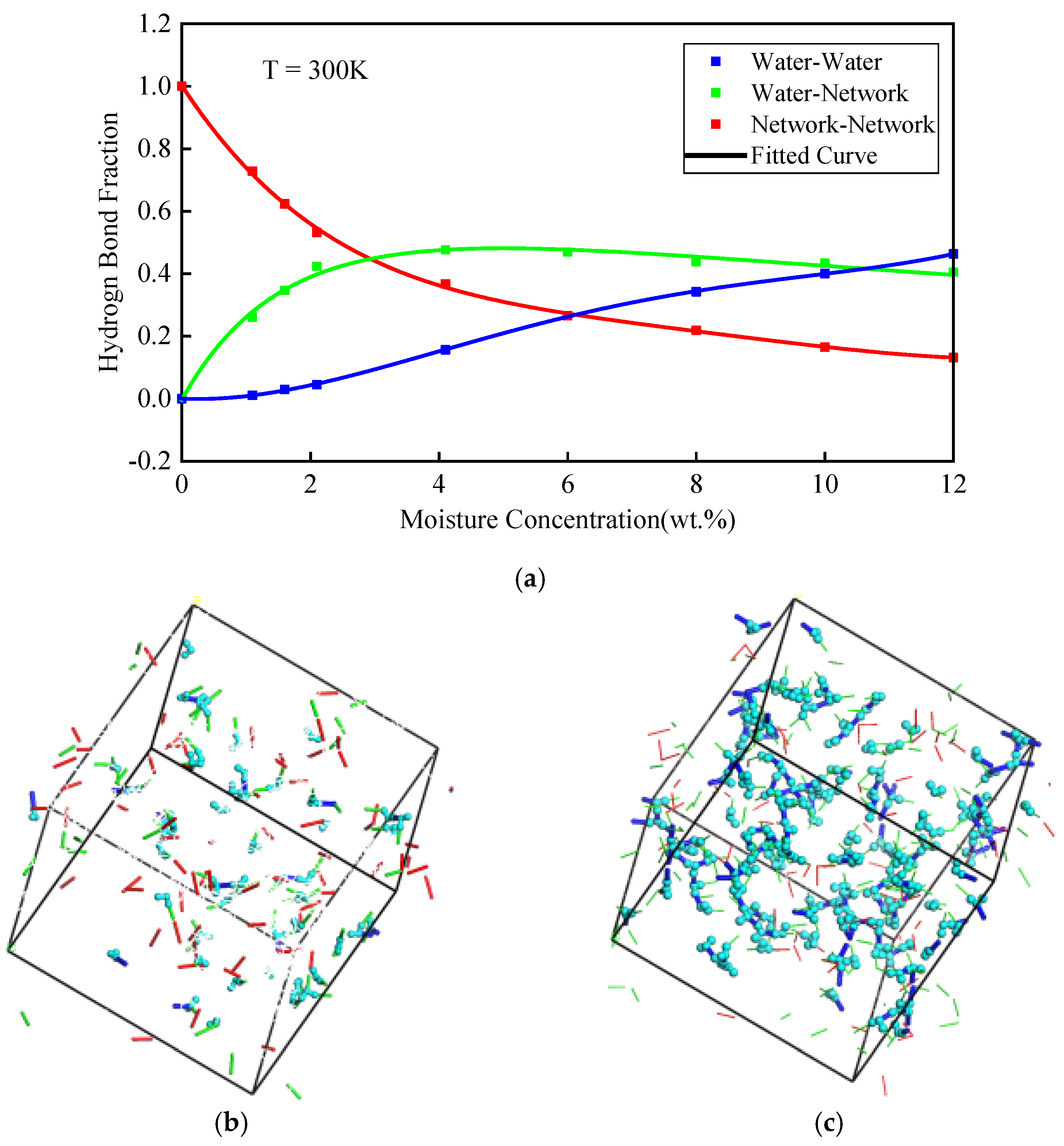
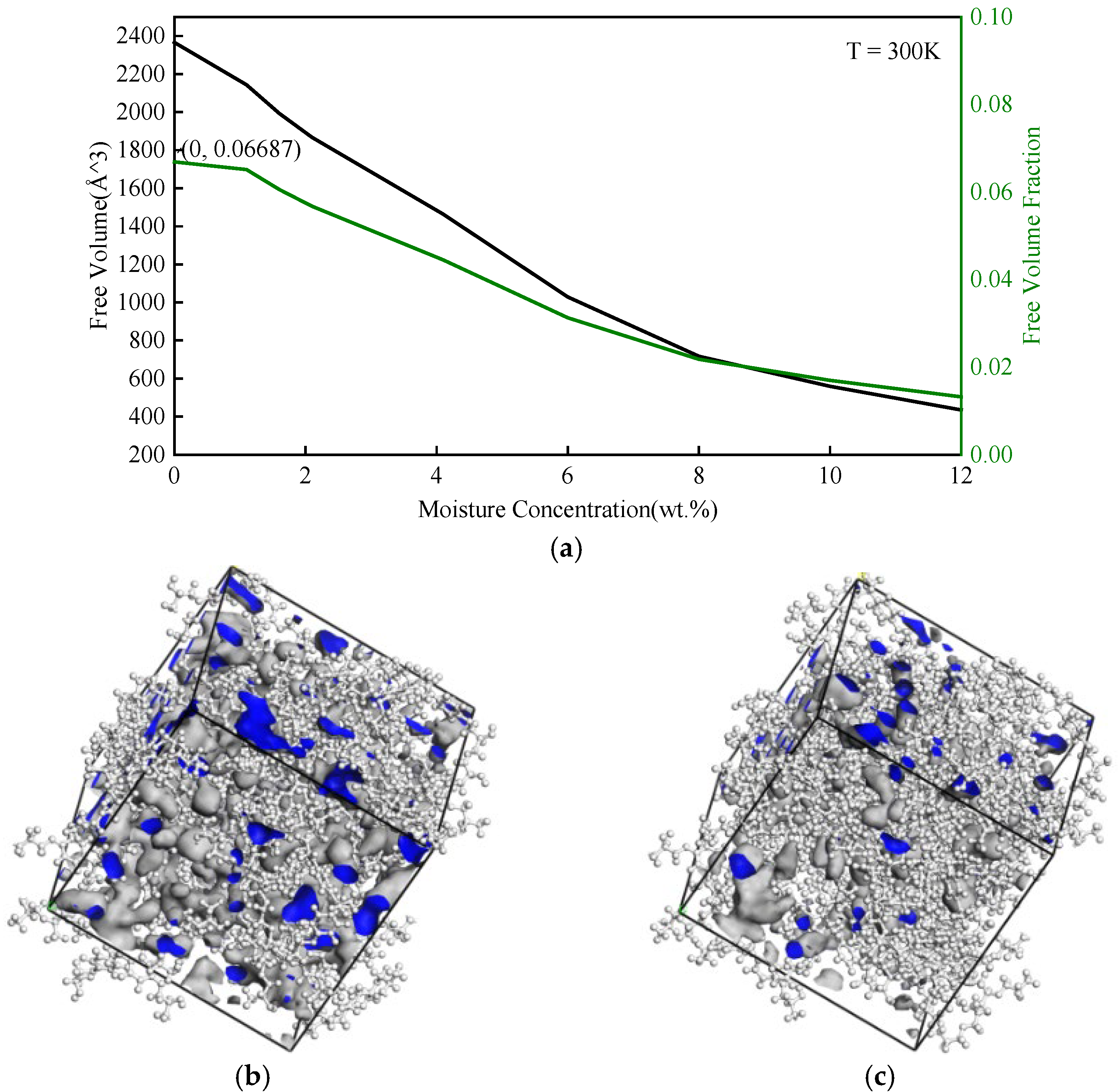

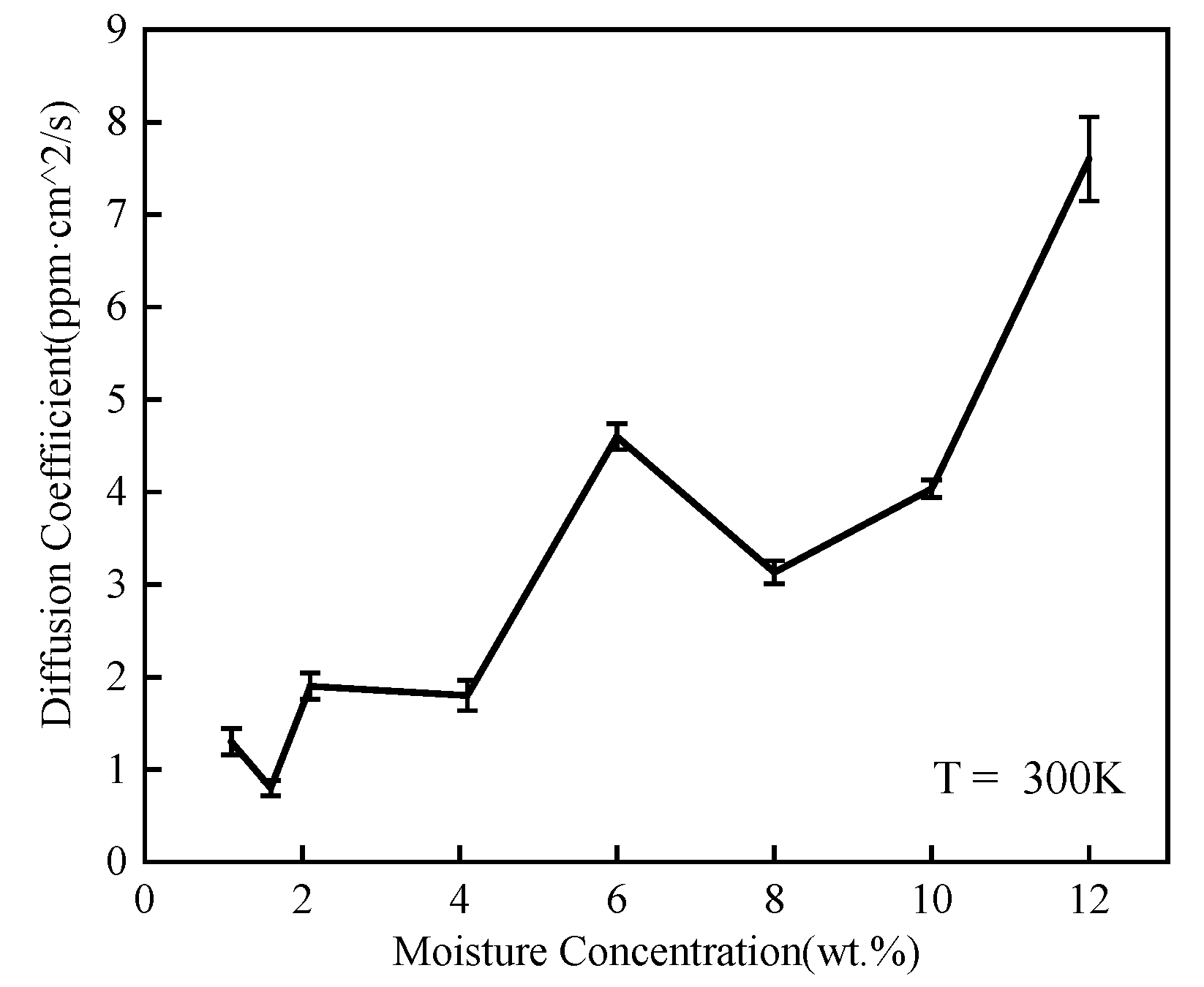
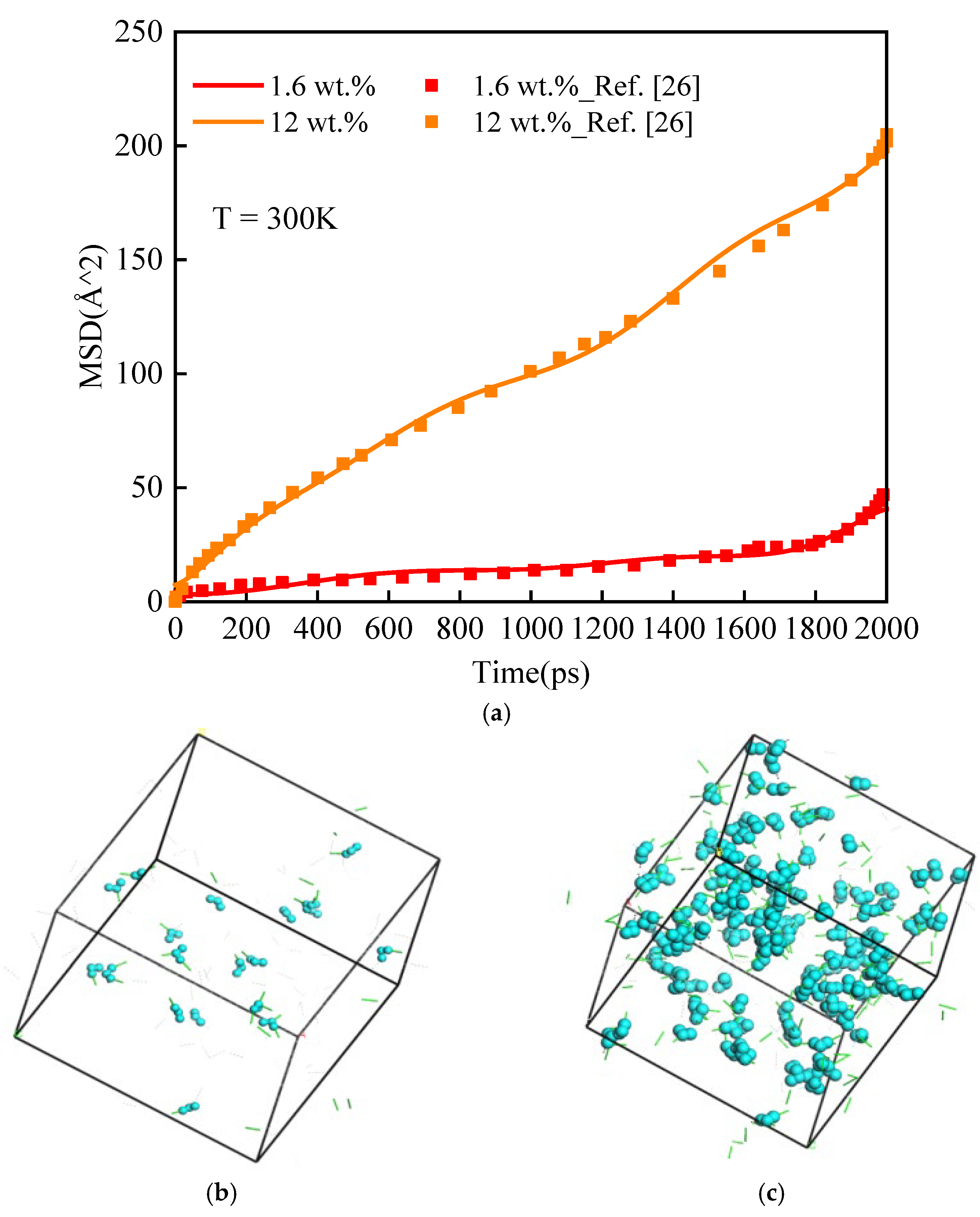


| Moisture Concentration (wt.%) | WN Hydrogen Bond | NN Hydrogen Bond | WW Hydrogen Bond | Sum |
|---|---|---|---|---|
| 0 | 0 | 70 | 0 | 70 |
| 1.1 | 24 | 67 | 1 | 92 |
| 1.6 | 35 | 63 | 3 | 101 |
| 2.1 | 47 | 59 | 5 | 111 |
| 4.1 | 70 | 54 | 23 | 147 |
| 6 | 92 | 52 | 52 | 196 |
| 8 | 100 | 50 | 78 | 228 |
| 10 | 118 | 45 | 109 | 272 |
| 12 | 132 | 43 | 151 | 326 |
| Source | Young’s Modulus (GPa) | Yield Strength (GPa) |
|---|---|---|
| DREIDING | 2.134 ± 0.522 | 0.081 ± 0.010 |
| COMPASS | 1.995 ± 0.824 | 0.113 ± 0.013 |
| Other simulations | 2.087–5.5 GPa [22,34,56] | 0.082–0.138 [34] |
| Experiment | 2.47–3.10 [53,54,55,57] | 0.057–0.092 [53,54,55] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheng, C.; Wu, G.; Sun, X.; Liu, S. Molecular Dynamics Investigation of the Thermo-Mechanical Properties of the Moisture Invaded and Cross-Linked Epoxy System. Polymers 2022, 14, 103. https://doi.org/10.3390/polym14010103
Sheng C, Wu G, Sun X, Liu S. Molecular Dynamics Investigation of the Thermo-Mechanical Properties of the Moisture Invaded and Cross-Linked Epoxy System. Polymers. 2022; 14(1):103. https://doi.org/10.3390/polym14010103
Chicago/Turabian StyleSheng, Can, Gai Wu, Xiang Sun, and Sheng Liu. 2022. "Molecular Dynamics Investigation of the Thermo-Mechanical Properties of the Moisture Invaded and Cross-Linked Epoxy System" Polymers 14, no. 1: 103. https://doi.org/10.3390/polym14010103
APA StyleSheng, C., Wu, G., Sun, X., & Liu, S. (2022). Molecular Dynamics Investigation of the Thermo-Mechanical Properties of the Moisture Invaded and Cross-Linked Epoxy System. Polymers, 14(1), 103. https://doi.org/10.3390/polym14010103






