Formulation Development, Characterization and Antifungal Evaluation of Chitosan NPs for Topical Delivery of Voriconazole In Vitro and Ex Vivo
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
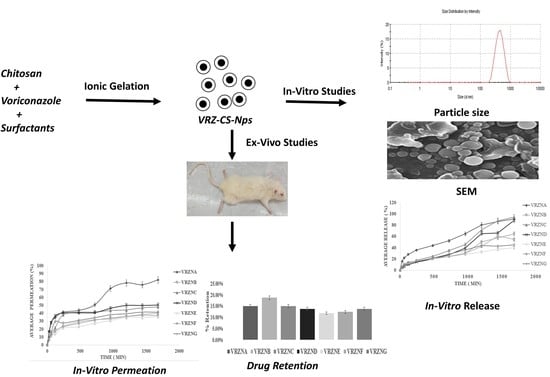
2.2. Preparation of NPs
2.3. Characterization of NPs
2.3.1. Particle Size and Zeta Potential
2.3.2. Percentage of Drug Entrapment Efficiency (%EE) Determination
2.3.3. Percentage of Drug Content (%DC) Determination
2.3.4. FTIR Analysis
2.3.5. Surface Morphology
2.4. In Vitro Release
2.5. Skin Permeation and Retention
2.5.1. Skin Preparation
2.5.2. Skin Permeation Analysis
2.5.3. Skin Retention Analysis
2.6. In Vitro Antifungal Activity
2.7. Statistical Analysis
3. Results and Discussions
3.1. Physicochemical Characterization of NPs
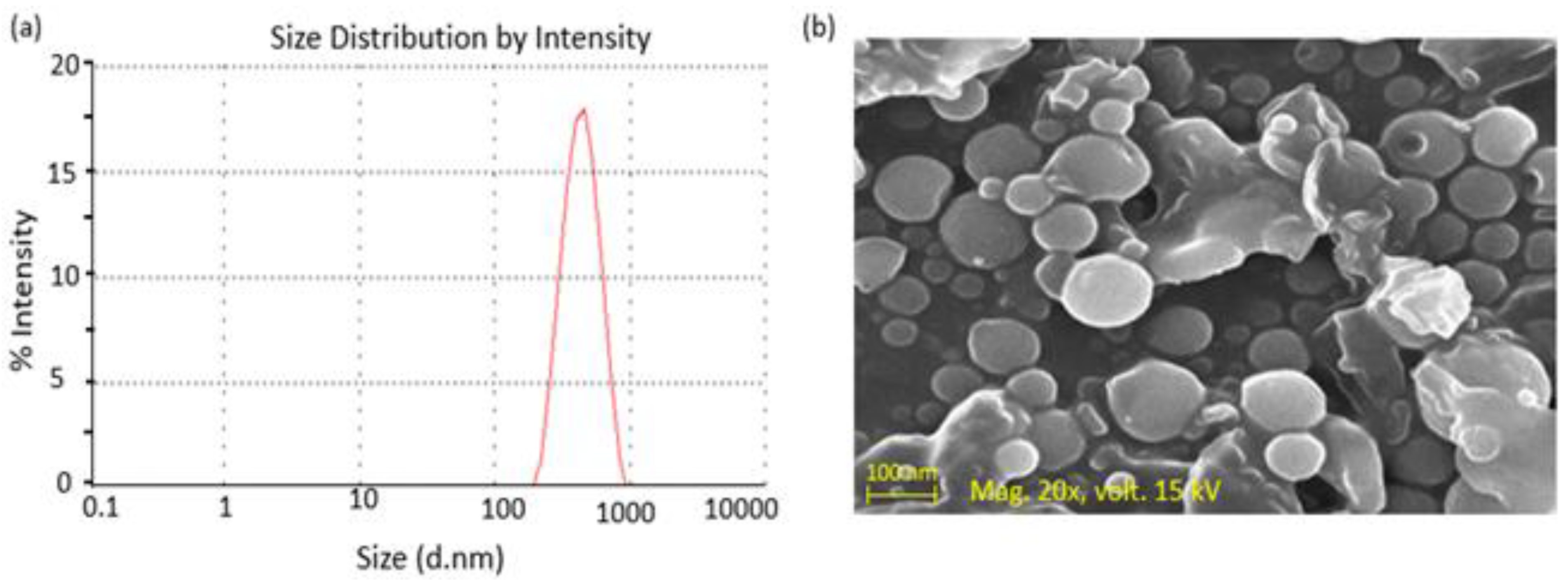
3.1.1. Size and Zeta Potential
3.1.2. Entrapment Efficiency (%EE)
3.1.3. Drug Content (%DC)
3.1.4. FTIR Analysis
3.1.5. Surface Morphology
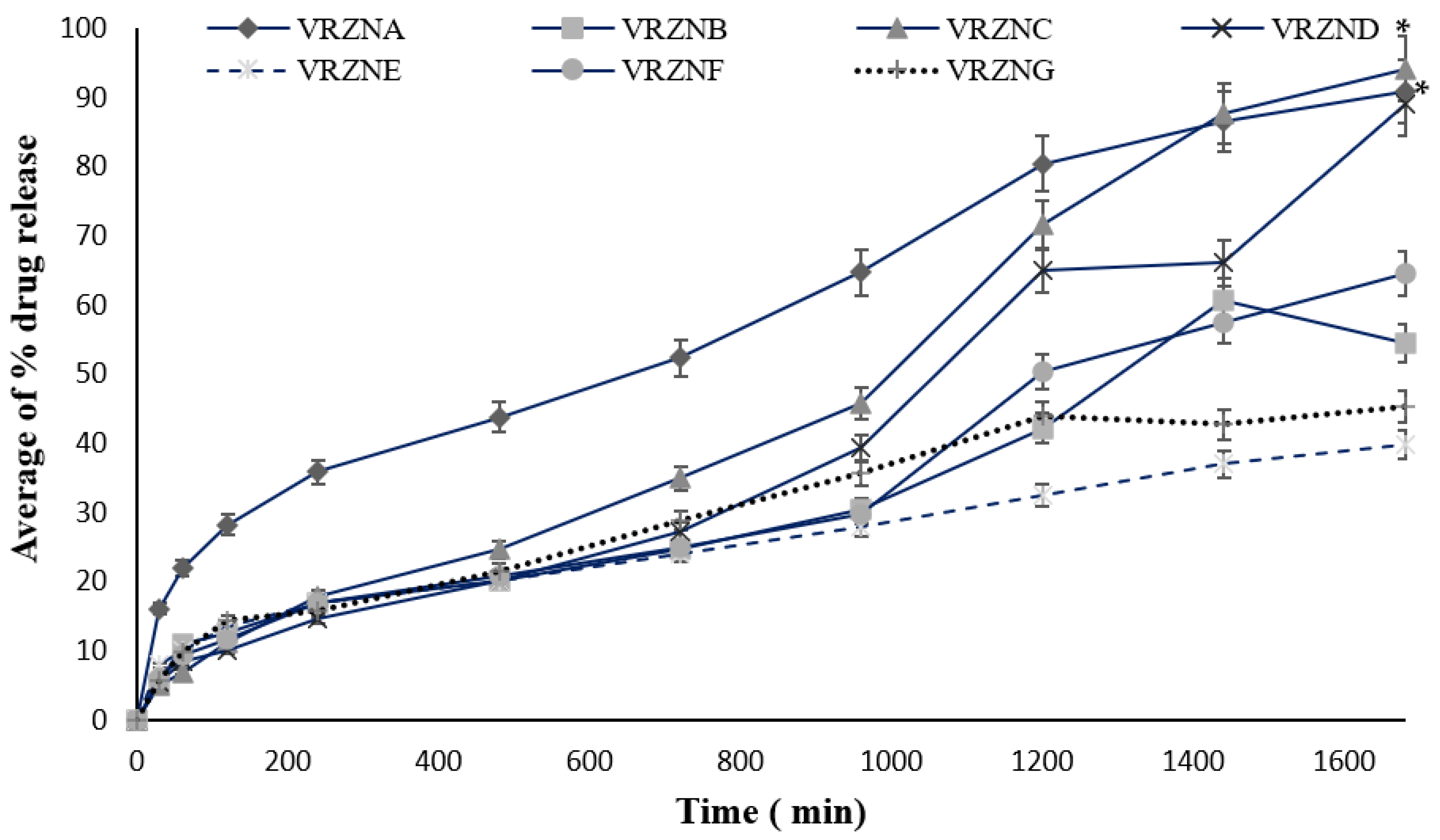
3.2. In Vitro Release
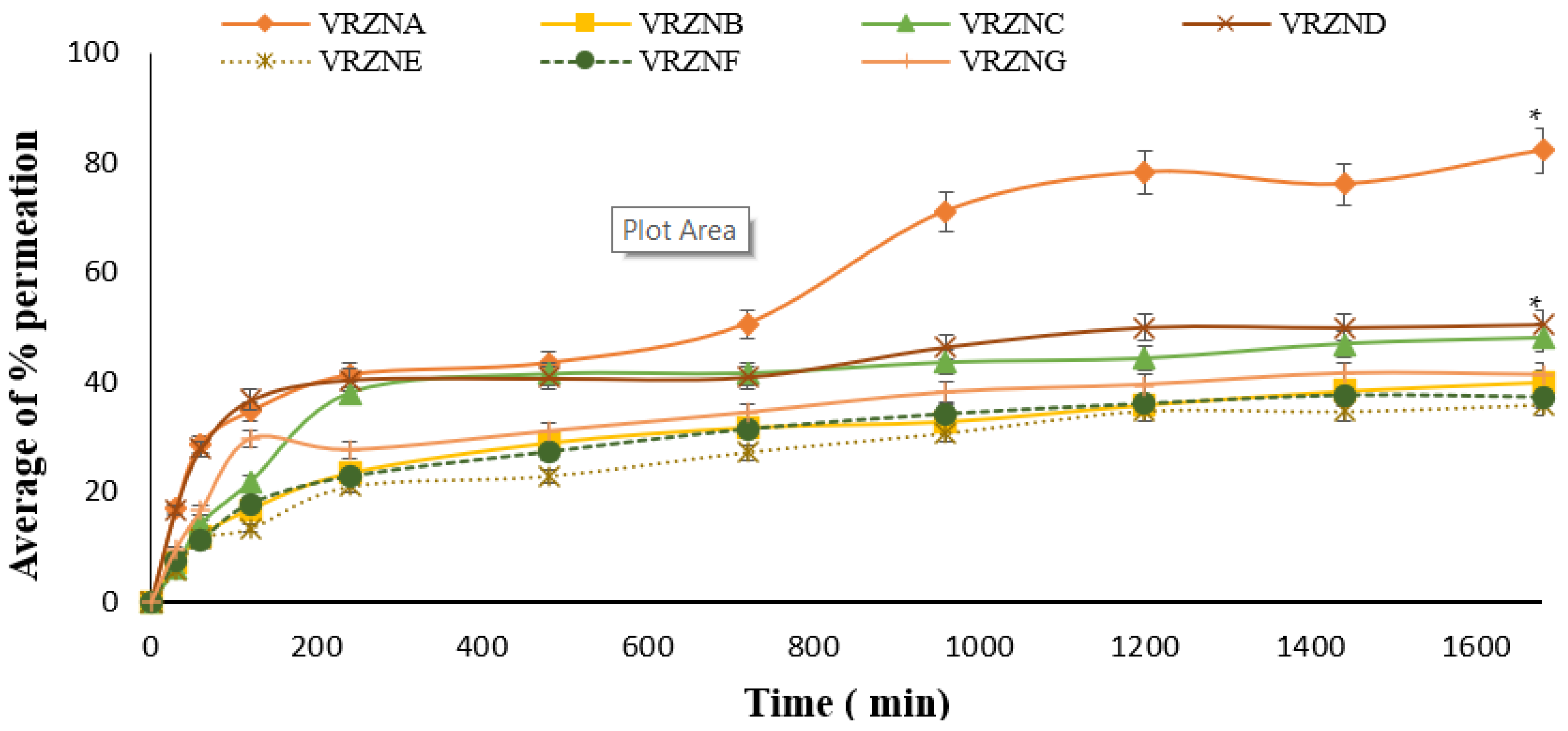
3.3. Skin Permeation
3.4. Drug Retention
3.5. Analysis of Skin Epidermis and Dermis by ATR-FTIR
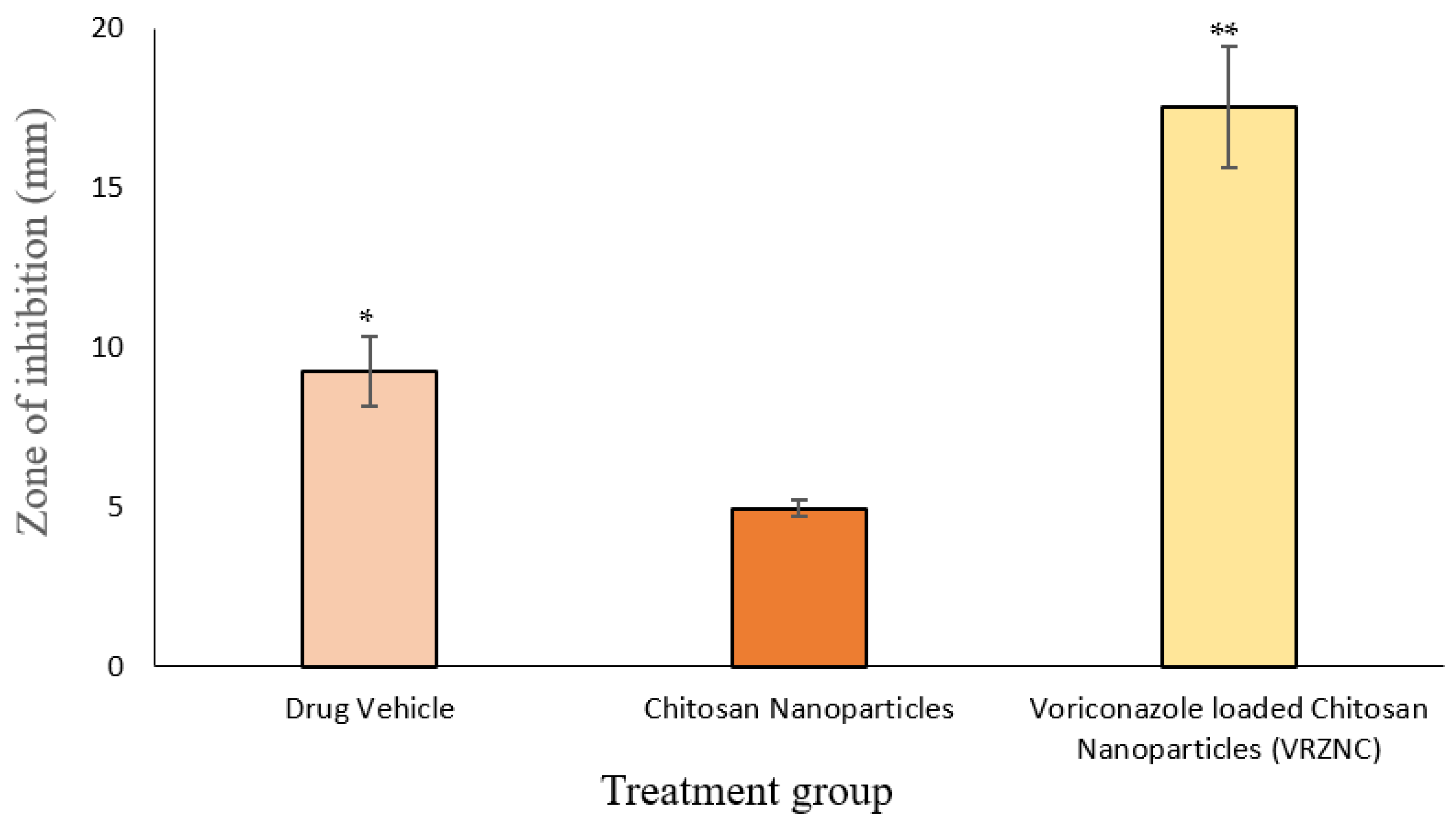
3.6. In Vitro Antifungal Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qurt, M.S.; Esentürk, İ.; Tan, S.B.; Erdal, M.S.; Araman, A.; Güngör, S. Voriconazole and sertaconazole loaded colloidal nano-carriers for enhanced skin deposition and improved topical fungal treatment. J. Drug Deliv. Sci. Technol. 2018, 48, 215–222. [Google Scholar] [CrossRef]
- Kumar, R.; Sinha, V.R. Preparation and optimization of voriconazole microemulsion for ocular delivery. Colloids Surf. B Biointerfaces 2014, 117, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Latif, M.S.; Azad, A.K.; Nawaz, A.; Rashid, S.A.; Rahman, M.; Al Omar, S.Y.; Bungau, S.G.; Aleya, L.; Abdel-Daim, M.M. Ethyl Cellulose and Hydroxypropyl Methyl Cellulose Blended Methotrexate-Loaded Transdermal Patches: In Vitro and Ex Vivo. Polymers 2021, 13, 3455. [Google Scholar] [CrossRef]
- Zonios, D.I.; Bennett, J.E. Update on azole antifungals. Semin. Respir. Crit. Care Med. 2008, 29, 198–210. [Google Scholar] [CrossRef] [PubMed]
- El-Hadidy, G.N.; Ibrahim, H.K.; Mohamed, M.I.; El-Milligi, M.F. Microemulsions as vehicles for topical administration of voriconazole: Formulation and in vitro evaluation. Drug Dev. Ind. Pharm. 2012, 38, 64–72. [Google Scholar] [CrossRef]
- Raju, Y.P.; Hyndavi, N.; Harini Chowdary, V.; Nair, R.S.; Basha, D.J.; Tejeswari, N. In vitro assessment of non-irritant microemulsified voriconazole hydrogel system. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1539–1547. [Google Scholar] [CrossRef]
- Sable, C.A.; Strohmeier, K.M.; Chodakewitz, J.A. Advances in antifungal therapy. Annu. Rev. Med. 2008, 59, 361–379. [Google Scholar] [CrossRef] [PubMed]
- Ghai, R.; Nagarajan, K.; Arora, M.; Grover, P.; Ali, N.; Kapoor, G. Current strategies, and novel drug approaches for Alzheimer disease. CNS Neurol. Disord. Drug Targets 2020, 19, 676–690. [Google Scholar] [CrossRef] [PubMed]
- Bandopadhyay, S.; Manchanda, S.; Chandra, A.; Ali, J.; Deb, P.K. Overview of different carrier systems for advanced drug delivery. In Drug Delivery Systems; Academic Press: Cambridge, MA, USA, 2020; pp. 179–233. [Google Scholar]
- Wani, T.U.; Mohi-ud-Din, R.; Majeed, A.; Kawoosa, S.; Pottoo, F.H. Skin permeation of NPs: Mechanisms involved and critical factors governing topical drug delivery. Curr. Pharm. Des. 2020, 26, 4601–4614. [Google Scholar] [CrossRef]
- Sarma, A.; Bania, R.; Devi, J.R.; Deka, S. Therapeutic nanostructures and nanotoxicity. J. Appl. Toxicol. 2021, 41, 1494–1517. [Google Scholar] [CrossRef]
- Arenz, M.; Mayrhofer, K.J.; Stamenkovic, V.; Blizanac, B.B.; Tomoyuki, T.; Ross, P.N.; Markovic, N.M. The effect of the particle size on the kinetics of CO electrooxidation on high surface area Pt catalysts. J. Am. Chem. Soc. 2005, 127, 6819–6829. [Google Scholar] [CrossRef]
- Alexander, A.; Khichariya, A.; Gupta, S.; Patel, R.J.; Giri, T.K.; Tripathi, D.K. Recent expansions in an emergent novel drug delivery technology: Emulgel. J. Control. Release 2013, 171, 122–132. [Google Scholar]
- Seetharam, A.A.; Choudhry, H.; Bakhrebah, M.A.; Abdulaal, W.H.; Gupta, M.S.; Rizvi, S.M.D.; Moin, A. Microneedles drug delivery systems for treatment of cancer: A recent update. Pharmaceutics 2020, 12, 1101. [Google Scholar] [CrossRef]
- Sutradhar, K.B.; Amin, M.L. Nanoemulsions: Increasing possibilities in drug delivery. Eur. J. Nanomed. 2013, 5, 97–110. [Google Scholar] [CrossRef]
- Prokai, L.; Nguyen, V.; Jasti, B.R.; Ghosh, T.K. Principles and applications of surface phenomena. In Theory and Practice of Contemporary Pharmaceutics; CRC Press: Boca Raton, FL, USA, 2021; pp. 165–196. [Google Scholar]
- Akhlaq, M.; Azad, A.K.; Fuloria, S.; Meenakshi, D.U.; Raza, S.; Safdar, M.; Nawaz, A.; Subramaniyan, V.; Sekar, M.; Sathasivam, K.V.; et al. Fabrication of Tizanidine Loaded Patches Using Flaxseed Oil and Coriander Oil as a Penetration Enhancer for Transdermal Delivery. Polymers 2021, 13, 4217. [Google Scholar] [CrossRef]
- Lin, X.; Wang, Z.; Ou, H.; Mitragotri, S.; Chen, M. Correlations between skin barrier integrity and delivery of hydrophilic molecules in the presence of penetration enhancers. Pharm. Res. 2020, 37, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Seenuvasan, M.; Sarojini, G.; Dineshkumar, M. Recovery of chitosan from natural biotic waste. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 115–133. [Google Scholar]
- Parhi, R. Drug delivery applications of chitin and chitosan: A review. Environ. Chem. Lett. 2020, 18, 577–594. [Google Scholar] [CrossRef]
- Anantrao, J.H.; Nath, P.A.; Nivrutti, P.R. Drug penetration enhancement techniques in transdermal drug delivery system: A review. J. Pharm. Res. Int. 2021, 33, 46–61. [Google Scholar] [CrossRef]
- Borchard, G.; Som, C.; Zinn, M.; Ostafe, V.; Borges, O.; Perale, G.; Wick, P. Polymeric nano-biomaterials for medical applications: Advancements in developing and implementation considering safety-by-design concepts. Front. Bioeng. Biotechnol. 2020, 8, 599950. [Google Scholar] [CrossRef]
- Esentürk, İ.; Balkan, T.; Özhan, G.; Döşler, S.; Güngör, S.; Erdal, M.S.; Sarac, A.S. Voriconazole incorporated nanofiber formulations for topical application: Preparation, characterization, and antifungal activity studies against Candida species. Pharm. Dev. Technol. 2020, 25, 440–453. [Google Scholar] [CrossRef] [PubMed]
- Chachuli, S.H.M.; Nawaz, A.; Shah, K.; Naharudin, I.; Wong, T.W. In vitro investigation of influences of chitosan NPs on fluorescein permeation into alveolar macrophages. Pharm. Res. 2016, 33, 1497–1508. [Google Scholar] [CrossRef]
- Azad, A.K.; Al-Mahmood, S.M.A.; Chatterjee, B.; Wan Sulaiman, W.M.A.; Elsayed, T.M.; Doolaanea, A.A. Encapsulation of black seed oil in alginate beads as a pH-sensitive carrier for intestine-targeted drug delivery: In vitro, in vivo and ex vivo study. Pharmaceutics 2020, 12, 219. [Google Scholar] [CrossRef] [PubMed]
- Bera, H.; Yasir, F.A.; Virendra, G.; Kok, F.L.; Pramod, K.; Prajakta, T.; Azad, A.K.; Dongmei, C.; Mingshi, Y. Carboxymethyl fenugreek galactomannan-g-poly (N-isopropylacrylamide-co-N, N′-methylene-bis-acrylamide)-clay based pH/temperature-responsive nanocomposites as drug-carriers. Mater. Sci. Eng. C 2020, 110, 110628. [Google Scholar] [CrossRef] [PubMed]
- Peltonen, L.; Aitta, J.; Hyvönen, S.; Karjalainen, M.; Hirvonen, J. Improved entrapment efficiency of hydrophilic drug substance during nanoprecipitation of poly (I) lactide NPs. AAPS PharmSciTech 2004, 5, 115–120. [Google Scholar]
- Azad, A.K.; Al-Mahmood, S.M.A.; Kennedy, J.F.; Chatterjee, B.; Bera, H. Electro-hydrodynamic assisted synthesis of lecithin-stabilized peppermint oil-loaded alginate microbeads for intestinal drug delivery. Int. J. Biol. Macromol. 2021, 185, 861–875. [Google Scholar] [CrossRef]
- Akhlaq, M.; Azad, A.K.; Ullah, I.; Nawaz, A.; Safdar, M.; Bhattacharya, T.; Uddin, A.B.; Abbas, S.A.; Mathews, A.; Kundu, S.K.; et al. Methotrexate-loaded gelatin and polyvinyl alcohol (Gel/PVA) hydrogel as a pH-sensitive matrix. Polymers 2021, 13, 2300. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, A.; Wong, T. Quantitative characterization of chitosan in the skin by Fourier-transform infrared spectroscopic imaging and ninhydrin assay: Application in transdermal sciences. J. Microsc. 2016, 263, 34–42. [Google Scholar] [CrossRef]
- Khan, T.A.; Azad, A.K.; Fuloria, S.; Nawaz, A.; Subramaniyan, V.; Akhlaq, M.; Safdar, M.; Sathasivam, K.V.; Sekar, M.; Porwal, O.; et al. Chitosan-Coated 5-Fluorouracil Incorporated Emulsions as Transdermal Drug Delivery Matrices. Polymers 2021, 13, 3345. [Google Scholar] [CrossRef]
- Irfan, M.M.; Shah, S.U.; Khan, I.U.; Munir, M.U.; Khan, N.R.; Shah, K.U.; Mahmood, S. Physicochemical characterization of finasteride nanosystem for enhanced topical delivery. Int. J. Nanomed. 2021, 16, 1207. [Google Scholar] [CrossRef]
- Finnin, B.; Walters, K.A.; Franz, T.J. In vitro skin permeation methodology. In Transdermal and Topical Drug Delivery: Principles and Practice; John Wiley & Sons: Hoboken, NJ, USA, 2012; pp. 85–108. [Google Scholar]
- Chandrasekar, V.; Knabel, S.J.; Anantheswaran, R.C. Modeling development of inhibition zones in an agar diffusion bioassay. Food Sci. Nutr. 2015, 3, 394–403. [Google Scholar] [CrossRef]
- Redhead, H.M.; Davis, S.S.; Illum, L. Drug delivery in poly (lactide-co-glycolide) NPs surface modified with poloxamer 407 and poloxamine 908: In vitro characterisation and in vivo evaluation. J. Control Release 2001, 70, 353–363. [Google Scholar] [CrossRef]
- Pal, S.L.; Jana, U.; Manna, P.K.; Mohanta, G.P.; Manavalan, R. Nanoparticle: An overview of preparation and characterization. J. Appl. Pharm. Sci. 2011, 1, 228–234. [Google Scholar]
- Urbán-Morlán, Z.; Ganem-Rondero, A.; Melgoza-Contreras, L.M.; Escobar-Chávez, J.J.; Nava-Arzaluz, M.G.; Quintanar-Guerrero, D. Preparation and characterization of solid lipid NPs containing cyclosporine by the emulsification-diffusion method. Int. J. Nanomed. 2010, 5, 611. [Google Scholar]
- Alvarez-Trabado, J.; Diebold, Y.; Sanchez, A. Designing lipid NPs for topical ocular drug delivery. Int. J. Pharm. 2017, 532, 204–217. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.R.; Harun, M.S.; Nawaz, A.; Harjoh, N.; Wong, T.W. Nanocarriers and their actions to improve skin permeability and transdermal drug delivery. Curr. Pharm. Des. 2015, 21, 2848–2866. [Google Scholar] [CrossRef] [PubMed]
- Chu, B.; Zhang, L.; Qu, Y.; Chen, X.; Peng, J.; Huang, Y.; Qian, Z. Synthesis characterization and drug loading property of Monomethoxy-Poly (ethylene glycol)-Poly (ε-caprolactone)-Poly (D, L-lactide) (MPEG-PCLA) copolymers. Sci. Rep. 2013, 6, 1–15. [Google Scholar] [CrossRef]
- Olbrich, C.; Kayser, O.; Müller, R.H. Lipase degradation of Dynasan 114 and 116 solid lipid NPs (SLN)—Effect of surfactants, storage time and crystallinity. Int. J. Pharm. 2002, 237, 119–128. [Google Scholar] [CrossRef]
- Budhian, A.; Siegel, S.J.; Winey, K.I. Production of haloperidol loaded PLGA NPs for extended controlled drug release of haloperidol. J. Microencapsul. 2005, 22, 773–785. [Google Scholar] [CrossRef]
- Mohammed, M.A.; Syeda, J.; Wasan, K.M.; Wasan, E.K. An overview of chitosan NPs and its application in non-parenteral drug delivery. Pharmaceutics 2017, 9, 53. [Google Scholar] [CrossRef]
- Üner, M.; Yener, G. Importance of solid lipid NPs (SLN) in various administration routes and future perspectives. Int. J. Nanomed. 2007, 2, 289. [Google Scholar]
- Som, I.; Bhatia, K.; Yasir, M. Status of surfactants as penetration enhancers in transdermal drug delivery. J. Pharm. Bioallied Sci. 2012, 4, 2. [Google Scholar] [PubMed]
- Khare, A.; Singh, I.; Pawar, P.; Grover, K. Design and evaluation of voriconazole loaded solid lipid nanoparticles for ophthalmic application. J. Drug. Deliv. 2016, 2016, 6590361. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Mittal, A.; Chauhan, N.; Alam, S. Role of surfactants as penetration enhancer in transdermal drug delivery system. J. Mol. Pharm. Org. Process. Res. 2014, 2, 2–7. [Google Scholar] [CrossRef]
- Schmidberger, M.; Nikolic, I.; Pantelic, I.; Lunter, D. Optimization of rheological behaviour and skin penetration of thermogelling emulsions with enhanced substantivity for potential application in treatment of chronic skin diseases. Pharmaceutics 2019, 11, 361. [Google Scholar] [CrossRef]
- Pajić, N.B.; Vucen, S.; Ilić, T.; O’Mahony, C.; Dobričić, V.; Savić, S. Comparative efficacy evaluation of different penetration enhancement strategies for dermal delivery of poorly soluble drugs—A case with sertaconazole nitrate. Eur. Pharm. Sci. 2021, 164, 105895. [Google Scholar] [CrossRef]
- Akhtar, N.; Rehman, M.U.; Khan, H.M.S.; Rasool, F.; Saeed, T.; Murtaz, G. Penetration enhancing effect of polysorbate 20 and 80 on the in vitro percutaneous absorption of lascorbic acid. Trop. J. Pharm. Res. 2011, 10, 281–288. [Google Scholar] [CrossRef]
- D’souza, A.A.; Shegokar, R. Polyethylene glycol (PEG): A versatile polymer for pharmaceutical applications. Expert Opin. Drug. Deliv. 2016, 13, 1257–1275. [Google Scholar] [CrossRef]
- Fantini, A.; Demurtas, A.; Nicoli, S.; Padula, C.; Pescina, S.; Santi, P. In vitro skin retention of crisaborole after topical application. Pharmaceutics 2020, 12, 491. [Google Scholar] [CrossRef]
- Sudhakar, K.; Fuloria, S.; Subramaniyan, V.; Sathasivam, K.V.; Azad, A.K.; Swain, S.S.; Sekar, M.; Karupiah, S.; Porwal, O.; Sahoo, A.; et al. Ultraflexible Liposome Nanocargo as a Dermal and Transdermal Drug Delivery System. Nanomaterials 2021, 11, 2557. [Google Scholar] [CrossRef]
- Uchechi, O.; Ogbonna, J.D.; Attama, A.A. NPs for dermal and transdermal drug delivery. In Application of Nanotechnology in Drug Delivery; IntechOpen: London, UK, 2014; Volume 4, pp. 193–227. [Google Scholar]
- Panapisal, V.; Charoensri, S.; Tantituvanont, A. Formulation of microemulsion systems for dermal delivery of silymarin. AAPS PharmSciTech 2012, 13, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Lopes, L.B. Overcoming the cutaneous barrier with microemulsions. Pharmaceutics 2014, 6, 52–77. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Yan, Q.; Wang, J.; Ding, C.; Sai, S. Enhanced antifungal activity of voriconazole-loaded nanostructured lipid carriers against Candida albicans with a dimorphic switching model. Int. J. Nanomed. 2017, 12, 7131. [Google Scholar] [CrossRef] [PubMed][Green Version]




| F. Code | Drug % w/w | Chitosan % w/w | Coconut Oil % w/w | Surfactants and Polymers | ||||
|---|---|---|---|---|---|---|---|---|
| T80 % w/w | SLS % w/w | T20 % w/w | PEG4000 % w/w | PG % w/w | ||||
| VRZNA | 1 | 0.5 | 0.25 | - | - | - | - | - |
| VRZNB | 1 | 0.5 | - | 0.6 | - | - | - | - |
| VRZNC | 1 | 0.5 | 0.25 | 0.6 | - | - | - | - |
| VRZND | 1 | 0.5 | 0.25 | - | 0.6 | - | - | - |
| VRZNE | 1 | 0.5 | 0.25 | - | - | 0.6 | - | - |
| VRZNF | 1 | 0.5 | 0.25 | - | - | - | 0.6 | - |
| VRZNG | 1 | 0.5 | 0.25 | - | - | - | - | 0.6 |
| F. Code | Average Size (nm) | Zeta Potential (mV) | % EE | % DC |
|---|---|---|---|---|
| VRZNA | 475 ± 15.30 | 45 ± 3.11 | 38.11 ± 0.51 | 63.22 ± 0.58 ** |
| VRZNB | 167 ± 8.23 ** | 44 ± 2.70 | 58.48 ± 0.85 ** | 85.05 ± 0.63 * |
| VRZNC | 210 ± 9.11 * | 41 ± 1.92 | 41.49 ± 1.13 | 76.95 ± 0.50 |
| VRZND | 189 ± 7.34 * | 39 ± 2.56 | 52.44 ± 0.35 * | 91.40 ± 0.10 * |
| VRZNE | 202 ± 9.12 * | 40 ± 1.82 | 49.54 ± 0.41 | 91.02 ± 0.25 * |
| VRZNF | 279 ± 11.41 | 42 ± 1.64 | 41.89 ± 0.39 | 75.02 ± 0.75 |
| VRZNG | 267 ± 10.17 | 41.5 ± 2.91 | 51.71 ± 0.25 * | 90.52 ± 0.39 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, M.K.A.; Azad, A.K.; Nawaz, A.; Ullah, S.; Latif, M.S.; Rahman, H.; Alsharif, K.F.; Alzahrani, K.J.; El-Kott, A.F.; Albrakati, A.; et al. Formulation Development, Characterization and Antifungal Evaluation of Chitosan NPs for Topical Delivery of Voriconazole In Vitro and Ex Vivo. Polymers 2022, 14, 135. https://doi.org/10.3390/polym14010135
Shah MKA, Azad AK, Nawaz A, Ullah S, Latif MS, Rahman H, Alsharif KF, Alzahrani KJ, El-Kott AF, Albrakati A, et al. Formulation Development, Characterization and Antifungal Evaluation of Chitosan NPs for Topical Delivery of Voriconazole In Vitro and Ex Vivo. Polymers. 2022; 14(1):135. https://doi.org/10.3390/polym14010135
Chicago/Turabian StyleShah, Muhammad Khurshid Alam, Abul Kalam Azad, Asif Nawaz, Shafi Ullah, Muhammad Shahid Latif, Habibur Rahman, Khalaf F. Alsharif, Khalid J. Alzahrani, Attalla F. El-Kott, Ashraf Albrakati, and et al. 2022. "Formulation Development, Characterization and Antifungal Evaluation of Chitosan NPs for Topical Delivery of Voriconazole In Vitro and Ex Vivo" Polymers 14, no. 1: 135. https://doi.org/10.3390/polym14010135
APA StyleShah, M. K. A., Azad, A. K., Nawaz, A., Ullah, S., Latif, M. S., Rahman, H., Alsharif, K. F., Alzahrani, K. J., El-Kott, A. F., Albrakati, A., & Abdel-Daim, M. M. (2022). Formulation Development, Characterization and Antifungal Evaluation of Chitosan NPs for Topical Delivery of Voriconazole In Vitro and Ex Vivo. Polymers, 14(1), 135. https://doi.org/10.3390/polym14010135