Selective Recognition of Gallic Acid Using Hollow Magnetic Molecularly Imprinted Polymers with Double Imprinting Surfaces
Abstract
:1. Introduction
2. Experimental
2.1. Chemicals and Apparatus
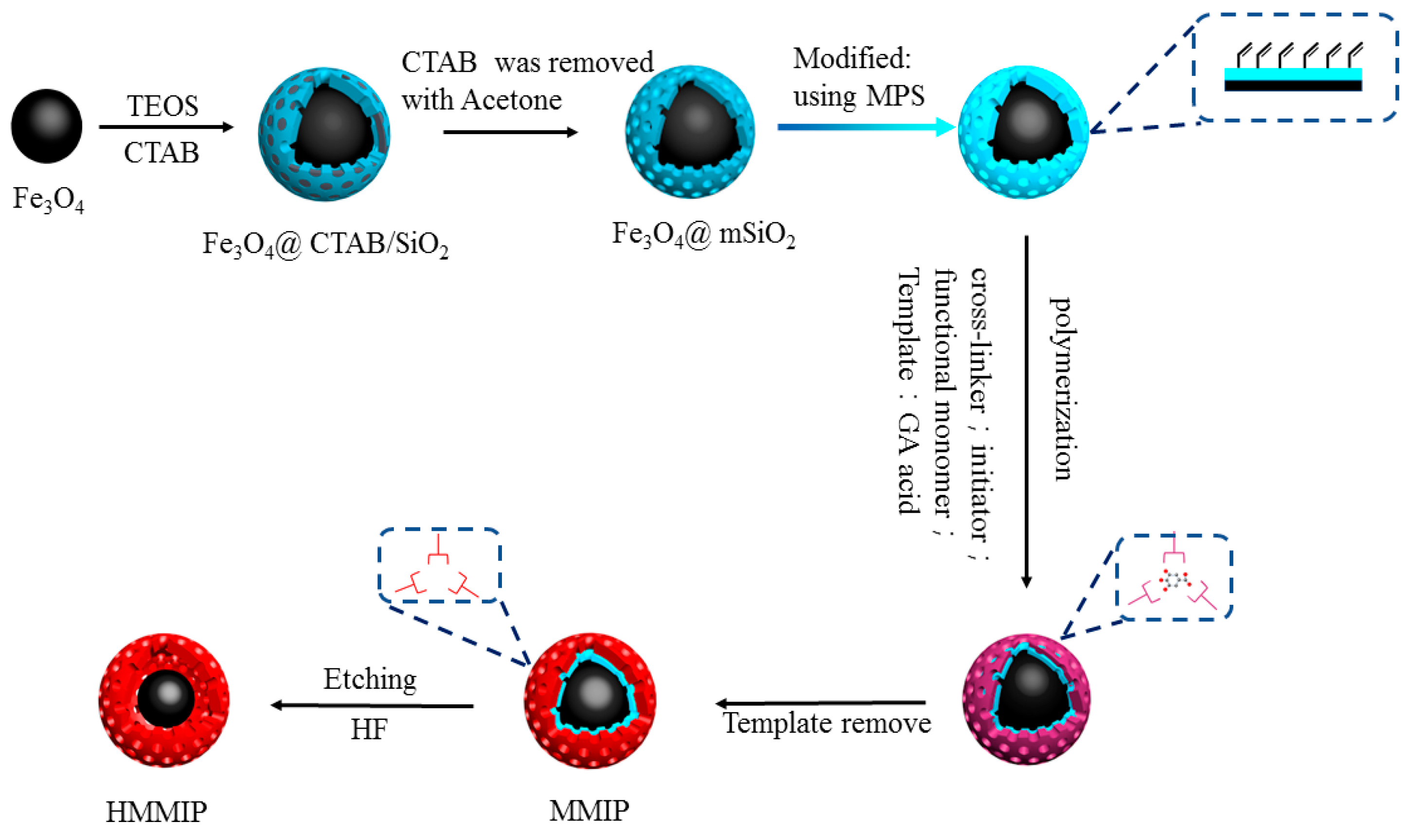
2.2. Preparation of Core-Shell Hollow Magnetic Molecularly Imprinted Polymers
2.2.1. Preparation of Core-Shell Magnetic Molecularly Imprinted Polymers
2.2.2. Preparation of Hollow Magnetic Molecularly Imprinted Polymers
2.3. Characterization and HPLC Analysis
2.4. Adsorption Performance
2.4.1. Kinetics Adsorption Experiment
2.4.2. Thermodynamic Adsorption Experiment
2.4.3. Selective Adsorption Experiment
2.4.4. Real Sample Analysis
3. Results and Discussion
3.1. Characterization of HMMIP and MMIP
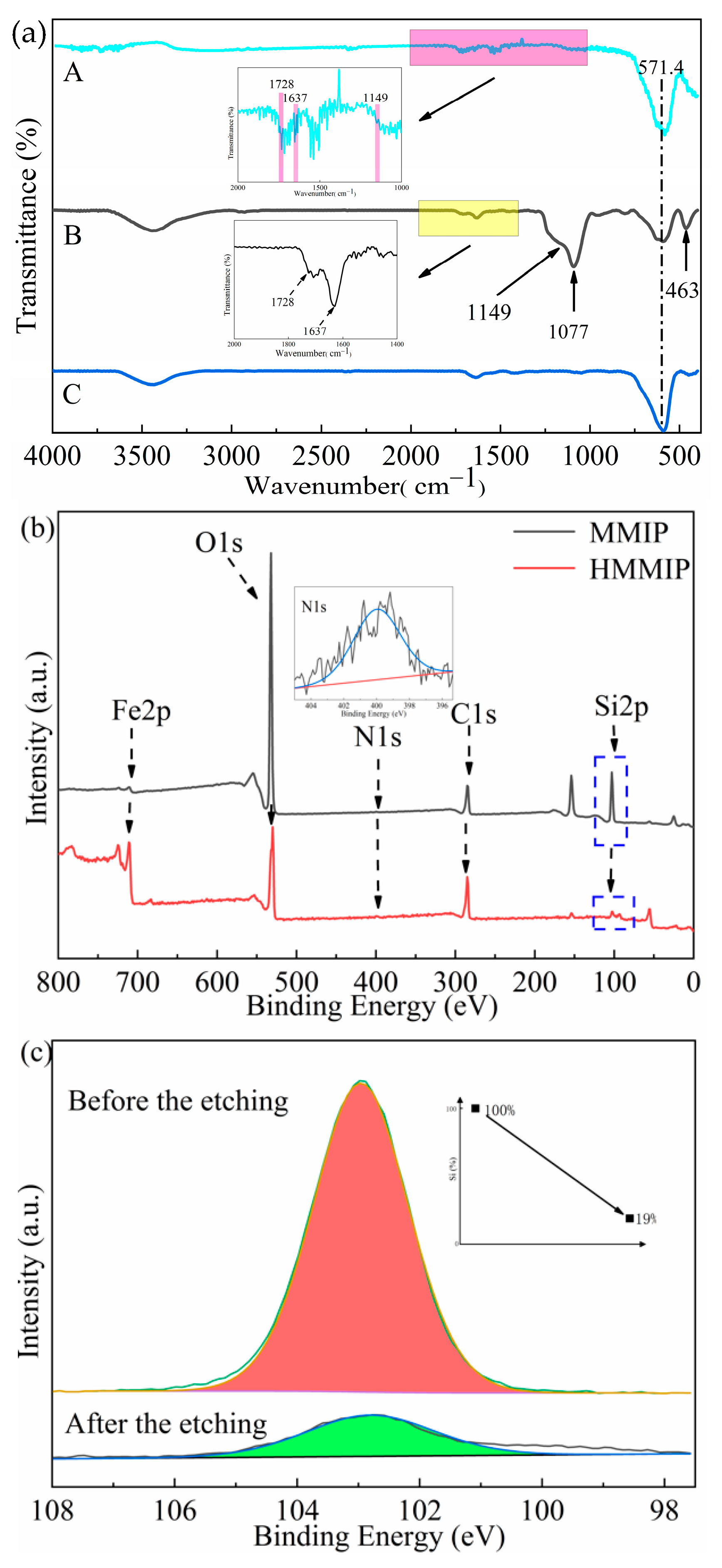
3.1.1. Analysis of FT-IR and XPS Results
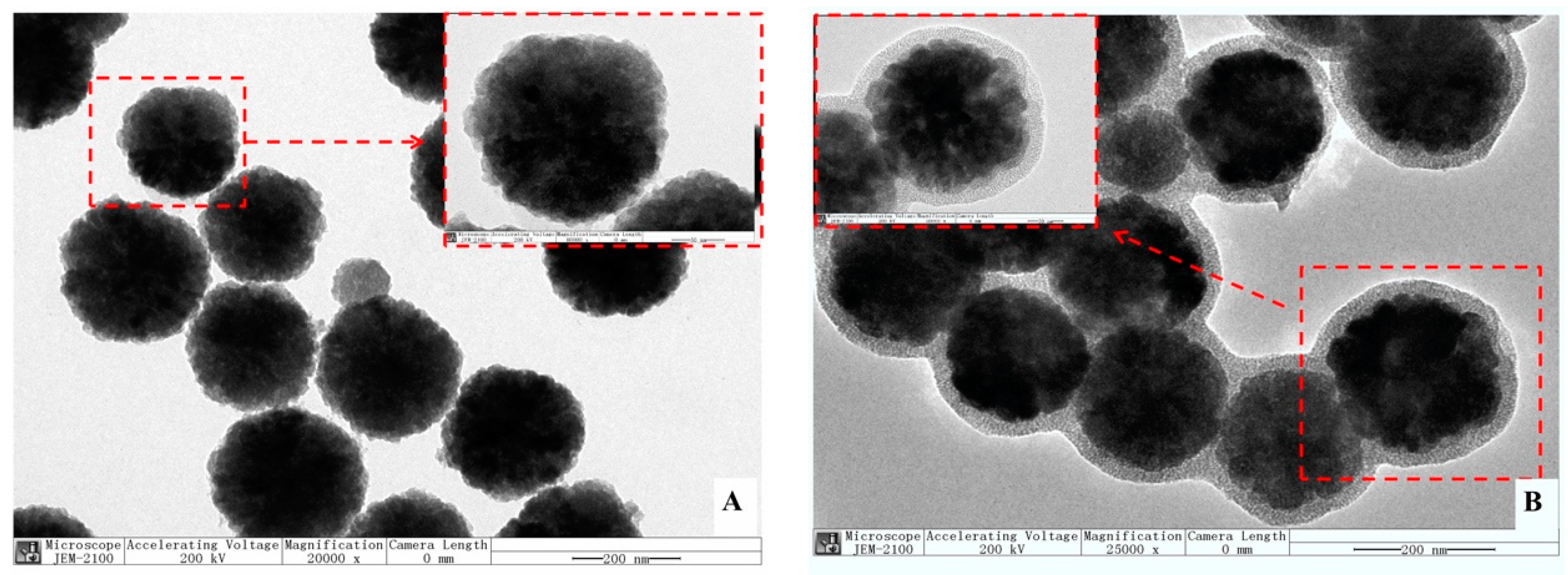
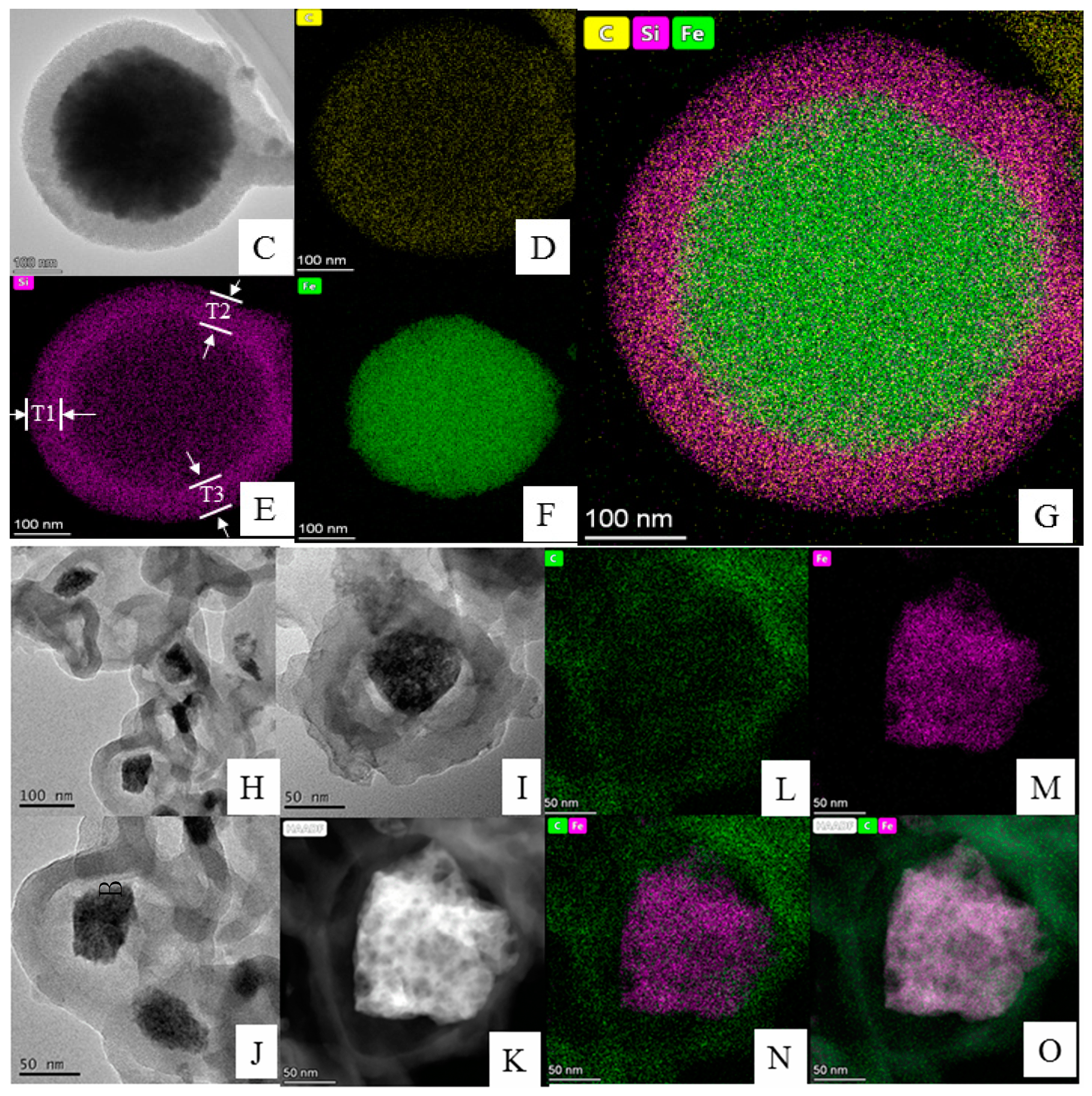
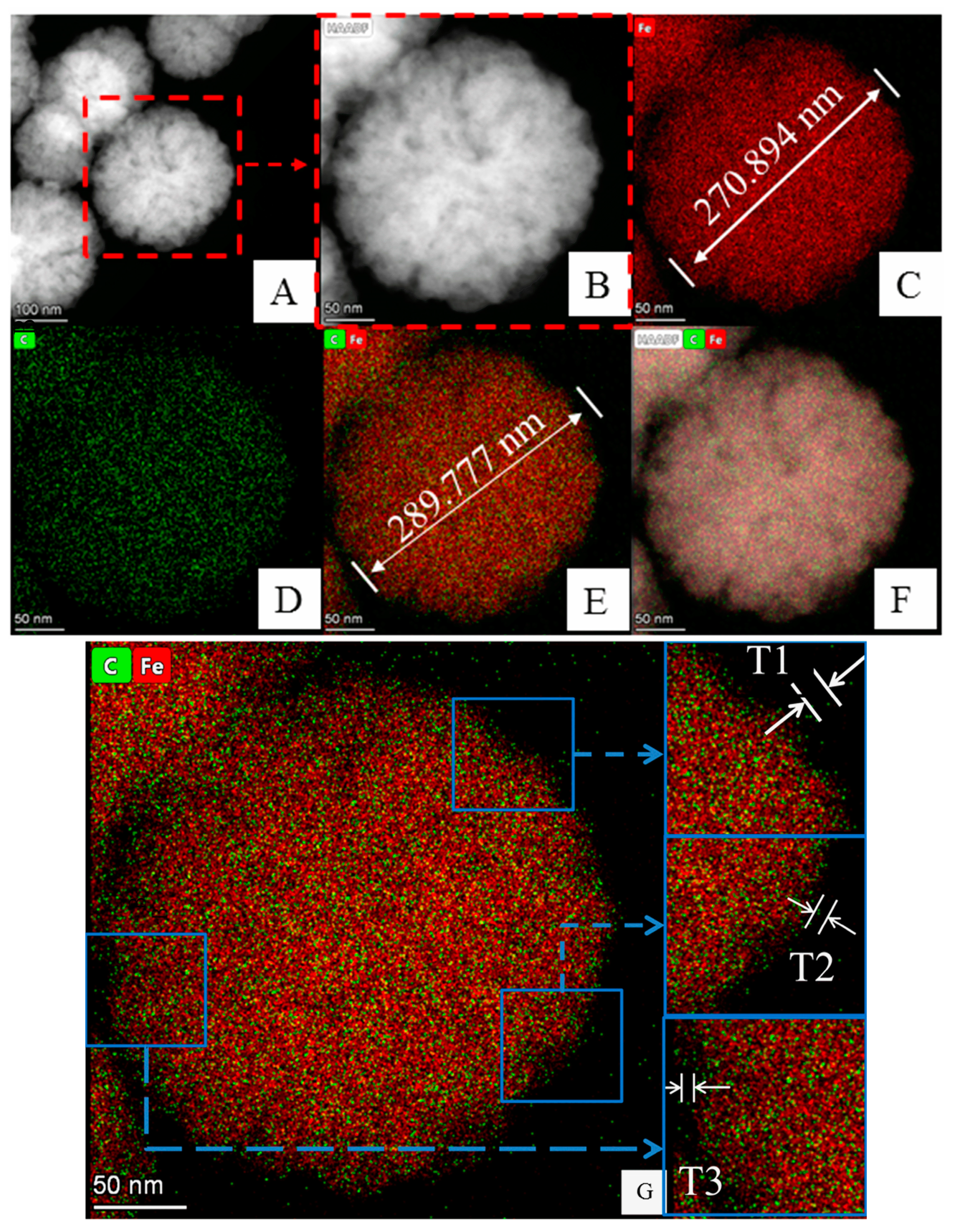
3.1.2. Analysis of TEM and Element Mapping
3.1.3. Determination of Specific Surface Area and Mesoporous Pore Size
3.1.4. Analysis of Magnetic Response Intensity of HMMIP
3.1.5. TGA Analysis
3.2. Adsorption Performance of HMMIP
3.2.1. Kinetics of Adsorption
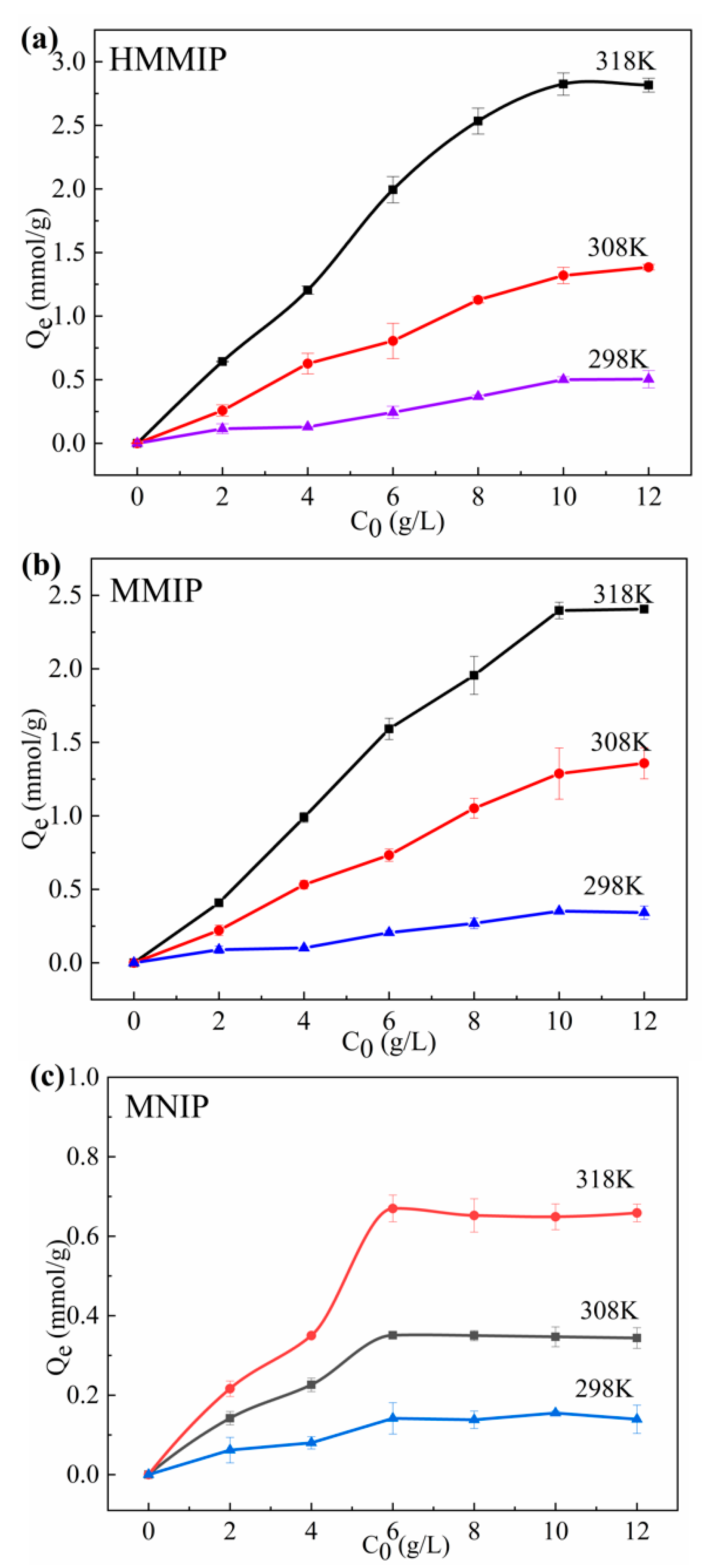
3.2.2. Thermodynamics of Adsorption
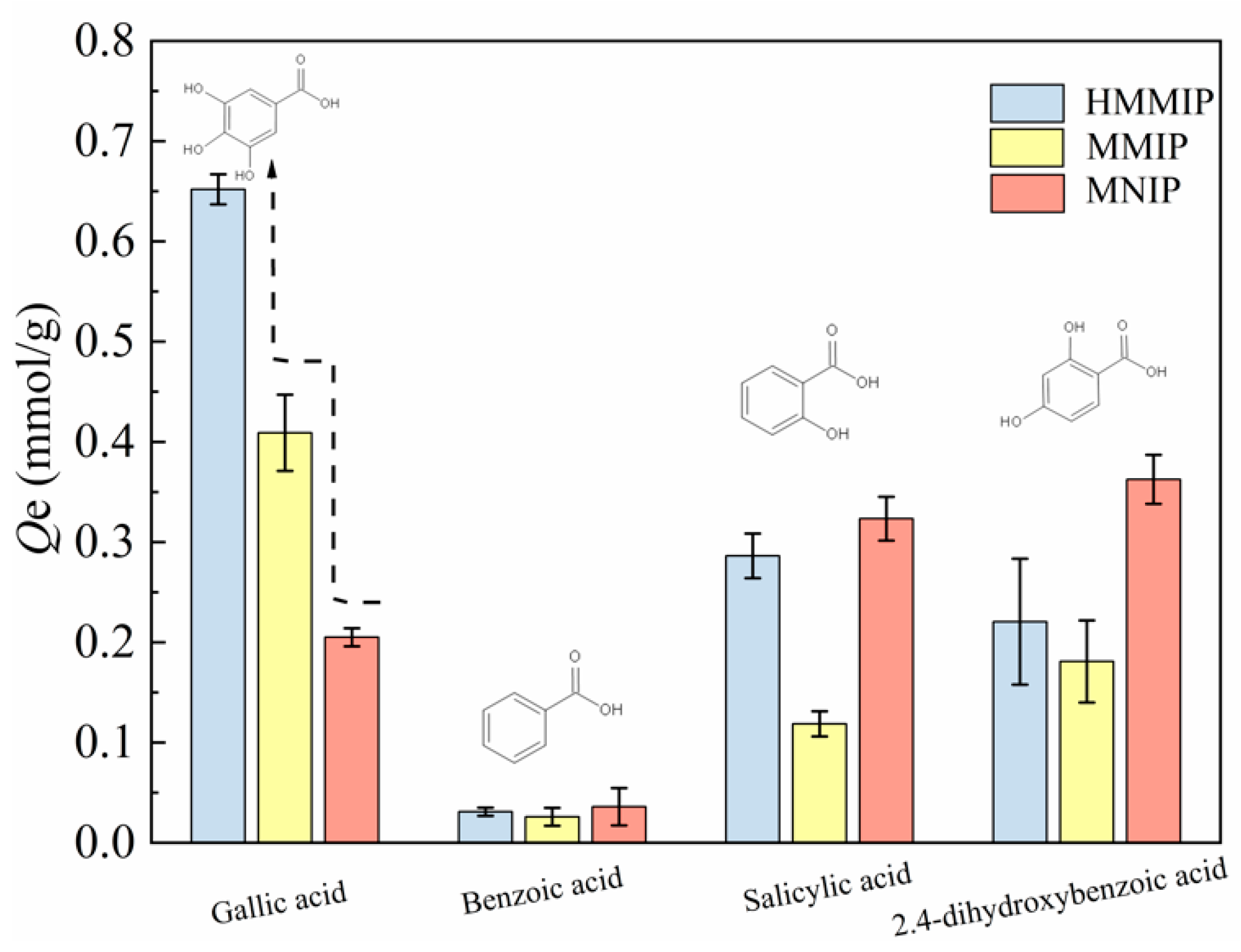
3.2.3. Selectivity of Adsorption
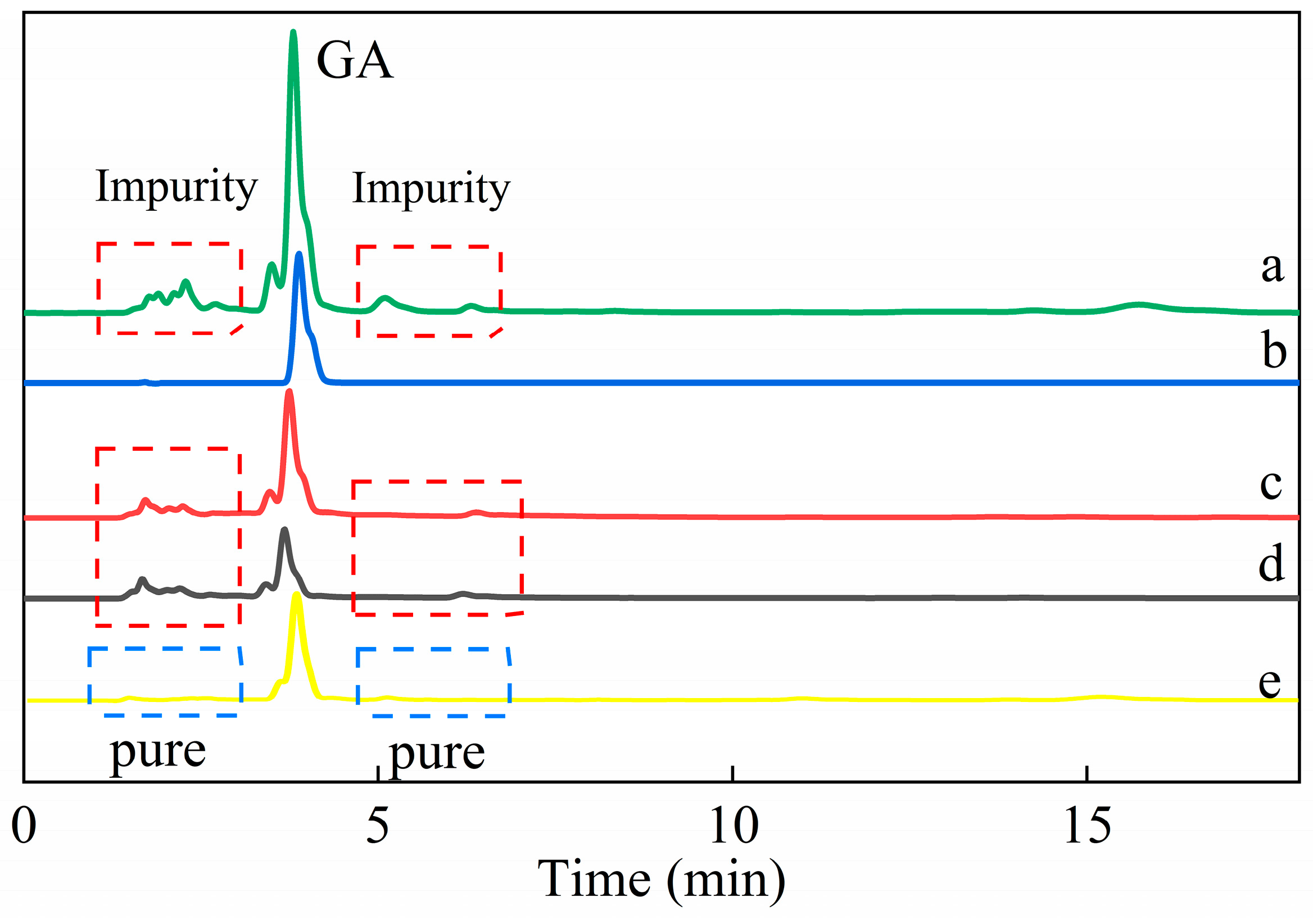
3.2.4. Specific Adsorption of Gallic Acid from Green Tea
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bashar, S.M.; Elhadidy, M.G.; Mostafa, A.F.; Hamed, S.; Helmy, H.A. Abd-Elmoniem, Hepatoprotective effect of gallic acid against type 2-induced diabetic liver injury in male rats through modulation of fetuin-A and GLP-1 with involvement of ERK1/2/NF-kappa B and Wnt1/beta-catenin signaling pathways. Gen. Physiol. Biophys. 2021, 40, 221–234. [Google Scholar] [CrossRef]
- Karatas, O.; Gevrek, F. 3,4,5-Trihydroxybenzoic acid attenuates ligature-induced periodontal disease in wistar rats, Anti-inflammatory. anti-allergy agents. Med. Chem. 2021, 20, 51–60. [Google Scholar]
- Sanjay, S.; Girish, C.; Toi, P.C.; Bobby, Z. Gallic acid attenuates isoniazid and rifampicin-induced liver injury by improving hepatic redox homeostasis through influence on Nrf2 and NF-kappa B signalling cascades in Wistar Rats. J. Pharm. Pharmacol. 2021, 73, 473–486. [Google Scholar] [CrossRef]
- Chung, D.J.; Wu, Y.L.; Yang, M.Y.; Chan, K.C.; Lee, H.J.; Wang, C.J. Nelumbo nuciferaleaf polyphenol extract and gallic acid inhibit TNF-alpha-induced vascular smooth muscle cell proliferation and migration involving the regulation of miR-21, miR-143 and miR-145. Food. Funct. 2020, 11, 8602–8611. [Google Scholar] [CrossRef]
- Fan, G.F.; Yu, Z.G.; Liang, Y.B.; Xu, Z.G.; Tang, J. Effect of pomegranate exaract.gallic aicd on the proliferation of prostate cancer cells by peomoting the expression of IGFBP7. Appl. Ecol. Environ. Res. 2020, 18, 6233–6241. [Google Scholar] [CrossRef]
- Batool, R.; Aziz, E.; Mahmood, T.; Tan, B.K.; Chow, V.T.K. Inhibitory activities of extracts of Rumex dentatus, Commelina benghalensis, Ajuga bracteosa, Ziziphus mauritiana as well as their compounds of gallic acid and emodin against dengue virus. Asian Pac. J. Trop. Med. 2018, 11, 265–271. [Google Scholar]
- Garcia-Mendoza, M.d.P.; Espinosa-Pardo, F.A.; Savoire, R.; Etchegoyen, C.; Harscoat-Schiavo, C.; Subra-Paternault, P. Recovery and antioxidant activity of phenolic compounds extracted from walnut press-cake using various methods and conditions. Ind. Crop. Prod. 2021, 167, 113546. [Google Scholar] [CrossRef]
- Bagheri, A.R.; Ghaedi, M. Application of Cu-based metal-organic framework (Cu-BDC) as a sorbent for dispersive solid-phase extraction of gallic acid from orange juice samples using HPLC-UV method. Arab. J. Chem. 2020, 13, 5218–5228. [Google Scholar] [CrossRef]
- Mahindrakar, K.V.; Rathod, V.K. Ultrasonic assisted aqueous extraction of catechin and gallic acid from Syzygium cumini seed kernel and evaluation of total phenolic, flavonoid contents and antioxidant activity. Chem. Eng. Process. 2020, 149, 107841. [Google Scholar] [CrossRef]
- Houng, P.; Murakami, Y.; Shimoyama, Y. Micro-mixing in flow-type process for supercritical CO2 extraction of ferulic acid and gallic acid from aqueous solution. J. CO2 Util. 2021, 47, 101503. [Google Scholar] [CrossRef]
- Khodaie, F.; Ghoreishi, S.M. Experimental extraction of gallic acid from brown sumac seed (Rhus coriaria) using supercritical carbon dioxide and ethanol as co-solvent: Modeling and optimization. J. Supercrit. Fluid 2021, 175, 105266. [Google Scholar] [CrossRef]
- Wulff, G.; Sarhan, A. Use of polymers with enzyme-analogous structures for resolution of racemates. Angrew. Chem. Internat. Edit. 1972, 11, 341. [Google Scholar]
- Janczura, M.; Lulinski, P.; Sobiech, M. Imprinting technology for effective sorbent fabrication: Current state-of-art and future prospects. Materials 2021, 14, 1850. [Google Scholar] [CrossRef]
- Marfa, J.; Pupin, R.; Sotomayor, R.; Pividori, M.I. Magnetic-molecularly imprinted polymers in electrochemical sensors and biosensors. Anal. Bioanal. Chem. 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Shi, H.; Han, Y.Y.; Ruixin, W.; Jiying, M. Molecularly imprinted polymers by the surface imprinting technique. Eur. Polym. J. 2021, 145, 110231. [Google Scholar] [CrossRef]
- Jalilian, N.; Ebrahimzadeh, H.; Asgharinezhad, A.A.; Khodayari, P. Magnetic molecularly imprinted polymer for the selective dispersive micro solid phase extraction of phenolphthalein in urine samples and herbal slimming capsules prior to HPLC-PDA analysis. Microchem. J. 2021, 160, 105712. [Google Scholar] [CrossRef]
- Song, H.J.; Zhang, H.P.; He, Y.L.; Gao, R.X.; Wang, Y.; Wang, W.T.; Pfefferle, L.D.; Tang, X.S.; Tang, Y.H. Novel bayberry-and-honeycomb-like magnetic surface molecularly imprinted polymers for the selective enrichment of rutin from Sophora Japonica. Food Chem. 2021, 356, 129722. [Google Scholar] [CrossRef] [PubMed]
- Song, X.Q.; Turiel, E.; He, L.M.; Martin-Esteban, A. Synthesis of molecularly imprinted polymers for the selective extraction of polymyxins from environmental water samples. Polymers 2020, 12, 131. [Google Scholar] [CrossRef] [Green Version]
- Ji, K.; Luo, X.; He, L.; Liao, S.; Hu, L.; Jingwen, H.; Can, C.; Yaqing, L.; Ni, T. Preparation of hollow magnetic molecularly imprinted polymer and its application in silybin recognition and controlled release. J. Pharmaceut. Biomed. 2020, 180, 113036. [Google Scholar] [CrossRef]
- Wang, A.; Lu, H.; Xu, S. Preparation of Magnetic Hollow Molecularly Imprinted Polymers for Detection of Triazines in Food Samples. J. Agric. Food Chem. 2016, 64, 5110. [Google Scholar] [CrossRef]
- Wu, M.; Fan, Y.J.; Li, J.W.; Lu, D.Q.; Guo, Y.P.; Xie, L.W.; Wu, Y.Q. Vinyl phosphate-functionalized, magnetic, molecularly-imprinted polymeric microspheres enrichment and carbon dots fluorescence-detection of organophosphorus pesticide residues. Polymers 2019, 11, 1770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, L.; Guo, J.; Zhang, Y.; Hu, Y.; You, Q.; Shi, S. Novel molecular imprinted polymers over magnetic mesoporous silica microspheres for selective and efficient determination of protocatechuic acid in Syzygium aromaticum. Food Chem. 2015, 178, 18–25. [Google Scholar] [CrossRef]
- Fan, D.; Li, H.; Shi, S.; Chen, X. Hollow molecular imprinted polymers towards rapid, effective and selective extraction of caffeic acid from fruits. J. Chromatogr. A 2016, 1470, 27–32. [Google Scholar] [CrossRef]
- Kargar, S.; Elhamifar, D.; Zarnegaryan, A. Core-shell structured Fe3O4@SiO2-supported IL/Mo6O19: A novel and magnetically recoverable nanocatalyst for the preparation of biologically active dihydropyrimidinones. J. Phys. Chem. Solids 2020, 146, 109601. [Google Scholar] [CrossRef]
- Qiu, L.; Jaria, G.; Gil, M.V.; Feng, J.D.; Dai, Y.D.; Esteves, V.I.; Otero, M.; Calisto, V. Core-shell molecularly imprinted polymers on magnetic yeast for the removal of sulfamethoxazole from water. Polymers 2020, 12, 109601. [Google Scholar] [CrossRef]
- Sardarian, A.R.; Kazemnejadi, M.; Esmaeilpour, M. Functionalization of superparamagnetic Fe3O4@SiO2 nanoparticles with a Cu (II) binuclear schiff base complex as an efficient and reusable nanomagnetic catalyst for N-arylation of alpha-amino acids and nitrogen-containing heterocycles with aryl halides. Appl. Organomet. Chem. 2021, 35, 6051. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Christa, J.; Shainy, F. Magnetic titanium dioxide embedded molecularly imprinted polymer nanocomposite for the degradation of diuron under visible light. React. Funct. Polym. 2020, 152, 104597. [Google Scholar] [CrossRef]
- Cai, X.; Huang, Q.; Hong, Z.; Zhang, Y.; Hu, H.; Huang, Z.; Liang, J.; Qin, Y. Cu anchored on manganese residue through mechanical activation to prepare a Fe-Cu@ SiO2/starch-derived carbon composites with highly stable and active visible light photocatalytic performance. J. Environ. Chem. Eng. 2021, 9, 104710. [Google Scholar] [CrossRef]
- Kiziltas, H.; Tekin, T. Synthesis, characterization of Fe3O4@SiO2@ZnO composite with a core-shell structure and evaluation of its photocatalytic activity. J. Environ. Chem. Eng. 2020, 8, 104160. [Google Scholar] [CrossRef]
- Wang, L.C.; Shen, C.Y.; Cao, Y.H. PVP modified Fe3O4@SiO2 nanoparticles as a new adsorbent for hydrophobic substances. J. Phys. Chem. Solids 2019, 133, 28–34. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Wang, H.M.; Wang, H.L.; Wu, C.C.; Li, M.L.; Li, L. Fabrication and evaluation of molecularly imprinted magnetic nanoparticles for selective recognition and magnetic separation of lysozyme in human urine. Analyst 2018, 143, 5849–5856. [Google Scholar] [CrossRef]
- Deng, Y.; Qi, D.; Deng, C.; Zhang, X.; Zhao, D. Superparamagnetic high-magnetization microspheres with an Fe3O4@SiO2 core and perpendicularly aligned mesoporous SiO2 shell for removal of microcystins. J. Am. Chem. Soc. 2008, 130, 28–29. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Pei, Y.; Hou, Y.; Pei, Z. Fabrication of core-shell magnetic molecularly imprinted nanospheres towards hypericin via click polymerization. Polymers 2019, 11, 313. [Google Scholar] [CrossRef] [Green Version]
- Brahmbhatt, H.A.; Surtees, A.; Tierney, C.; Ige, O.A.; Piletska, E.V.; Swift, T.; Turner, N.W. Effect of polymerisation by microwave on the physical properties of molecularly imprinted polymers (MIPs) specific for caffeine. Polym. Chem. 2020, 11, 5778–5789. [Google Scholar] [CrossRef]
- Guo, S.Z.; Zhang, F.; Li, D.; Jiao, P.P. Highly efficient and selective removal of cadmium from aqueous solutions based on magnetic graphitic carbon nitride materials with molecularly imprinted polymers. J. Mol. Struct. 2020, 1221, 128887. [Google Scholar] [CrossRef]
- Liu, Z.W.; Wang, Y.Z.; Xu, F.T.; Wei, X.X.; Chen, J.; Li, H.Q.; He, X.Y.; Zhou, Y.G. A new magnetic molecularly imprinted polymer based on deep eutectic solvents as functional monomer and cross-linker for specific recognition of bovine hemoglobin. Anal. Chim. Acta 2020, 1129, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.N.; Du, D.D.; Li, Q.N.; Chen, W.J.; Li, Q.C.; Zhang, Q.Z.; Liang, N. Dummy-surface molecularly imprinted polymers based on magnetic graphene oxide for selective extraction and quantification of pyrethroids pesticides in fruit juices. Microchem. J. 2020, 159, 105411. [Google Scholar] [CrossRef]
- Zhang, J.; Li, B.; Yue, H.; Wang, J.; Zheng, Y. Highly selective and efficient imprinted polymers based on carboxyl-functionalized magnetic nanoparticles for the extraction of gallic acid from pomegranate rind. Adv. Polym. Technol. 2018, 41, 540–547. [Google Scholar] [CrossRef]
- Fu, H.; Liu, J.P.; Xu, W.; Wang, H.X.; Liao, S.H.; Chen, G.T. A new type of magnetic molecular imprinted material combined with beta-cyclodextrin for the selective adsorption of zearalenone. J. Mater. Chem. B 2020, 8, 10966–10976. [Google Scholar] [CrossRef]
- Tian, D.T.; Zhou, Y.C.; Xiong, L.; Lu, F.T. Synthesis and properties of caffeine molecularly imprinted polymers based on konjac glucomannan. Adv. Polym. Technol. 2017, 36, 68–76. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.X.; He, J.; Song, L.X.; Wang, H.G.; Huang, Z.P.; Sun, Q.Y.; Ba, X.; Li, L.Y.Y.; You, Q.; Zhang, S.S. Application of surface-imprinted polymers supported by hydroxyapatite in the extraction of zearalenone in various cereals. Anal. Bioanal. Chem. 2020, 412, 4045–4055. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Rwo, K.H. Hydrophilic molecularly imprinted chitosan based on deep eutectic solvents for the enrichment of gallic acid in red ginseng tea. Polymers 2019, 11, 1434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Zhou, X.; Yan, Y.; Shen, D.; Lu, D.; Guo, Y.; Xie, L.; Deng, B. Selective Recognition of Gallic Acid Using Hollow Magnetic Molecularly Imprinted Polymers with Double Imprinting Surfaces. Polymers 2022, 14, 175. https://doi.org/10.3390/polym14010175
Li J, Zhou X, Yan Y, Shen D, Lu D, Guo Y, Xie L, Deng B. Selective Recognition of Gallic Acid Using Hollow Magnetic Molecularly Imprinted Polymers with Double Imprinting Surfaces. Polymers. 2022; 14(1):175. https://doi.org/10.3390/polym14010175
Chicago/Turabian StyleLi, Jiawei, Xinji Zhou, Yu Yan, Dianling Shen, Danqing Lu, Yaping Guo, Lianwu Xie, and Bin Deng. 2022. "Selective Recognition of Gallic Acid Using Hollow Magnetic Molecularly Imprinted Polymers with Double Imprinting Surfaces" Polymers 14, no. 1: 175. https://doi.org/10.3390/polym14010175






