Probing the Nanoscale Heterogeneous Mixing in a High-Performance Polymer Blend
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation and Handling
2.2. AFM-IR and nanoTA Measurements
2.3. FTIR Spectra
2.4. Similarity Index Calculations
3. Results and Discussion
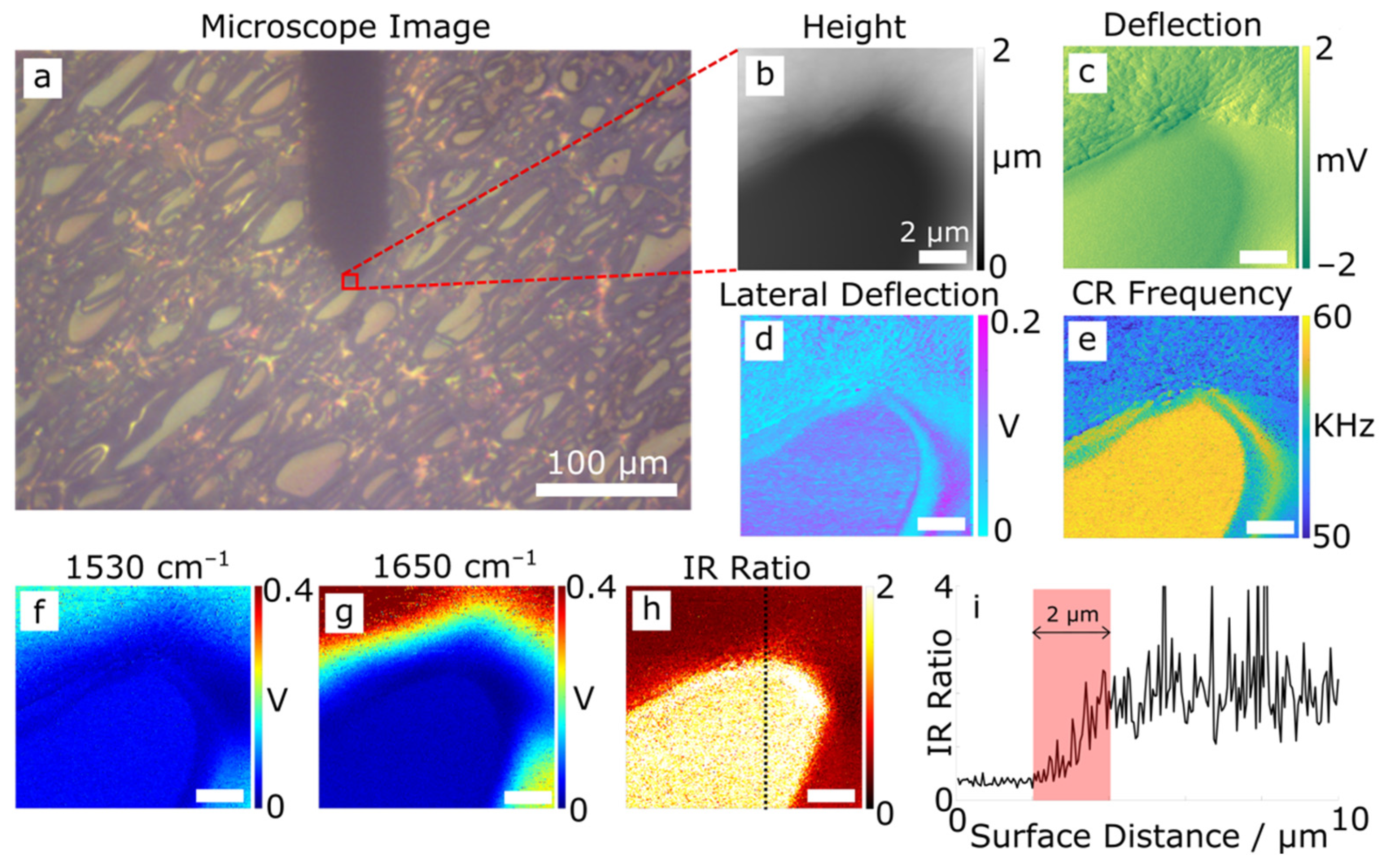
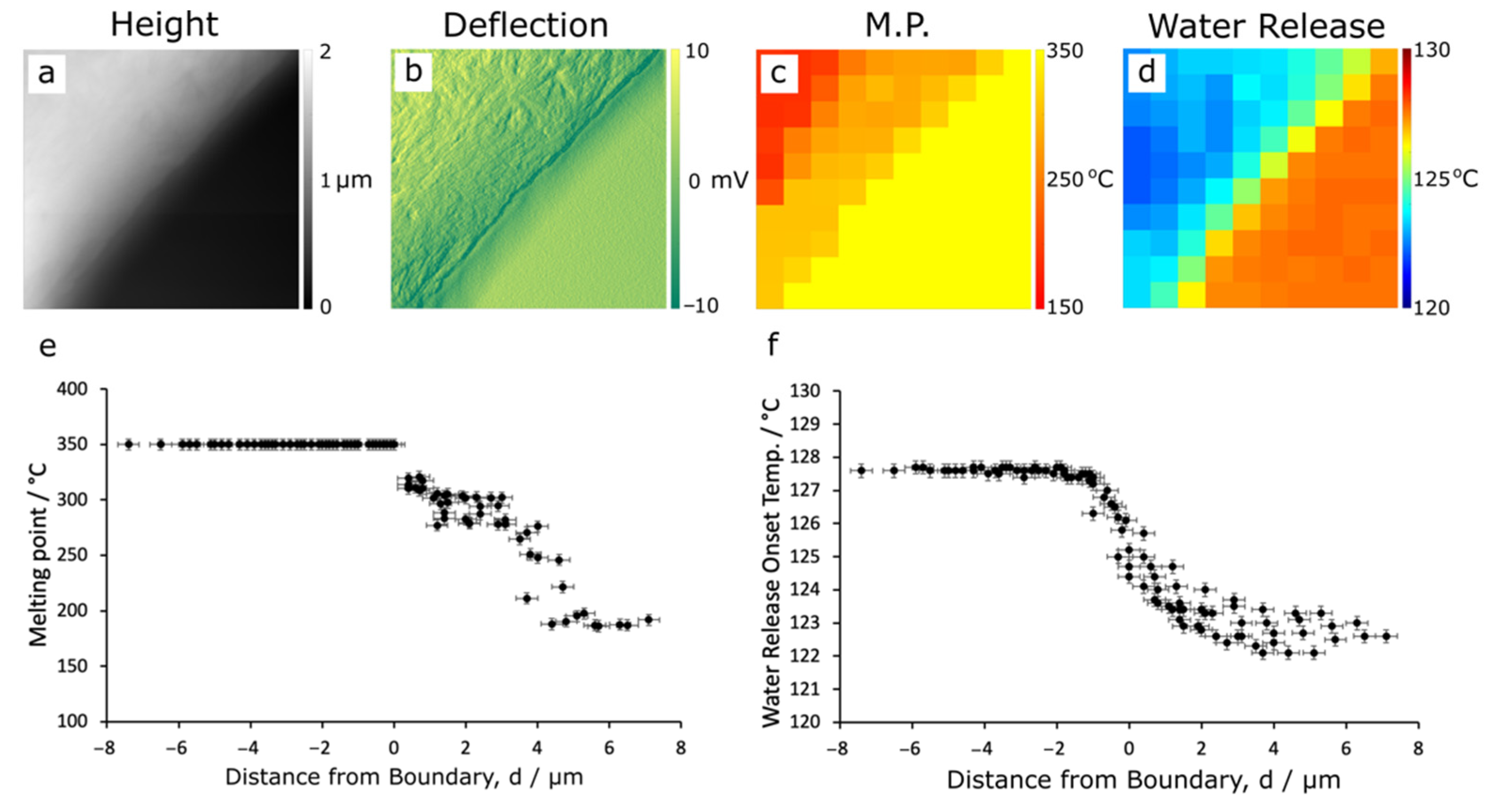
3.1. AFM and Infrared Nanospectroscopy
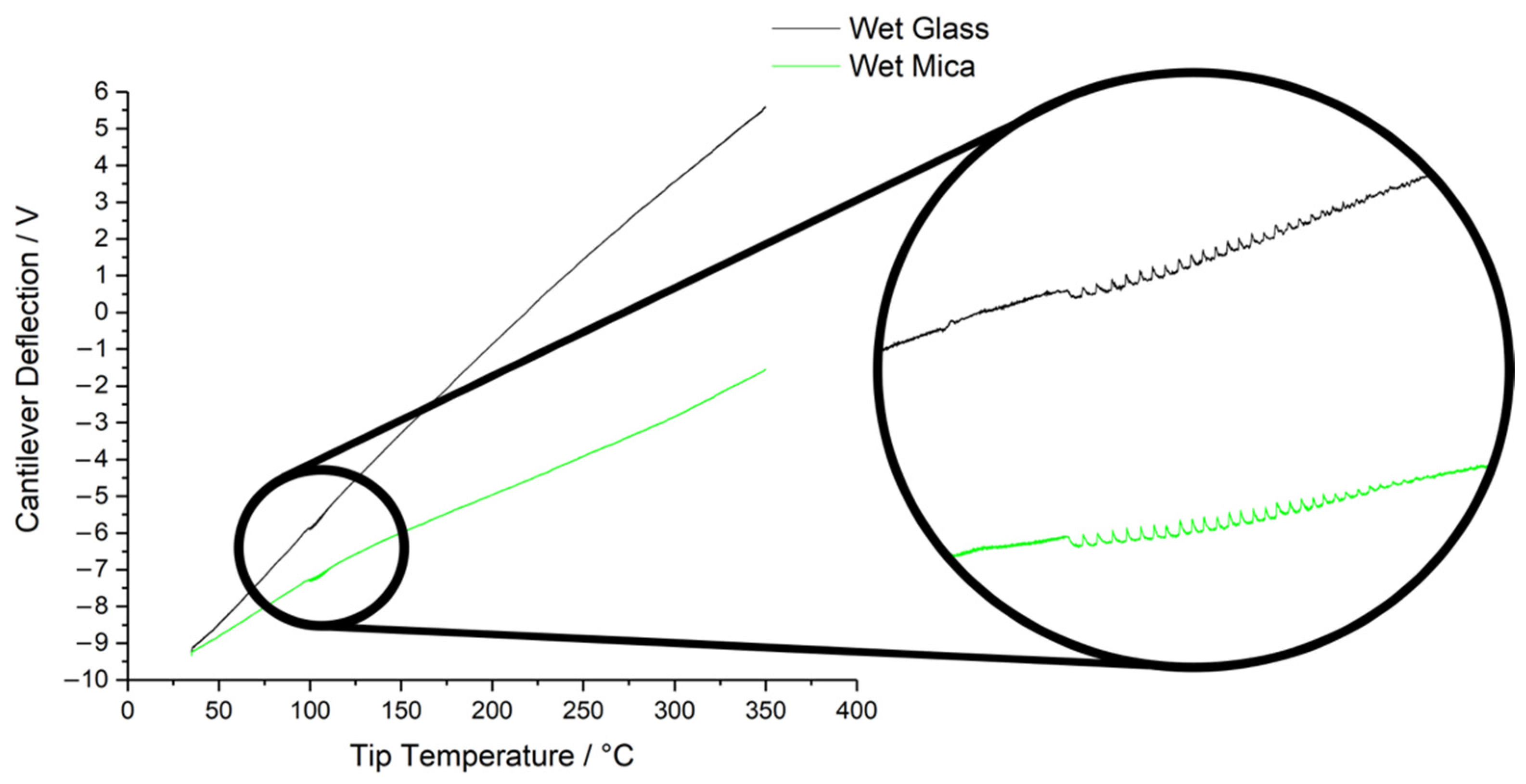
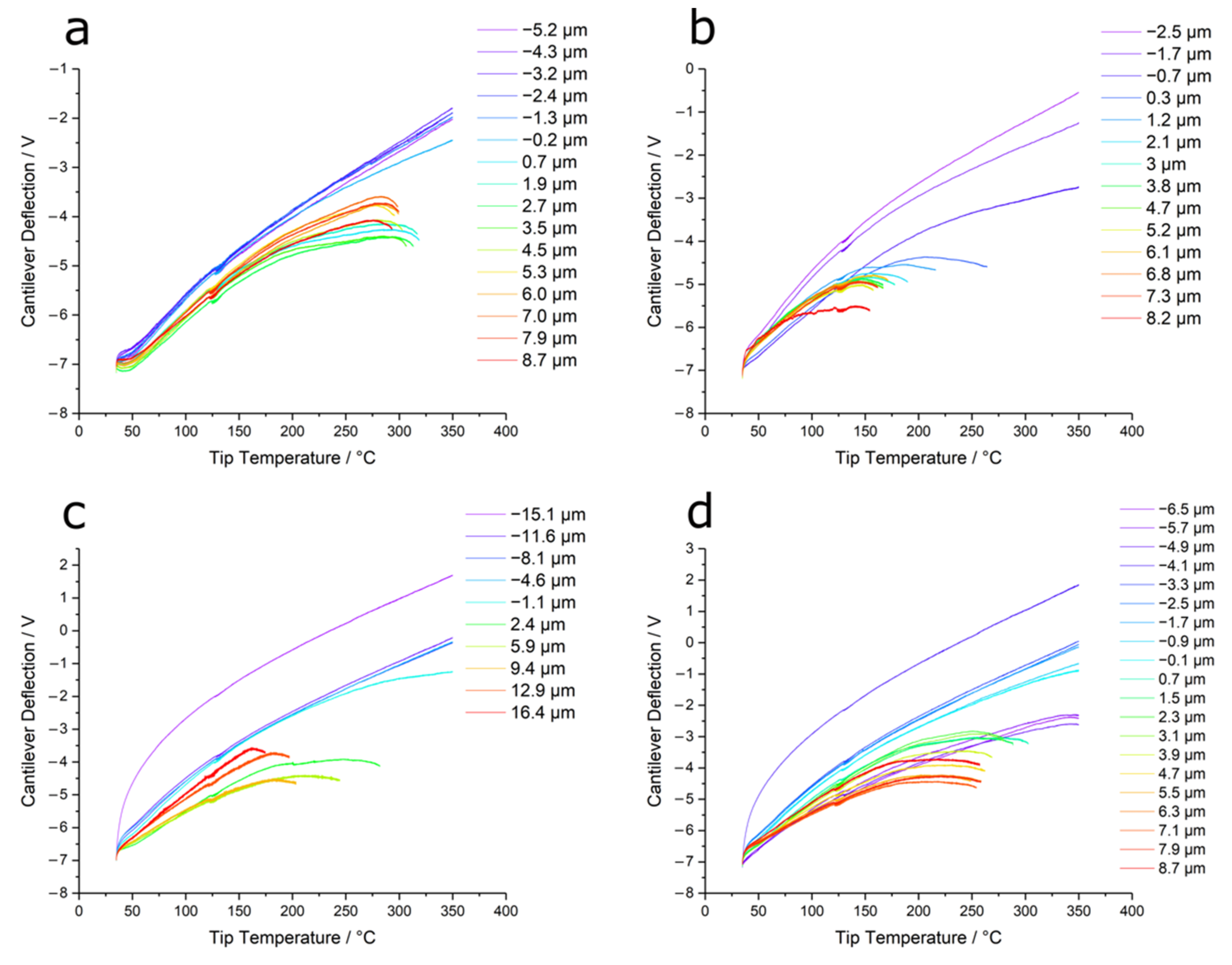
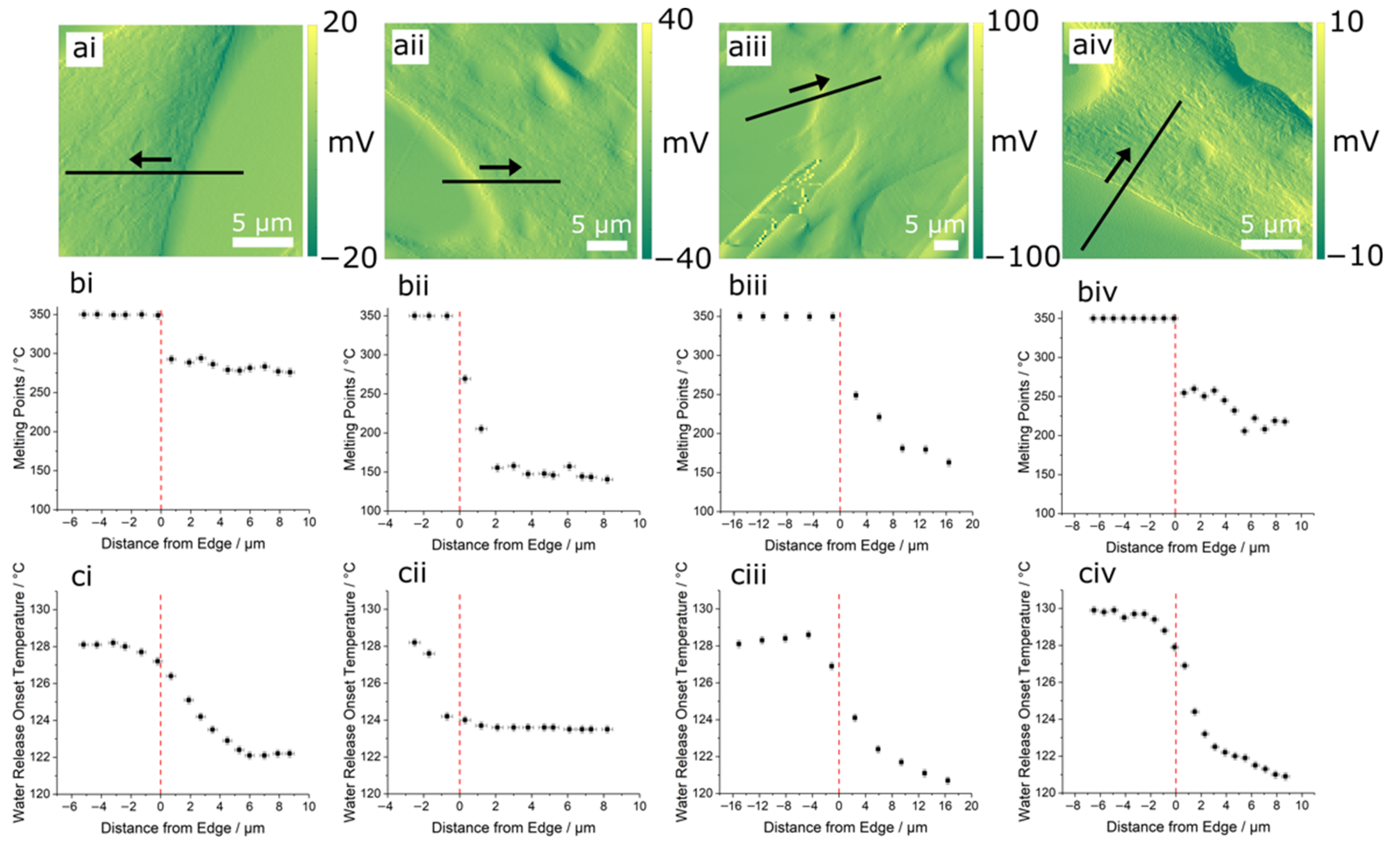
3.2. Thermal Nanomicroscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Band/cm−1 | Assignment | |
|---|---|---|
| PEEK | 1155 | Aromatic in-plane C-H deformation |
| 1190 | Asymmetric C-O-C stretching | |
| 1215 | Aromatic in-plane C-H deformation | |
| 1277 | Asymmetric C-O-C stretching | |
| 1305 | Ketone bend vibration | |
| 1410 | Skeletal ring vibration | |
| 1485 | Skeletal ring vibration | |
| 1500 | Skeletal ring vibration | |
| 1593 | Skeletal ring vibration | |
| 1653 | C=O Ketone stretching | |
| PBI | 1215–1235 | In-plane C-H deformation |
| 1277–1287 | Ring breathing mode | |
| 1395–1528 | In-plane ring deformation | |
| 1590–1650 | C=N/C=C stretching |






References
- Jean-Fulcrand, A.; Masen, M.A.; Bremner, T.; Wong, J.S.S. Effect of temperature on tribological performance of polyetheretherketone-polybenzimidazole blend. Tribol. Int. 2019, 129, 5–15. [Google Scholar] [CrossRef]
- Jean-Fulcrand, A.; Masen, M.A.; Bremner, T.; Wong, J.S.S. High temperature tribological properties of polybenzimidazole (PBI). Polymer 2017, 128, 159–168. [Google Scholar] [CrossRef]
- Hafiz Muhammad Saleem, I. Performance Evaluation of Polybenzimidazole for Potential Aerospace Applications; Technische Universiteit Delft: Delft, The Netherlands, 2014. [Google Scholar]
- Puhan, D.; Wong, J.S.S. Properties of Polyetheretherketone (PEEK) transferred materials in a PEEK-steel contact. Tribol. Int. 2019, 135, 189–199. [Google Scholar] [CrossRef]
- Bijwe, J.; Gupta, M.K.; Parida, T.; Trivedi, P. Design and development of advanced polymer composites as high performance tribo-materials based on blends of PEK and ABPBI. Wear 2015, 342–343, 65–76. [Google Scholar] [CrossRef]
- Puhan, D.; Jiang, S.; Wong, J.S.S. Effect of carbon fiber inclusions on polymeric transfer film formation on steel. Compos. Sci. Technol. 2022, 217, 109084. [Google Scholar] [CrossRef]
- Kurdi, A.; Kan, W.H.; Chang, L. Tribological behaviour of high performance polymers and polymer composites at elevated temperature. Tribol. Int. 2019, 130, 94–105. [Google Scholar] [CrossRef]
- Liu, P.; Mullins, M.; Bremner, T.; Benner, N.; Sue, H.J. Interfacial Phenomena and Mechanical Behavior of Polyetheretherketone/Polybenzimidazole Blend under Hygrothermal Environment. J. Phys. Chem. B 2017, 121, 5396–5406. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, X.; Zhao, C.; Fu, T.; Shi, Y.; Na, H. Composite membranes based on highly sulfonated PEEK and PBI: Morphology characteristics and performance. J. Memb. Sci. 2008, 308, 66–74. [Google Scholar] [CrossRef]
- Celazole® PBI U-60 Typical Properties. Available online: https://pbipolymer.com/wp-content/uploads/2016/05/Celazole-PBI-U-60-Typical-Properties.pdf (accessed on 17 March 2019).
- VICTREX® PEEK 650G. Available online: https://www.victrex.com/~/media/datasheets/victrex_tds_650g.pdf (accessed on 14 May 2018).
- Celazole® TU-60 Unreinforced Melt Processable PBI. Available online: https://pbipolymer.com/wp-content/uploads/2016/05/Celazole-PBI-TU-60-Typical-Properties.pdf (accessed on 17 March 2019).
- Adedeji, A.; Lyu, S.; Macosko, W. Block copolymers in homopolymer blends: Interface vs micelles. Macromolecules 2001, 34, 8663–8668. [Google Scholar] [CrossRef]
- Noolandi, J.; Hong, K.M. Interfacial Properties of Immiscible Homopolymer Blends in the Presence of Block Copolymers. Macromolecules 1982, 15, 482–492. [Google Scholar] [CrossRef]
- Nguyen-Tri, P.; Ghassemi, P.; Carriere, P.; Nanda, S.; Assadi, A.A.; Nguyen, D.D. Recent applications of advanced atomic force microscopy in polymer science: A review. Polymers 2020, 12, 1142. [Google Scholar] [CrossRef] [PubMed]
- Resch, J.; Dreier, J.; Bonten, C.; Kreutzbruck, M. Miscibility and phase separation in pmma/san blends investigated by nanoscale afm-ir. Polymers 2021, 13, 3809. [Google Scholar] [CrossRef]
- Handschuh-Wang, S.; Wang, T.; Zhou, X. Recent advances in hybrid measurement methods based on atomic force microscopy and surface sensitive measurement techniques. RSC Adv. 2017, 7, 47464–47499. [Google Scholar] [CrossRef] [Green Version]
- Dazzi, A.; Prater, C.B.; Hu, Q.; Chase, D.B.; Rabolt, J.F.; Marcott, C. AFM-IR: Combining atomic force microscopy and infrared spectroscopy for nanoscale chemical characterization. Appl. Spectrosc. 2012, 66, 1365–1384. [Google Scholar] [CrossRef] [Green Version]
- Dazzi, A.; Prater, C.B. AFM-IR: Technology and applications in nanoscale infrared spectroscopy and chemical imaging. Chem. Rev. 2017, 117, 5146–5173. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Tri, P.; Prud’homme, R.E. Nanoscale analysis of the photodegradation of polyester fibers by AFM-IR. J. Photochem. Photobiol. A Chem. 2019, 371, 196–204. [Google Scholar] [CrossRef]
- Tang, F.; Bao, P.; Su, Z. Analysis of Nanodomain Composition in High-Impact Polypropylene by Atomic Force Microscopy-Infrared. Anal. Chem. 2016, 88, 4926–4930. [Google Scholar] [CrossRef]
- Morsch, S.; Van Driel, B.A.; Van Den Berg, K.J.; Dik, J. Investigating the Photocatalytic Degradation of Oil Paint using ATR-IR and AFM-IR. ACS Appl. Mater. Interfaces 2017, 9, 10169–10179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fellows, A.P.; Casford, M.T.L.; Davies, P.B. Infrared Nanospectroscopy of Air-Sensitive Biological Substrates Protected by Thin Hydrogel Films. Biophys. J. 2020, 119, 1474–1480. [Google Scholar] [CrossRef]
- Goussous, S.A.; Fellows, A.P.; Casford, M.T.L.; Davies, P.B. A time domain study of surfactin penetrating a phospholipid monolayer at the air-water interface investigated using sum frequency generation spectroscopy, infrared reflection absorption spectroscopy, and AFM-nano infrared microscopy. Biochim. Biophys. Acta-Biomembr. 2019, 1861, 1568–1578. [Google Scholar] [CrossRef]
- Fellows, A.P.; Puhan, D.; Casford, M.T.L.; Davies, P.B. Understanding the Lubrication Mechanism of Poly(vinyl alcohol) Hydrogels using Infrared Nanospectroscopy. J. Phys. Chem. C 2020, 124, 18091–18101. [Google Scholar] [CrossRef]
- Fellows, A.P.; Casford, M.T.L.; Davies, P.B. Nanoscale Molecular Characterization of Hair Cuticle Cells Using Integrated Atomic Force Microscopy–Infrared Laser Spectroscopy. Appl. Spectrosc. 2020, 74, 1540–1550. [Google Scholar] [CrossRef]
- Fellows, A.P.; Casford, M.T.L.; Davies, P.B.; Gibson, J.S.; Brewin, J.N.; Rees, D.C. Nanoscale adhesion profiling and membrane characterisation in sickle cell disease using hybrid atomic force microscopy-IR spectroscopy. Colloids Surfaces B Biointerfaces 2021, 197, 111383. [Google Scholar] [CrossRef]
- Perez-Guaita, D.; Kochan, K.; Batty, M.; Doerig, C.; Garcia-Bustos, J.; Espinoza, S.; McNaughton, D.; Heraud, P.; Wood, B.R. Multispectral Atomic Force Microscopy-Infrared Nano-Imaging of Malaria Infected Red Blood Cells. Anal. Chem. 2018. [Google Scholar] [CrossRef]
- Ruggeri, F.S.; Šneideris, T.; Chia, S.; Vendruscolo, M.; Knowles, T.P.J. Characterizing individual protein aggregates by infrared nanospectroscopy and atomic force microscopy. J. Vis. Exp. 2019, 2019, e60108. [Google Scholar] [CrossRef] [Green Version]
- Fellows, A.P.; Casford, M.T.L.; Davies, P.B. Using hybrid atomic force microscopy and infrared spectroscopy (AFM-IR) to identify chemical components of the hair medulla on the nanoscale. J. Microsc. 2021, 284, 189–202. [Google Scholar] [CrossRef]
- Meng, J.; Levina, M.; Rajabi-Siahboomi, A.R.; Round, A.N.; Reading, M.; Craig, D.Q.M. The development of thermal nanoprobe methods as a means of characterizing and mapping plasticizer incorporation into ethylcellulose films. Pharm. Res. 2012, 29, 2128–2138. [Google Scholar] [CrossRef]
- Fellows, A.P.; Casford, M.T.L.; Davies, P.B. Spectral Analysis and Deconvolution of the Amide I Band of Proteins Presenting with High-Frequency Noise and Baseline Shifts. Appl. Spectrosc. 2020, 74, 597–615. [Google Scholar] [CrossRef]
- Chang, L.; Zhang, G.; Wang, H.; Fu, K. Comparative Study on the Wear Behaviour of Two High-Temperature-Resistant Polymers. Tribol. Lett. 2017, 65, 34. [Google Scholar] [CrossRef]
- Kumar, B.S.; Sana, B.; Unnikrishnan, G.; Jana, T.; Santhosh Kumar, K.S. Polybenzimidazole co-polymers: Their synthesis, morphology and high temperature fuel cell membrane properties. Polym. Chem. 2020, 11, 1043–1054. [Google Scholar] [CrossRef]
- Bellamy, L.J. Aromatic Compounds. In The Infra-red Spectra of Complex Molecules; Springer Netherlands: London, UK, 1975; pp. 72–104. [Google Scholar]
- Pope, J.C.; Sue, H.J.; Bremner, T.; Blümel, J. High-temperature steam-treatment of PBI, PEEK, and PEKK polymers with H2O and D2O: A solid-state NMR study. Polymer 2014, 55, 4577–4585. [Google Scholar] [CrossRef]
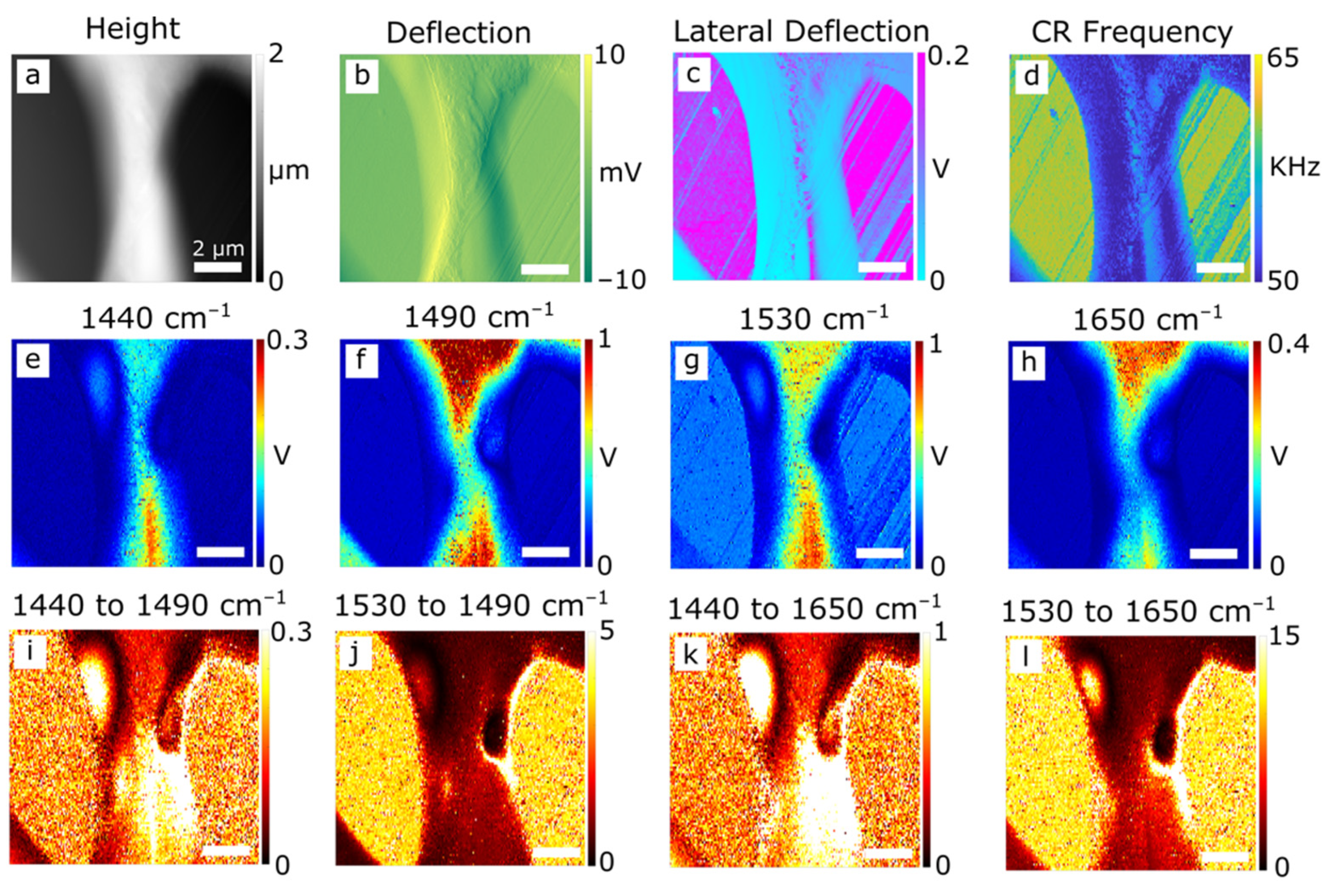
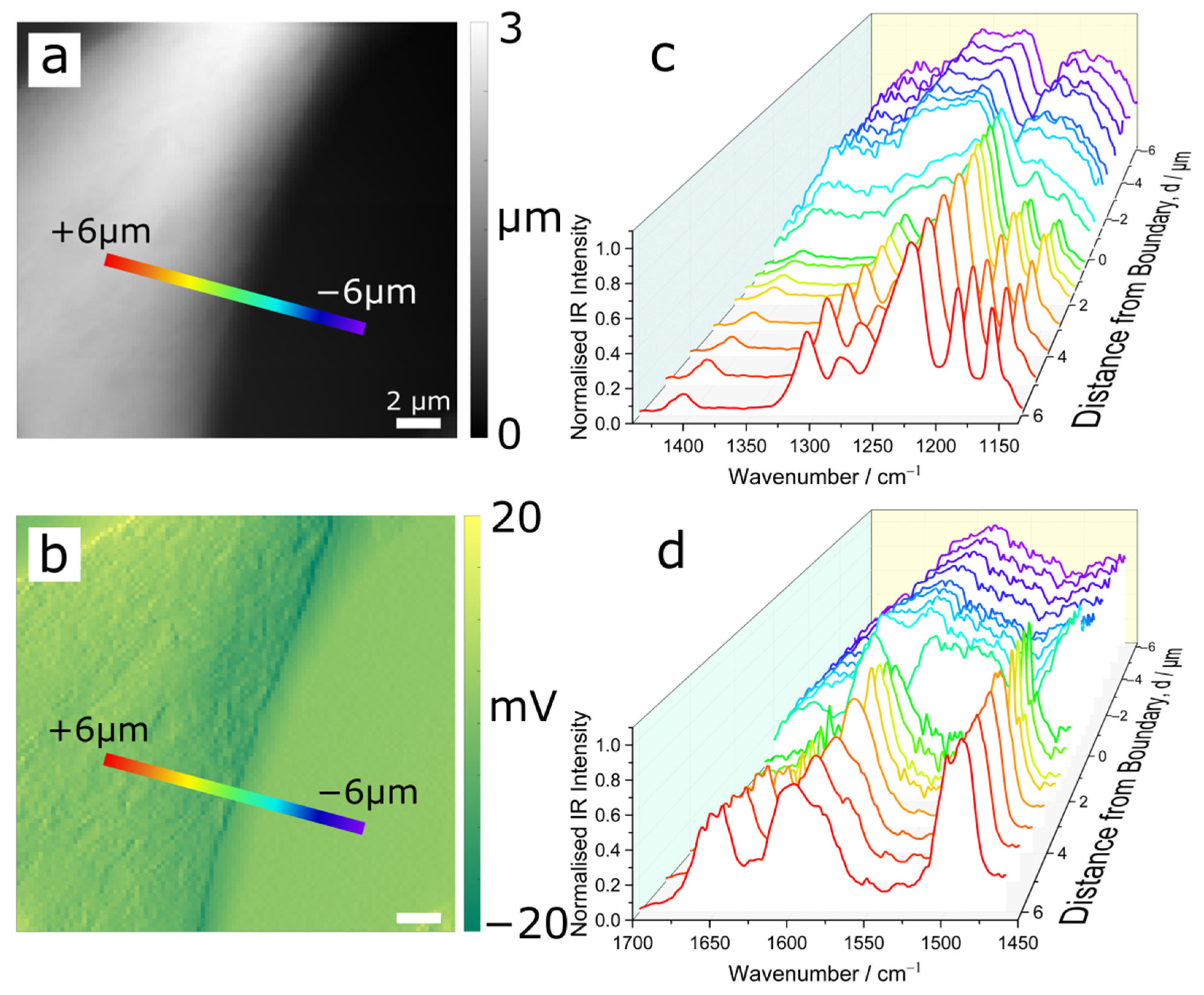
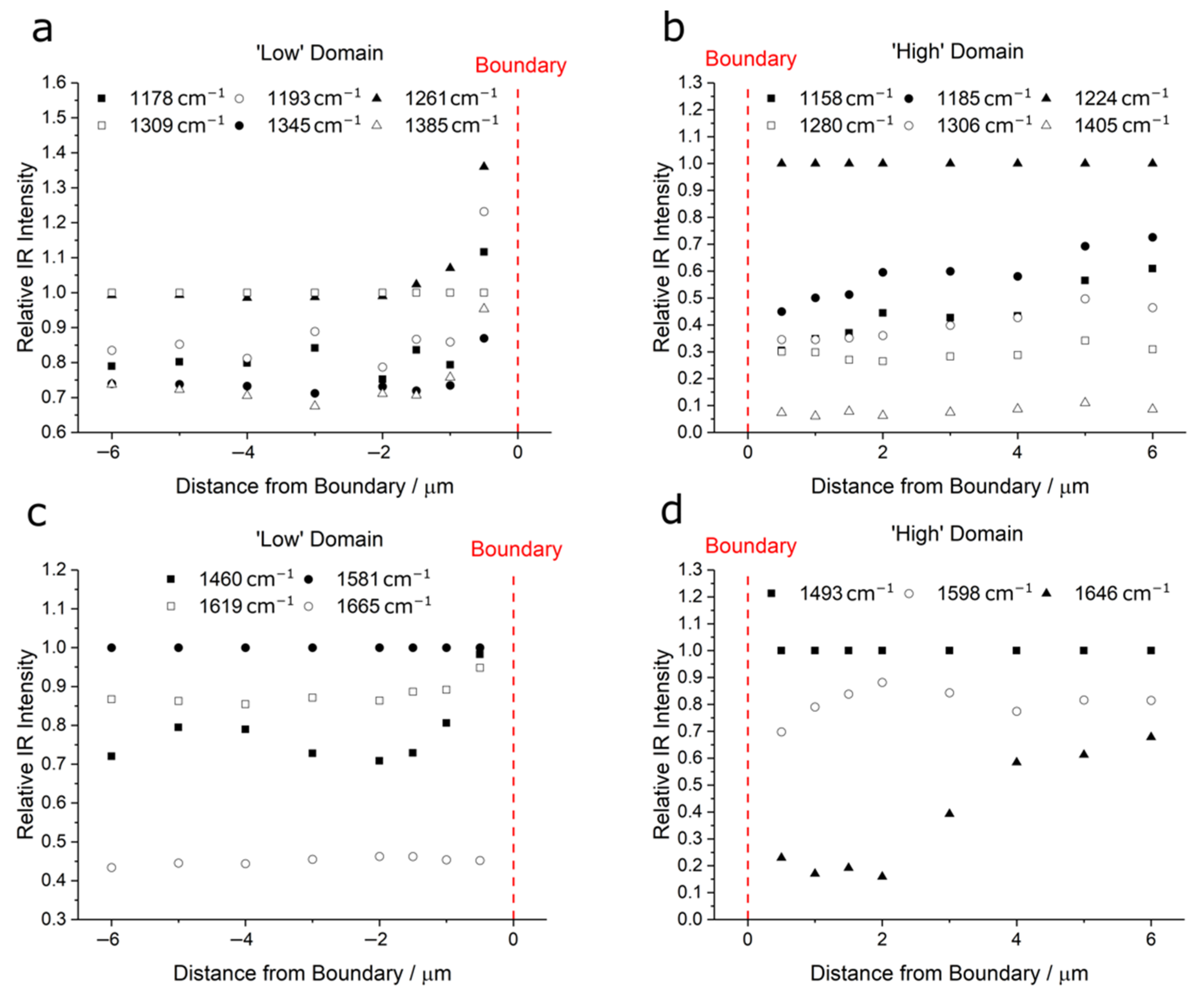

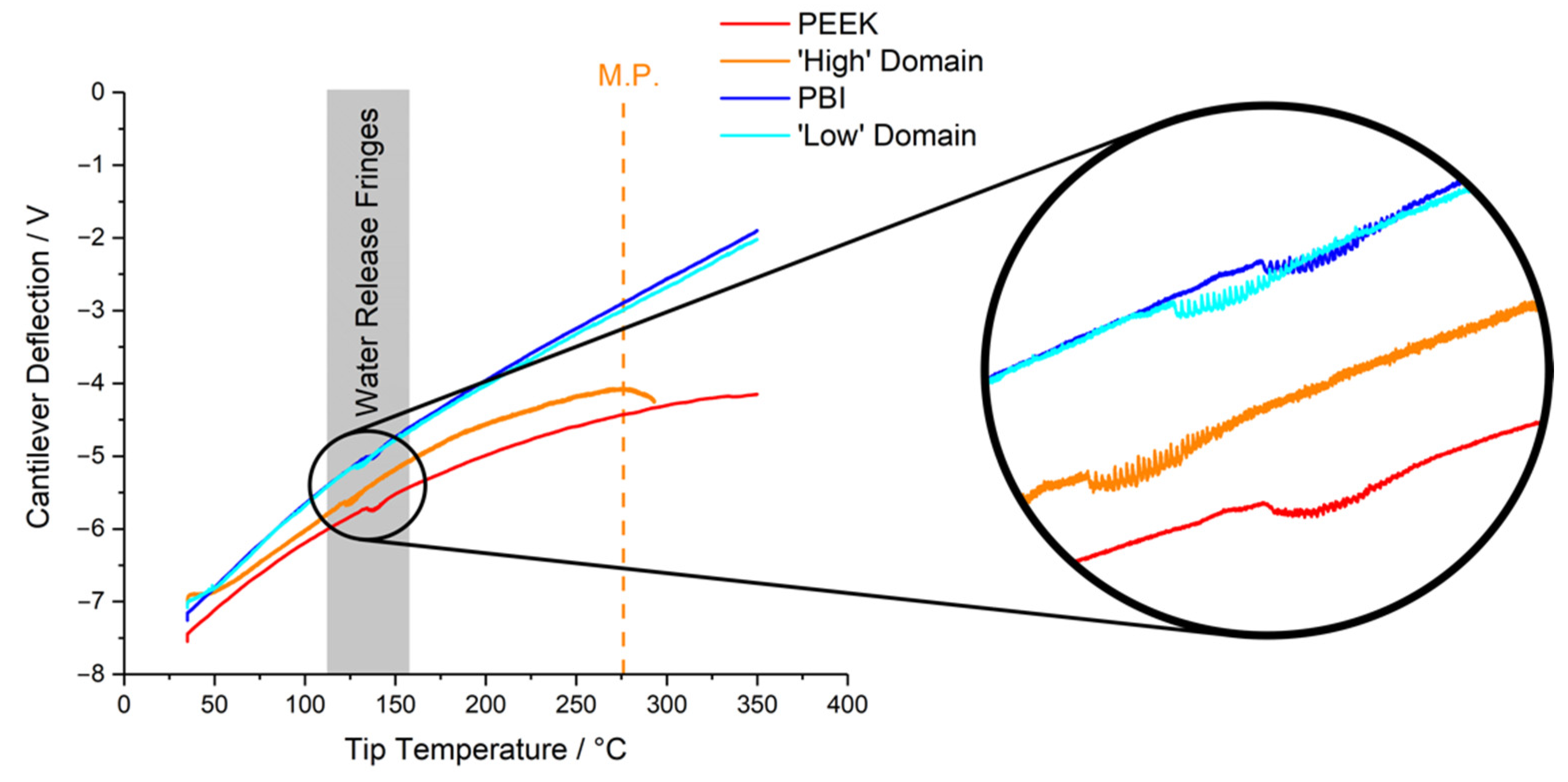
| PEEK | PBI | PPB | |
|---|---|---|---|
| PEEK | 1 | - | - |
| PBI | 0.77 ± 0.04 | 1 | - |
| PPB | 0.94 ± 0.03 | 0.83 ± 0.04 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fellows, A.P.; Puhan, D.; Wong, J.S.S.; Casford, M.T.L.; Davies, P.B. Probing the Nanoscale Heterogeneous Mixing in a High-Performance Polymer Blend. Polymers 2022, 14, 192. https://doi.org/10.3390/polym14010192
Fellows AP, Puhan D, Wong JSS, Casford MTL, Davies PB. Probing the Nanoscale Heterogeneous Mixing in a High-Performance Polymer Blend. Polymers. 2022; 14(1):192. https://doi.org/10.3390/polym14010192
Chicago/Turabian StyleFellows, Alexander Paul, Debashis Puhan, Janet S. S. Wong, Michael T. L. Casford, and Paul B. Davies. 2022. "Probing the Nanoscale Heterogeneous Mixing in a High-Performance Polymer Blend" Polymers 14, no. 1: 192. https://doi.org/10.3390/polym14010192
APA StyleFellows, A. P., Puhan, D., Wong, J. S. S., Casford, M. T. L., & Davies, P. B. (2022). Probing the Nanoscale Heterogeneous Mixing in a High-Performance Polymer Blend. Polymers, 14(1), 192. https://doi.org/10.3390/polym14010192






