Statistical Analysis of Synthesis Parameters to Fabricate PVDF/PVP/TiO2 Membranes via Phase-Inversion with Enhanced Filtration Performance and Photocatalytic Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of Neat Membranes
2.2. Fabrication of TiO2-Modified Membranes
2.3. Membrane Characterization
2.3.1. Filtration
2.3.2. Morphology
2.3.3. Photocatalytic Activity
3. Results and Discussion
3.1. Characterization of Neat Membranes
3.2. Characterization of TiO2-Modified Membranes
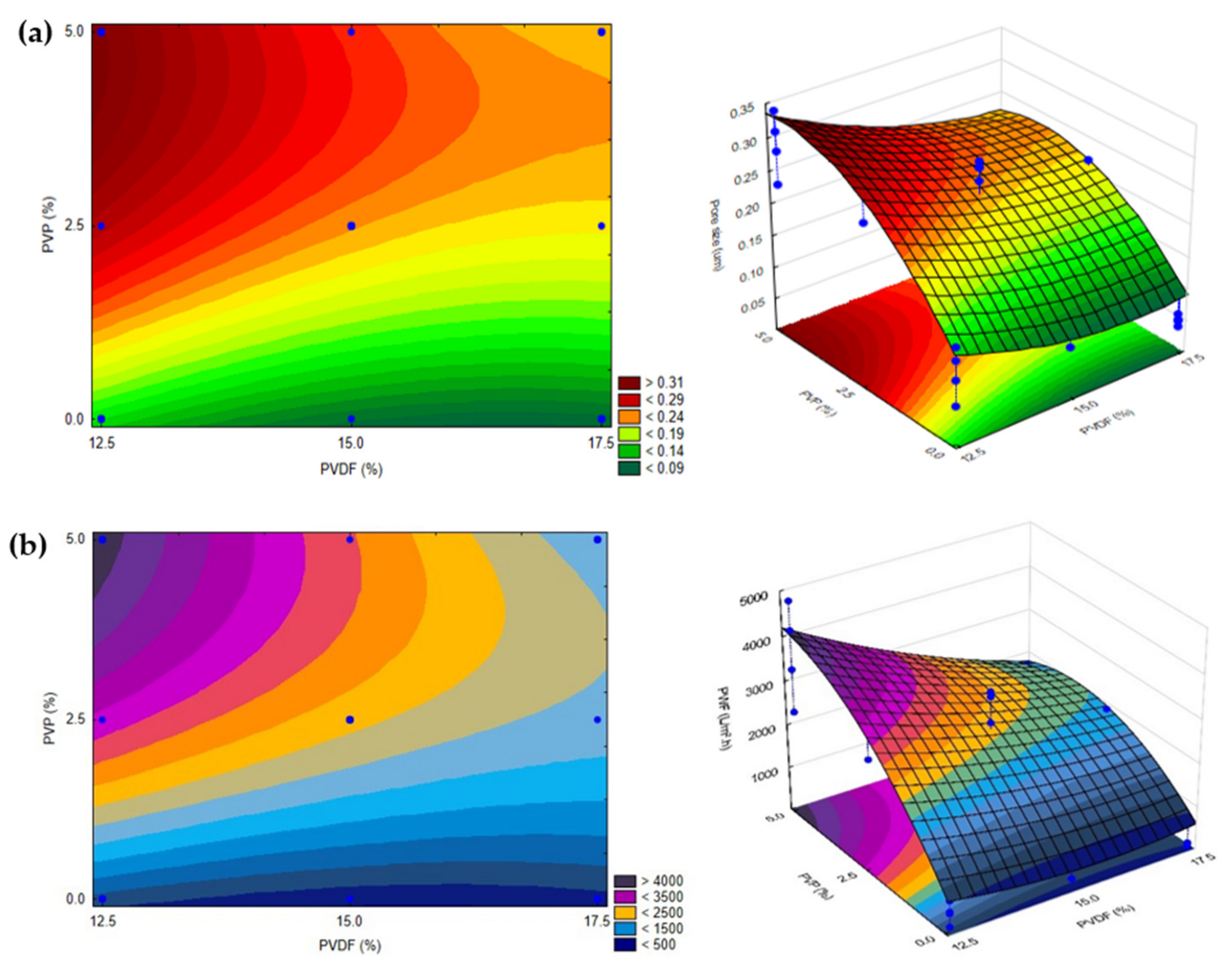
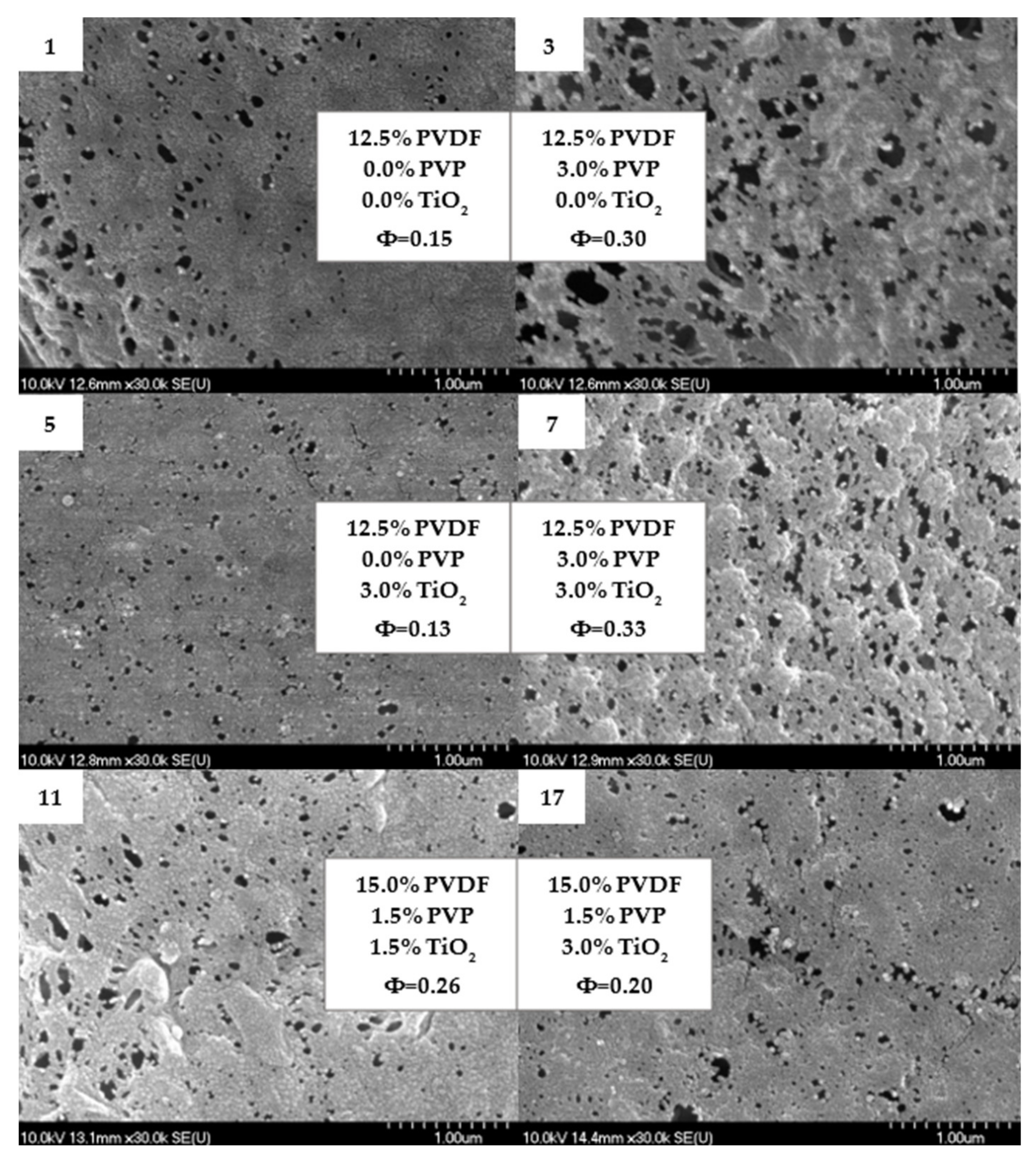
3.2.1. Filtration and Morphology
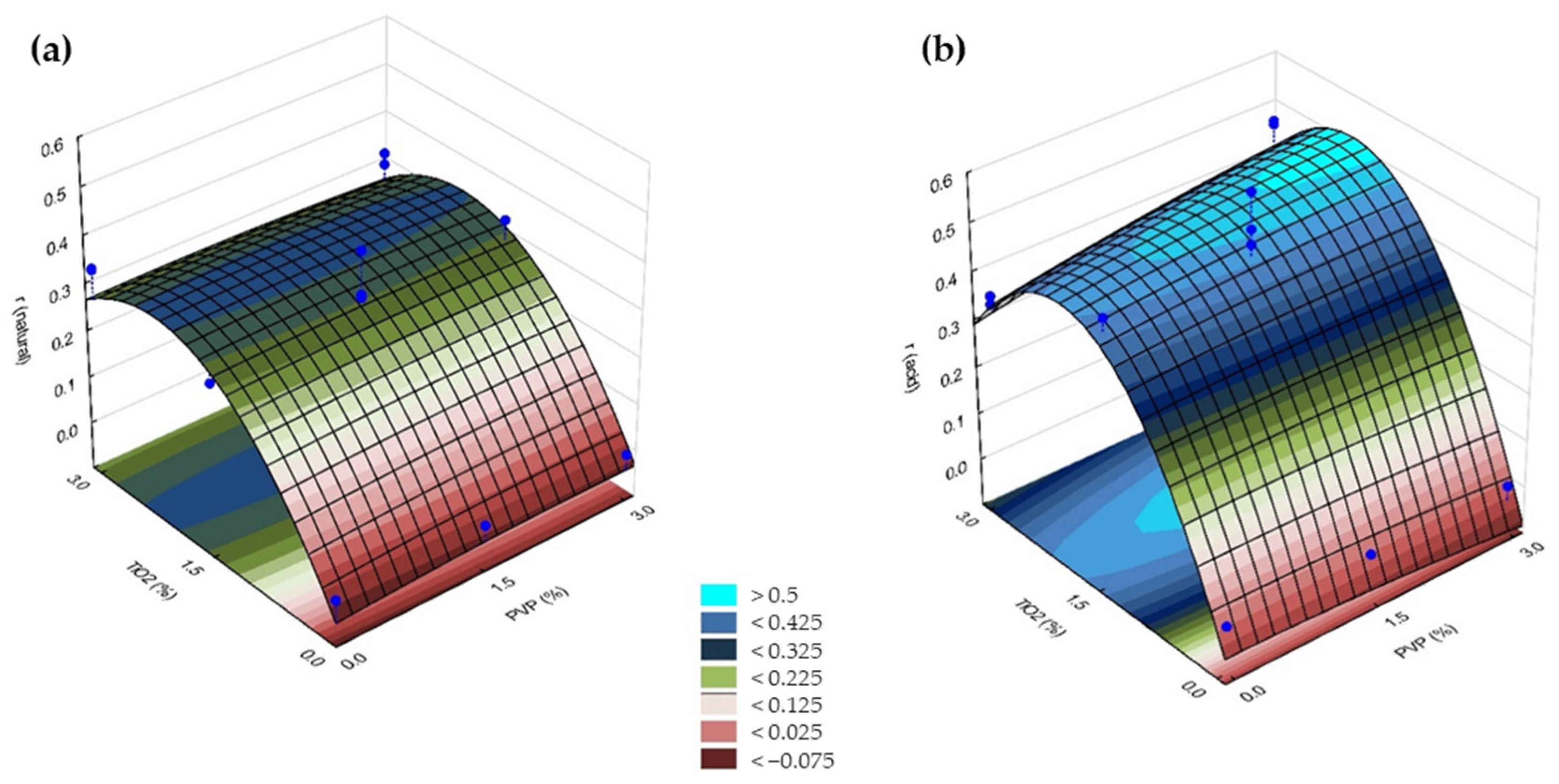
3.2.2. Photocatalytic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abdullah, N.; Rahman, M.A.; Othman, M.H.D.; Jaafar, J.; Ismail, A.F. Membranes and Membrane Processes: Fundamentals; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128135495. [Google Scholar]
- Zarghami, S.; Mohammadi, T.; Sadrzadeh, M.; Van Der Bruggen, B. Progress in Polymer Science Superhydrophilic and underwater superoleophobic membranes-A review of synthesis methods. Prog. Polym. Sci. 2019, 98, 101166. [Google Scholar] [CrossRef]
- Tasselli, F. Non-solvent Induced Phase Separation Process (NIPS) for Membrane Preparation. Encycl. Membr. 2014, 1–3. [Google Scholar] [CrossRef]
- Hołda, A.K.; Vankelecom, I.F.J. Understanding and guiding the phase inversion process for synthesis of solvent resistant nanofiltration membranes. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Guillen, G.R.; Pan, Y.; Li, M.; Hoek, E.M.V. Preparation and characterization of membranes formed by nonsolvent induced phase separation: A review. Ind. Eng. Chem. Res. 2011, 50, 3798–3817. [Google Scholar] [CrossRef]
- Liu, F.; Hashim, N.A.; Liu, Y.; Abed, M.R.M.; Li, K. Progress in the production and modification of PVDF membranes. J. Membr. Sci. 2011, 375, 1–27. [Google Scholar] [CrossRef]
- Gryta, M.; Karakulski, K.; Morawski, A.W. Purification of oily wastewater by hybrid UF/MD. Water Res. 2001, 35, 3665–3669. [Google Scholar] [CrossRef]
- Chin, S.S.; Chiang, K.; Fane, A.G. The stability of polymeric membranes in a TiO2 photocatalysis process. J. Membr. Sci. 2006, 275, 202–211. [Google Scholar] [CrossRef]
- Safari, N.H.M.; Hassan, A.R.; Che Wan Takwa, C.W.I.; Rozali, S. Deduction of surfactants effect on performance, morphology, thermal and molecular properties of polymeric polyvinylidene fluoride (PVDF) based ultrafiltration membrane. Period. Polytech. Chem. Eng. 2019, 63, 27–35. [Google Scholar] [CrossRef]
- Samsudin, M.F.R.; Mahmood, A.; Sufian, S. Enhanced photocatalytic degradation of wastewater over RGO-TiO2/BiVO4 photocatalyst under solar light irradiation. J. Mol. Liq. 2018, 268, 26–36. [Google Scholar] [CrossRef]
- Pagidi, A.; Saranya, R.; Arthanareeswaran, G.; Ismail, A.F.; Matsuura, T. Enhanced oil-water separation using polysulfone membranes modified with polymeric additives. Desalination 2014, 344, 280–288. [Google Scholar] [CrossRef]
- Khulbe, K.C.; Matsuura, T. Membrane modification. In Nanotechnology in Membrane Processes. Lecture Notes in Nanoscale Science and Technology; Springer: Cham, Switzerland, 2021. [Google Scholar]
- Miller, D.; Dreyer, D.; Bielawski, C.; Paul, D.; Freeman, B. Surface Modification of Water Purification Membranes: A Review Autoren. Angew. Chem. Int. Ed. 2016, 56, 4662–4711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nascimbén Santos, É.; László, Z.; Hodúr, C.; Arthanareeswaran, G.; Veréb, G.; Nascimben Santos, E.; László, Z.; Hodúr, C.; Arthanareeswaran, G.; Veréb, G.; et al. Photocatalytic membrane filtration and its advantages over conventional approaches in the treatment of oily wastewater: A review. Asia-Pac. J. Chem. Eng. 2020, 15, e2533. [Google Scholar] [CrossRef]
- Kallem, P.; Ibrahim, Y.; Hasan, S.W.; Show, P.L.; Banat, F. Fabrication of novel polyethersulfone (PES) hybrid ultrafiltration membranes with superior permeability and antifouling properties using environmentally friendly sulfonated functionalized polydopamine nanofillers. Sep. Purif. Technol. 2021, 261, 118311. [Google Scholar] [CrossRef]
- Ognibene, G.; Gangemi, C.M.A.; Spitaleri, L.; Gulino, A.; Purrello, R.; Cicala, G.; Fragalà, M.E. Role of the surface composition of the polyethersulfone–TiiP–H 2 T 4 fibers on lead removal: From electrostatic to coordinative binding. J. Mater. Sci. 2019, 54, 8023–8033. [Google Scholar] [CrossRef]
- Ognibene, G.; Cristaldi, D.A.; Fiorenza, R.; Blanco, I.; Cicala, G.; Scirè, S.; Fragalà, M.E. Photoactivity of hierarchically nanostructured ZnO-PES fibre mats for water treatments. RSC Adv. 2016, 6, 42778–42785. [Google Scholar] [CrossRef]
- Zimbone, M.; Cacciato, G.; Spitaleri, L.; Egdell, R.G.; Grimaldi, M.G.; Gulino, A. Sb-Doped Titanium Oxide: A Rationale for Its Photocatalytic Activity for Environmental Remediation. ACS Omega 2018, 3, 11270–11277. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Cui, J.; Xie, A.; Lang, J.; Li, C.; Yan, Y. PVDF composite membrane with robust UV-induced self-cleaning performance for durable oil / water emulsions separation. J. Taiwan Inst. Chem. Eng. 2020, 110, 1–10. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, G.; Bai, R.; Shen, S.; Zhou, X.; Wyman, I. Fabrication of superhydrophilic and underwater superoleophobic membranes via an in situ crosslinking blend strategy for highly efficient oil/water emulsion separation. J. Membr. Sci. 2019, 569, 60–70. [Google Scholar] [CrossRef]
- Benhabiles, O.; Galiano, F.; Marino, T.; Mahmoudi, H.; Lounici, H.; Figoli, A. Preparation and characterization of TiO2-PVDF/PMMA blend membranes using an alternative non-toxic solvent for UF/MF and photocatalytic application. Molecules 2019, 24, 724. [Google Scholar] [CrossRef] [Green Version]
- Arthanareeswaran, G.; Thanikaivelan, P.; Srinivasn, K.; Mohan, D.; Rajendran, M. Synthesis, characterization and thermal studies on cellulose acetate membranes with additive. Eur. Polym. J. 2004, 40, 2153–2159. [Google Scholar] [CrossRef]
- Arthanareeswaran, G.; Mohan, D.; Raajenthiren, M. Preparation, characterization and performance studies of ultrafiltration membranes with polymeric additive. J. Membr. Sci. 2010, 350, 130–138. [Google Scholar] [CrossRef]
- Wei, C.; Dai, F.; Lin, L.; An, Z.; He, Y.; Chen, X.; Chen, L.; Zhao, Y. Simplified and robust adhesive-free superhydrophobic SiO2-decorated PVDF membranes for efficient oil/water separation. J. Membr. Sci. 2018, 555, 220–228. [Google Scholar] [CrossRef]
- Shi, H.; He, Y.; Pan, Y.; Di, H.; Zeng, G.; Zhang, L.; Zhang, C. A modified mussel-inspired method to fabricate TiO2 decorated superhydrophilic PVDF membrane for oil/water separation. J. Membr. Sci. 2016, 506, 60–70. [Google Scholar] [CrossRef]
- Kovács, I.; Veréb, G.; Kertész, S.; Hodúr, C.; László, Z. Fouling mitigation and cleanability of TiO2 photocatalyst-modified PVDF membranes during ultrafiltration of model oily wastewater with different salt contents. Environ. Sci. Pollut. Res. 2018, 25, 34912–34921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molinari, R.; Lavorato, C.; Argurio, P. Recent progress of photocatalytic membrane reactors in water treatment and in synthesis of organic compounds. A review. Catal. Today 2017, 281, 144–164. [Google Scholar] [CrossRef]
- Sun, X.X.; Liu, G.; Li, R.; Meng, Y.; Wu, J. Polyporous PVDF/TiO2 photocatalytic composites for photocatalyst fixation, recycle, and repair. J. Am. Ceram. Soc. 2021, 104, 6290–6298. [Google Scholar] [CrossRef]
- Neghi, N.; Kumar, M.; Burkhalov, D. Synthesis and application of stable, reusable TiO2 polymeric composites for photocatalytic removal of metronidazole: Removal kinetics and density functional analysis. Chem. Eng. J. 2019, 359, 963–975. [Google Scholar] [CrossRef]
- Hyun, S.; Hak, J.; Young, J.; Won, J.; Soo, Y. Influence of the addition of PVP on the morphology of asymmetric polyimide phase inversion membranes : Effect of PVP molecular weight. J. Membr. Sci. 2004, 236, 203–207. [Google Scholar] [CrossRef]
- Nunes, S.P.; Peinemann, K.-V. Membrane preparation. In Membrane Technology in the Chemical Industry; Wiley: Hoboken, NJ, USA, 2001; pp. 6–11. ISBN 3527284850. [Google Scholar]
- Buonomenna, M.G.; Macchi, P.; Davoli, M.; Drioli, E. Poly(vinylidene fluoride) membranes by phase inversion: The role the casting and coagulation conditions play in their morphology, crystalline structure and properties. Eur. Polym. J. 2007, 43, 1557–1572. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, L.; Sun, D.; An, Q.; Chen, H. Effect of Coagulation Bath Temperature on Formation Mechanism of Poly(vinylidene fluoride) Membrane. J. Appl. Polym. Sci. 2008, 110, 1656–1663. [Google Scholar] [CrossRef]
- Thürmer, M.B.; Poletto, P.; Marcolin, M.; Duarte, J.; Zeni, M. Effect of non-solvents used in the coagulation bath on morphology of PVDF membranes. Mater. Res. 2012, 15, 884–890. [Google Scholar] [CrossRef]
- Hir, Z.A.M.; Moradihamedani, P.; Abdullah, A.H.; Mohamed, M.A. Immobilization of TiO2 into polyethersulfone matrix as hybrid film photocatalyst for effective degradation of methyl orange dye. Mater. Sci. Semicond. Process. 2017, 57, 157–165. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Ideris, N.; Ooi, B.S.; Low, S.C.; Ismail, A. Optimization of polyvinylidene fluoride (PVDF) membrane fabrication for protein binding using statistical experimental design. J. Immunoass. Immunochem. 2016, 37, 421–437. [Google Scholar] [CrossRef] [PubMed]
- Back, J.O.; Brandstätter, R.; Spruck, M.; Koch, M.; Penner, S.; Rupprich, M. Parameter screening of PVDF/PVP multi-channel capillary membranes. Polymers 2019, 11, 463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orooji, Y.; Ghasali, E.; Emami, N.; Noorisafa, F.; Razmjou, A. ANOVA Design for the Optimization of TiO2 Coating on polyether sulfone membranes. Molecules 2019, 24, 2924. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.; Xu, X.; Chen, J.; Yang, F. Optimization of preparation conditions of poly(vinylidene fluoride)/graphene oxide microfiltration membranes by the Taguchi experimental design. Desalination 2014, 334, 17–22. [Google Scholar] [CrossRef]
- Rawindran, H.; Lim, J.W.; Goh, P.S.; Subramaniam, M.N.; Ismail, A.F.; Daud, N.M.R.B.N.M.; Arzhandi, M.R.-D. Simultaneous separation and degradation of surfactants laden in produced water using PVDF/TiO2 photocatalytic membrane. J. Clean. Prod. 2019, 221, 490–501. [Google Scholar] [CrossRef]
- Ghandashtani, M.B.; Tavangar, T.; Ashtiani, F.Z.; Karimi, M.; Fouladitajar, A. Experimental investigation and mathematical modeling of nano-composite membrane fabrication process: Focus on the role of solvent type. Asia-Pac. J. Chem. Eng. 2018, 13, 1–15. [Google Scholar] [CrossRef]
- Vatanpour, V.; Darrudi, N.; Sheydaei, M. A comprehensive investigation of effective parameters in continuous submerged photocatalytic membrane reactors by RSM. Chem. Eng. Process. Process Intensif. 2020, 157, 108144. [Google Scholar] [CrossRef]
- Fouladitajar, A.; Zokaee Ashtiani, F.; Dabir, B.; Rezaei, H.; Valizadeh, B. Response surface methodology for the modeling and optimization of oil-in-water emulsion separation using gas sparging assisted microfiltration. Environ. Sci. Pollut. Res. 2015, 22, 2311–2327. [Google Scholar] [CrossRef]
- Sadeghian, M.; Sadeghi, M.; Hesampour, M.; Moheb, A. Application of response surface methodology (RSM) to optimize operating conditions during ultrafiltration of oil-in-water emulsion. Desalin. Water Treat. 2015, 55, 615–623. [Google Scholar] [CrossRef]
- Tan, Y.H.; Goh, P.S.; Ismail, A.F.; Ng, B.C.; Lai, G.S. Decolourization of aerobically treated palm oil mill effluent (AT-POME) using polyvinylidene fluoride (PVDF) ultrafiltration membrane incorporated with coupled zinc-iron oxide nanoparticles. Chem. Eng. J. 2017, 308, 359–369. [Google Scholar] [CrossRef]
- Vatanpour, V.; Madaeni, S.S.; Moradian, R.; Zinadini, S.; Astinchap, B. Novel antibifouling nanofiltration polyethersulfone membrane fabricated from embedding TiO2 coated multiwalled carbon nanotubes. Sep. Purif. Technol. 2012, 90, 69–82. [Google Scholar] [CrossRef]
- Zhou, Y.; Xi, D. Effect of PVP Additive on PVDF/TPU Blend Hollow Fibre Membranes by Phase Inversion Yuan. Iran. Polym. J. 2007, 16, 241–250. [Google Scholar]
- Marino, T.; Galiano, F.; Simone, S.; Figoli, A. DMSO EVOLTM as novel non-toxic solvent for polyethersulfone membrane preparation. Environ. Sci. Pollut. Res. 2019, 26, 14774–14785. [Google Scholar] [CrossRef]
- Chen, W.; Peng, J.; Su, Y.; Zheng, L.; Wang, L.; Jiang, Z. Separation of oil/water emulsion using Pluronic F127 modified polyethersulfone ultrafiltration membranes. Sep. Purif. Technol. 2009, 66, 591–597. [Google Scholar] [CrossRef]
- Wang, Q.; Cui, J.; Liu, S.; Gao, J.; Lang, J.; Li, C. Facile preparation of halloysite nanotube-modified polyvinylidene fluoride composite membranes for highly efficient oil / water emulsion separation. J. Mater. Sci. 2019, 54, 8332–8345. [Google Scholar] [CrossRef]
- Zhao, J.; Han, H.; Wang, Q.; Yan, C.; Li, D.; Yang, J.; Feng, X.; Yang, N.; Zhao, Y.; Chen, L. Hydrophilic and anti-fouling PVDF blend ultrafiltration membranes using polyacryloylmorpholine-based triblock copolymers as amphiphilic modifiers. React. Funct. Polym. 2019, 139, 92–101. [Google Scholar] [CrossRef]
- Abd Hamid, M.A.; Chung, Y.T.; Rohani, R.; Mohd. Junaidi, M.U. Miscible-blend polysulfone/polyimide membrane for hydrogen purification from palm oil mill effluent fermentation. Sep. Purif. Technol. 2019, 209, 598–607. [Google Scholar] [CrossRef]
- Zangeneh, H.; Rahimi, Z.; Zinatizadeh, A.A.; Razavizadeh, S.H.; Zinadini, S. L-Histidine doped-TiO2-CdS nanocomposite blended UF membranes with photocatalytic and self-cleaning properties for remediation of effluent from a local waste stabilization pond (WSP) under visible light. Process Saf. Environ. Prot. 2020, 136, 92–104. [Google Scholar] [CrossRef]
- Moslehyani, A.; Ismail, A.F.; Othman, M.H.D.; Matsuura, T. Design and performance study of hybrid photocatalytic reactor-PVDF/MWCNT nanocomposite membrane system for treatment of petroleum refinery wastewater. Desalination 2015, 363, 99–111. [Google Scholar] [CrossRef]
- Srivastava, H.P.; Arthanareeswaran, G.; Anantharaman, N.; Starov, V.M. Performance of modified poly(vinylidene fluoride) membrane for textile wastewater ultrafiltration. Desalination 2011, 282, 87–94. [Google Scholar] [CrossRef]
- Ananth, A.; Arthanareeswaran, G.; Wang, H. The influence of tetraethylorthosilicate and polyethyleneimine on the performance of polyethersulfone membranes. Desalination 2012, 287, 61–70. [Google Scholar] [CrossRef]
- Zangeneh, H.; Zinatizadeh, A.A.; Zinadini, S.; Feyzi, M.; Bahnemann, D.W. A novel photocatalytic self-cleaning PES nanofiltration membrane incorporating triple metal-nonmetal doped TiO2 (K-B-N-TiO2) for post treatment of biologically treated palm oil mill effluent. React. Funct. Polym. 2018, 127, 139–152. [Google Scholar] [CrossRef] [Green Version]
- Tofighy, M.A.; Mohammadi, T.; Sadeghi, M.H. High-flux PVDF/PVP nanocomposite ultrafiltration membrane incorporated with graphene oxide nanoribbones with improved antifouling properties. J. Appl. Polym. Sci. 2021, 138, e49718. [Google Scholar] [CrossRef]
- Gnanasekaran, G.; Balaguru, S.; Arthanareeswaran, G.; Das, D.B. Removal of hazardous material from wastewater by using metal organic framework (MOF) embedded polymeric membranes. Sep. Sci. Technol. 2019, 54, 434–446. [Google Scholar] [CrossRef] [Green Version]
- Hosseinifard, S.M.; Aroon, M.A.; Dahrazma, B. Application of PVDF/HDTMA-modified clinoptilolite nanocomposite membranes in removal of reactive dye from aqueous solution. Sep. Purif. Technol. 2020, 251, 117294. [Google Scholar] [CrossRef]
- Yu, H.; Gu, L.; Wu, S.; Dong, G.; Qiao, X.; Zhang, K.; Lu, X.; Wen, H.; Zhang, D. Hydrothermal carbon nanospheres assisted-fabrication of PVDF ultrafiltration membranes with improved hydrophilicity and antifouling performance. Sep. Purif. Technol. 2020, 247, 116889. [Google Scholar] [CrossRef]
- Barka, N.; Assabbane, A. Photocatalytic degradation of methyl orange with immobilized TiO2 nanoparticles: Effect of pH and some inorganic anions. Phys. Chem. News 2008, 41, 85–88. [Google Scholar]
- Bouarioua, A.; Zerdaoui, M. Photocatalytic activities of TiO2 layers immobilized on glass substrates by dip-coating technique toward the decolorization of methyl orange as a model organic pollutant. J. Environ. Chem. Eng. 2017, 5, 1565–1574. [Google Scholar] [CrossRef]
- Tombácz, E. Ph-dependent surface charging of metal oxides. Period. Polytech. Chem. Eng. 2009, 53, 77–86. [Google Scholar] [CrossRef]
- Chen, Q.; Yu, Z.; Li, F.; Yang, Y.; Pan, Y.; Peng, Y.; Yang, X.; Zeng, G. A novel photocatalytic membrane decorated with RGO-Ag-TiO2 for dye degradation and oil–water emulsion separation. J. Chem. Technol. Biotechnol. 2018, 93, 761–775. [Google Scholar] [CrossRef]
- Dutka, M.; Ditaranto, M.; Løvås, T. Application of a central composite design for the study of NOx emission performance of a low NOx burner. Energies 2015, 8, 3606–3627. [Google Scholar] [CrossRef] [Green Version]
- Ghelich, R.; Jahannama, M.R.; Abdizadeh, H.; Torknik, F.S.; Vaezi, M.R. Central composite design (CCD)-Response surface methodology (RSM) of effective electrospinning parameters on PVP-B-Hf hybrid nanofibrous composites for synthesis of HfB 2 -based composite nanofibers. Compos. Part B Eng. 2019, 166, 527–541. [Google Scholar] [CrossRef]
- Haponska, M.; Trojanowska, A.; Nogalska, A.; Jastrzab, R.; Gumi, T.; Tylkowski, B. PVDF membrane morphology-Influence of polymer molecularweight and preparation temperature. Polymers 2017, 9, 718. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.; Mohd Yunus, R.; Awang, M.; Mat, R. The Effects of Evaporation Time on Morphological Structure of Polysulfone/Cellulose Acetate Phthalate/Polyvinylpyrrolidone (PSf/CAP/PVP) Blend Membranes. Appl. Mech. Mater. 2014, 695, 77–80. [Google Scholar] [CrossRef]
- Vandezande, P.; Li, X.; Gevers, L.E.M.; Vankelecom, I.F.J. High throughput study of phase inversion parameters for polyimide-based SRNF membranes. J. Membr. Sci. 2009, 330, 307–318. [Google Scholar] [CrossRef]
- Gao, L.; Tang, B.; Wu, P. An experimental investigation of evaporation time and the relative humidity on a novel positively charged ultrafiltration membrane via dry-wet phase inversion. J. Membr. Sci. 2009, 326, 168–177. [Google Scholar] [CrossRef]
- Simone, S. Casting Solution Additives; Springer: Berlin/Heidelberg, Germany, 2016; ISBN 9783662443248. [Google Scholar]
- Fontananova, E.; Jansen, J.C.; Cristiano, A.; Curcio, E.; Drioli, E. Effect of additives in the casting solution on the formation of PVDF membranes. Desalination 2006, 192, 190–197. [Google Scholar] [CrossRef]
- Alsohaimi, I.H.; Kumar, M.; Algamdi, M.S.; Khan, M.A.; Nolan, K.; Lawler, J. Antifouling hybrid ultrafiltration membranes with high selectivity fabricated from polysulfone and sulfonic acid functionalized TiO2 nanotubes. Chem. Eng. J. 2017, 316, 573–583. [Google Scholar] [CrossRef]
- Nascimben Santos, E.; Ágoston, Á.; Kertész, S.; Hodúr, C.; László, Z.; Pap, Z.; Kása, Z.; Alapi, T.; Krishnan, S.A.G.; Arthanareeswaran, G.; et al. Investigation of the applicability of TiO2, BiVO4, and WO3 nanomaterials for advanced photocatalytic membranes used for oil-in-water emulsion separation. Asia-Pac. J. Chem. Eng. 2020, 15, e2549. [Google Scholar] [CrossRef]
- Yeow, M.L.; Liu, Y.T.; Li, K. Morphological Study of Poly (vinylidene fluoride ) Asymmetric Membranes : Effects of the Solvent, Additive, and Dope Temperature. J. Appl. Polym. Sci. 2004, 92, 1782–1789. [Google Scholar] [CrossRef]
- Venkatesh, K.; Arthanareeswaran, G.; Bose, A.C. PVDF mixed matrix nano-filtration membranes integrated with 1D-PANI/TiO2 NFs for oil-water emulsion separation. RSC Adv. 2016, 6, 18899–18908. [Google Scholar] [CrossRef]
- Rahimpour, A.; Jahanshahi, M.; Rajaeian, B.; Rahimnejad, M. TiO2 entrapped nano-composite PVDF/SPES membranes: Preparation, characterization, antifouling and antibacterial properties. Desalination 2011, 278, 343–353. [Google Scholar] [CrossRef]
- Kertèsz, S.; Cakl, J.; Jiránková, H. Submerged hollow fiber microfiltration as a part of hybrid photocatalytic process for dye wastewater treatment. Desalination 2014, 343, 106–112. [Google Scholar] [CrossRef]
- Leong, S.; Razmjou, A.; Wang, K.; Hapgood, K.; Zhang, X.; Wang, H. TiO2 based photocatalytic membranes: A review. J. Membr. Sci. 2014, 472, 167–184. [Google Scholar] [CrossRef]
- Rahimpour, A.; Madaeni, S.S.; Taheri, A.H.; Mansourpanah, Y. Coupling TiO2 nanoparticles with UV irradiation for modification of polyethersulfone ultrafiltration membranes. J. Membr. Sci. 2008, 313, 158–169. [Google Scholar] [CrossRef]




| Variable | Level | ||
|---|---|---|---|
| −1 | 0 | +1 | |
| Bath temperature (°C) | 15 | 20 | 25 |
| Evaporation time (s) | 0 | 30 | 60 |
| PVDF (wt%) | 12.5 | 15.0 | 17.5 |
| PVP (wt%) | 0 | 2.5 | 5.0 |
| Variable | Level | ||
|---|---|---|---|
| −1 | 0 | +1 | |
| PVDF (wt%) | 12.5 | 15.0 | 17.5 |
| PVP (wt%) | 0 | 1.5 | 3.0 |
| TiO2 (wt%) | 0 | 1.5 | 3.0 |
| Membrane | Variable Factors | Membrane Characterization | |||||
|---|---|---|---|---|---|---|---|
| Bath T | Evap. Time | PVDF | PVP | Porosity | Pure Water Flux | Pore Size | |
| (°C) | (s) | (%) | (%) | (%) | (L m−2 h−1) | (µm) | |
| 1 | 15 | 0 | 12.5 | 0.0 | 89.2 | 715 | 0.13 |
| 2 | 25 | 0 | 12.5 | 0.0 | 82.2 | 107 | 0.06 |
| 3 | 15 | 60 | 12.5 | 0.0 | 92.2 | 420 | 0.10 |
| 4 | 25 | 60 | 12.5 | 0.0 | 89.0 | 461 | 0.15 |
| 5 | 15 | 0 | 17.5 | 0.0 | 83.4 | 54 | 0.04 |
| 6 | 25 | 0 | 17.5 | 0.0 | 85.6 | 130 | 0.06 |
| 7 | 15 | 60 | 17.5 | 0.0 | 89.0 | 119 | 0.05 |
| 8 | 25 | 60 | 17.5 | 0.0 | 85.8 | 110 | 0.05 |
| 9 | 15 | 0 | 12.5 | 5.0 | 89.8 | 2309 | 0.23 |
| 10 | 25 | 0 | 12.5 | 5.0 | 91.4 | 4150 | 0.31 |
| 11 | 15 | 60 | 12.5 | 5.0 | 89.7 | 4801 | 0.34 |
| 12 | 25 | 60 | 12.5 | 5.0 | 90.3 | 3277 | 0.28 |
| 13 | 15 | 0 | 17.5 | 5.0 | 88.1 | 1102 | 0.16 |
| 14 | 25 | 0 | 17.5 | 5.0 | 87.3 | 1777 | 0.21 |
| 15 | 15 | 60 | 17.5 | 5.0 | 88.0 | 1063 | 0.16 |
| 16 | 25 | 60 | 17.5 | 5.0 | 87.0 | 1088 | 0.17 |
| 17 (C) | 20 | 30 | 15.0 | 2.5 | 88.6 | 2482 | 0.25 |
| 18 (C) | 20 | 30 | 15.0 | 2.5 | 88.5 | 3186 | 0.28 |
| 19 (C) | 20 | 30 | 15.0 | 2.5 | 89.1 | 3156 | 0.27 |
| 20 (S) | 15 | 30 | 15.0 | 2.5 | 89.0 | 1378 | 0.18 |
| 21 (S) | 25 | 30 | 15.0 | 2.5 | 94.5 | 2106 | 0.21 |
| 22 (S) | 20 | 0 | 15.0 | 2.5 | 89.3 | 3070 | 0.27 |
| 23 (S) | 20 | 60 | 15.0 | 2.5 | 89.7 | 480 | 0.11 |
| 24 (S) | 20 | 30 | 12.5 | 2.5 | 87.1 | 2517 | 0.25 |
| 25 (S) | 20 | 30 | 17.5 | 2.5 | 87.0 | 1949 | 0.22 |
| 26 (S) | 20 | 30 | 15.0 | 0.0 | 86.5 | 241 | 0.08 |
| 27 (S) | 20 | 30 | 15.0 | 5.0 | 89.0 | 2533 | 0.25 |
| Factor | Sum of Squares | Df | Mean Square | F-Value | Prob > F |
|---|---|---|---|---|---|
| 1. Bath (°C) (1 L) | 86,070 | 1 | 86,070 | 0.5431 | 0.537861 |
| Bath (°C) (1 Q) | 228,501 | 1 | 228,501 | 1.4420 | 0.352747 |
| 2. Ev. Time (s) (2 L) | 141,465 | 1 | 141,465 | 0.8927 | 0.444475 |
| Ev. time (s) (2 Q) | 180,617 | 1 | 180,617 | 1.1398 | 0.397493 |
| 3. PVDF (%) (3 L) | 7,175,580 | 1 | 7,175,580 | 45.2817 | 0.021378 a |
| PVDF (%) (3 Q) | 95,960 | 1 | 95,960 | 0.6056 | 0.517910 |
| 4. PVP (%) (4 L) | 21,655,116 | 1 | 21,655,116 | 136.6552 | 0.007238 a |
| PVP (%) (4 Q) | 1,096,213 | 1 | 1,096,213 | 6.9177 | 0.119247 |
| 1 L by 2 L | 744,338 | 1 | 744,338 | 4.6972 | 0.162524 |
| 1 L by 3 L | 64,643 | 1 | 64,643 | 0.4079 | 0.588403 |
| 1 L by 4 L | 143,831 | 1 | 143,831 | 0.9076 | 0.441288 |
| 2 L by 3 L | 348,395 | 1 | 348,395 | 2.1986 | 0.276366 |
| 2 L by 4 L | 38,711 | 1 | 38,711 | 0.2443 | 0.670080 |
| 3 L by 4 L | 4,219,943 | 1 | 4,219,943 | 26.6301 | 0.035561 a |
| Lack of Fit | 8,219,698 | 10 | 821,970 | 5.1871 | 0.172349 |
| Pure Error | 316,931 | 2 | 158,465 | ||
| Total SS | 48,585,198 | 26 |
| Factor | Sum of Squares | Df | Mean Square | F-Value | Prob > F |
|---|---|---|---|---|---|
| 1. Bath (°C) (1 L) | 0.000653 | 1 | 0.000653 | 2.8004 | 0.236216 |
| Bath (°C) (1 Q) | 0.000962 | 1 | 0.000962 | 4.1234 | 0.179399 |
| 2. Ev. Time (s) (2 L) | 0.000225 | 1 | 0.000225 | 0.9639 | 0.429724 |
| Ev. time (s) (2 Q) | 0.001873 | 1 | 0.001873 | 8.0255 | 0.105288 |
| 3. PVDF (%) (3 L) | 0.029595 | 1 | 0.029595 | 126.8349 | 0.007792 a |
| PVDF (%) (3 Q) | 0.001150 | 1 | 0.001150 | 4.9303 | 0.156549 |
| 4. PVP (%) (4 L) | 0.106963 | 1 | 0.106963 | 458.4112 | 0.002174 a |
| PVP (%) (4 Q) | 0.007183 | 1 | 0.007183 | 30.7827 | 0.030984 a |
| 1 L by 2 L | 0.000400 | 1 | 0.000400 | 1.7143 | 0.320634 |
| 1 L by 3 L | 0.000400 | 1 | 0.000400 | 1.7143 | 0.320634 |
| 1 L by 4 L | 0.000400 | 1 | 0.000400 | 1.7143 | 0.320634 |
| 2 L by 3 L | 0.002025 | 1 | 0.002025 | 8.6786 | 0.098496 |
| 2 L by 4 L | 0.000025 | 1 | 0.000025 | 0.1071 | 0.774506 |
| 3 L by 4 L | 0.003025 | 1 | 0.003025 | 12.9643 | 0.069222 |
| Lack of Fit | 0.030382 | 10 | 0.003038 | 13.0210 | 0.073383 |
| Pure Error | 0.000467 | 2 | 0.000233 | ||
| Total SS | 0.207600 | 26 |
| Membrane | Variable Factors | Membrane Characterization | ||||
|---|---|---|---|---|---|---|
| PVDF | PVP | TiO2 | Porosity | Pure Water Flux | Pore Size | |
| (%) | (%) | (%) | (%) | (L m−2 h−1) | (µm) | |
| 1 | 12.5 | 0.0 | 0.0 | 87.8 | 921 | 0.15 |
| 2 | 17.5 | 0.0 | 0.0 | 80.6 | 73 | 0.05 |
| 3 | 12.5 | 3.0 | 0.0 | 89.7 | 3737 | 0.30 |
| 4 | 17.5 | 3.0 | 0.0 | 87.0 | 1791 | 0.21 |
| 5 | 12.5 | 0.0 | 3.0 | 84.4 | 609 | 0.13 |
| 6 | 17.5 | 0.0 | 3.0 | 80.8 | 247 | 0.08 |
| 7 | 12.5 | 3.0 | 3.0 | 88.5 | 4592 | 0.33 |
| 8 | 17.5 | 3.0 | 3.0 | 61.6 | 1474 | 0.26 |
| 9 (C) | 15.0 | 1.5 | 1.5 | 86.9 | 1593 | 0.20 |
| 10 (C) | 15.0 | 1.5 | 1.5 | 87.3 | 2692 | 0.26 |
| 11 (C) | 15.0 | 1.5 | 1.5 | 87.1 | 2689 | 0.26 |
| 12 (S) | 12.5 | 1.5 | 1.5 | 89.0 | 2310 | 0.23 |
| 13 (S) | 17.5 | 1.5 | 1.5 | 84.9 | 1685 | 0.21 |
| 14 (S) | 15.0 | 0.0 | 1.5 | 84.1 | 372 | 0.10 |
| 15 (S) | 15.0 | 3.0 | 1.5 | 86.8 | 2092 | 0.23 |
| 16 (S) | 15.0 | 1.5 | 0.0 | 87.2 | 2358 | 0.24 |
| 17 (S) | 15.0 | 1.5 | 3.0 | 87.7 | 1651 | 0.20 |
| Factor | Sum of Squares | Df | Mean Square | F-Value | Prob > F |
|---|---|---|---|---|---|
| 1. PVDF (%) (1 L) | 0.010595 | 1 | 0.010595 | 9.11185 | 0.094455 |
| PVDF (%) (1 Q) | 0.000108 | 1 | 0.000108 | 0.09327 | 0.788915 |
| 2. PVP (%) (2 L) | 0.067912 | 1 | 0.067912 | 58.40530 | 0.016694 a |
| PVP (%) (2 Q) | 0.007158 | 1 | 0.007158 | 6.15642 | 0.131211 |
| 3. TiO2 (%) (3 L) | 0.000390 | 1 | 0.000390 | 0.33521 | 0.621125 |
| TiO2 (%) (3 Q) | 0.000085 | 1 | 0.000085 | 0.07345 | 0.811791 |
| 1 L by 2 L | 0.000007 | 1 | 0.000007 | 0.00614 | 0.944700 |
| 1 L by 3 L | 0.000710 | 1 | 0.000710 | 0.61103 | 0.516245 |
| 2 L by 3 L | 0.000649 | 1 | 0.000649 | 0.55833 | 0.532840 |
| Lack of Fit | 0.005018 | 5 | 0.001004 | 0.86316 | 0.614004 |
| Pure Error | 0.002326 | 2 | 0.001163 | ||
| Total SS | 0.096430 | 16 |
| Membrane | Natural pH (5.3~5.6) | Acid pH (3) | ||||||
|---|---|---|---|---|---|---|---|---|
| k | R2 | ϵ15 | r | k | R2 | ϵ15 | r | |
| 1 | ~0.0 | 0.0382 | 4 | ~0.0 | ~0.0 | 0.0057 | 3 | ~0.0 |
| 2 | ~0.0 | 0.4193 | 6 | ~0.0 | ~0.0 | 0.9093 | 6 | ~0.0 |
| 3 | ~0.0 | 0.6276 | 5 | ~0.0 | ~0.0 | 0.4191 | 0 | ~0.0 |
| 4 | ~0.0 | 0.7066 | 10 | ~0.0 | ~0.0 | 0.9439 | 7 | ~0.0 |
| 5 | −0.1034 | 0.9998 | 80 | 0.3381 | −0.1070 | 0.9997 | 81 | 0.3499 |
| 6 | −0.1043 | 0.9951 | 81 | 0.3411 | −0.1018 | 0.9984 | 75 | 0.3329 |
| 7 | −0.0999 | 0.9976 | 78 | 0.3267 | −0.1443 | 0.9969 | 84 | 0.4719 |
| 8 | −0.0926 | 0.9975 | 78 | 0.3028 | −0.1420 | 0.9962 | 85 | 0.4643 |
| 9 (C) | −0.0948 | 0.9998 | 76 | 0.3100 | −0.1296 | 0.9946 | 90 | 0.4238 |
| 10 (C) | −0.0729 | 0.9967 | 67 | 0.2384 | −0.1798 | 0.9999 | 93 | 0.5879 |
| 11 (C) | −0.0939 | 0.9992 | 73 | 0.3071 | −0.1566 | 0.9997 | 91 | 0.5121 |
| 12 (S) | −0.0959 | 0.9991 | 75 | 0.3136 | −0.1204 | 0.9998 | 84 | 0.3937 |
| 13 (S) | −0.1240 | 0.9991 | 83 | 0.4055 | −0.1471 | 0.9997 | 89 | 0.4810 |
| 14 (S) | −0.0825 | 0.9999 | 71 | 0.2698 | −0.1413 | 0.9997 | 88 | 0.4621 |
| 15 (S) | −0.1031 | 0.9991 | 78 | 0.3371 | −0.1122 | 0.9992 | 82 | 0.3669 |
| 16 (S) | ~0.0 | 0.9106 | 3 | ~0.0 | ~0.0 | 0.9104 | 2 | ~0.0 |
| 17 (S) | −0.0813 | 0.9990 | 70 | 0.2659 | −0.0904 | 0.9996 | 74 | 0.2956 |
| Factor | Sum of Squares | Df | Mean Square | F-Value | Prob > F |
|---|---|---|---|---|---|
| 1. PVDF (%) (1 L) | 0.000504 | 1 | 0.000504 | 0.3069 | 0.635249 |
| PVDF (%) (1 Q) | 0.006677 | 1 | 0.006677 | 4.0653 | 0.181309 |
| 2. PVP (%) (2 L) | 0.000031 | 1 | 0.000031 | 0.0189 | 0.903347 |
| PVP (%) (2 Q) | 0.000102 | 1 | 0.000102 | 0.0623 | 0.826221 |
| 3. TiO2 (%) (3 L) | 0.247937 | 1 | 0.247937 | 150.9559 | 0.006559 a |
| TiO2 (%) (3 Q) | 0.083634 | 1 | 0.083634 | 50.9203 | 0.019078 a |
| 1 L by 2 L | 0.000090 | 1 | 0.000090 | 0.0551 | 0.836300 |
| 1 L by 3 L | 0.000055 | 1 | 0.000055 | 0.0332 | 0.872132 |
| 2 L by 3 L | 0.000309 | 1 | 0.000309 | 0.1880 | 0.706881 |
| Lack of Fit | 0.009209 | 5 | 0.001842 | 1.1213 | 0.533581 |
| Pure Error | 0.003285 | 2 | 0.001642 | ||
| Total SS | 0.366334 | 16 |
| Factor | Sum of Squares | Df | Mean Square | F-Value | Prob > F |
|---|---|---|---|---|---|
| 1. PVDF (%) (1 L) | 0.000393 | 1 | 0.000393 | 0.05828 | 0.831726 |
| PVDF (%) (1 Q) | 0.000654 | 1 | 0.000654 | 0.09693 | 0.784996 |
| 2. PVP (%) (2 L) | 0.002503 | 1 | 0.002503 | 0.37104 | 0.604416 |
| PVP (%) (2 Q) | 0.000140 | 1 | 0.000140 | 0.02075 | 0.898660 |
| 3. TiO2 (%) (3 L) | 0.366569 | 1 | 0.366569 | 54.34502 | 0.017908 a |
| TiO2 (%) (3 Q) | 0.201042 | 1 | 0.201042 | 29.80503 | 0.031952 a |
| 1 L by 2 L | 0.000011 | 1 | 0.000011 | 0.00164 | 0.971398 |
| 1 L by 3 L | 0.000076 | 1 | 0.000076 | 0.01121 | 0.925327 |
| 2 L by 3 L | 0.008026 | 1 | 0.008026 | 1.18995 | 0.389238 |
| Lack of Fit | 0.034477 | 5 | 0.006895 | 1.02226 | 0.562020 |
| Pure Error | 0.013490 | 2 | 0.006745 | ||
| Total SS | 0.725524 | 16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nascimben Santos, E.; Fazekas, Á.; Hodúr, C.; László, Z.; Beszédes, S.; Scheres Firak, D.; Gyulavári, T.; Hernádi, K.; Arthanareeswaran, G.; Veréb, G. Statistical Analysis of Synthesis Parameters to Fabricate PVDF/PVP/TiO2 Membranes via Phase-Inversion with Enhanced Filtration Performance and Photocatalytic Properties. Polymers 2022, 14, 113. https://doi.org/10.3390/polym14010113
Nascimben Santos E, Fazekas Á, Hodúr C, László Z, Beszédes S, Scheres Firak D, Gyulavári T, Hernádi K, Arthanareeswaran G, Veréb G. Statistical Analysis of Synthesis Parameters to Fabricate PVDF/PVP/TiO2 Membranes via Phase-Inversion with Enhanced Filtration Performance and Photocatalytic Properties. Polymers. 2022; 14(1):113. https://doi.org/10.3390/polym14010113
Chicago/Turabian StyleNascimben Santos, Erika, Ákos Fazekas, Cecilia Hodúr, Zsuzsanna László, Sándor Beszédes, Daniele Scheres Firak, Tamás Gyulavári, Klára Hernádi, Gangasalam Arthanareeswaran, and Gábor Veréb. 2022. "Statistical Analysis of Synthesis Parameters to Fabricate PVDF/PVP/TiO2 Membranes via Phase-Inversion with Enhanced Filtration Performance and Photocatalytic Properties" Polymers 14, no. 1: 113. https://doi.org/10.3390/polym14010113
APA StyleNascimben Santos, E., Fazekas, Á., Hodúr, C., László, Z., Beszédes, S., Scheres Firak, D., Gyulavári, T., Hernádi, K., Arthanareeswaran, G., & Veréb, G. (2022). Statistical Analysis of Synthesis Parameters to Fabricate PVDF/PVP/TiO2 Membranes via Phase-Inversion with Enhanced Filtration Performance and Photocatalytic Properties. Polymers, 14(1), 113. https://doi.org/10.3390/polym14010113










