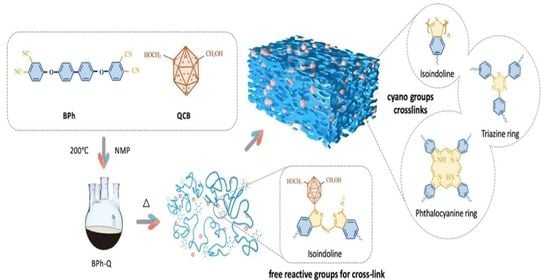
Catalytic Polymerization of Phthalonitrile Resins by Carborane with Enhanced Thermal Oxidation Resistance: Experimental and Molecular Simulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
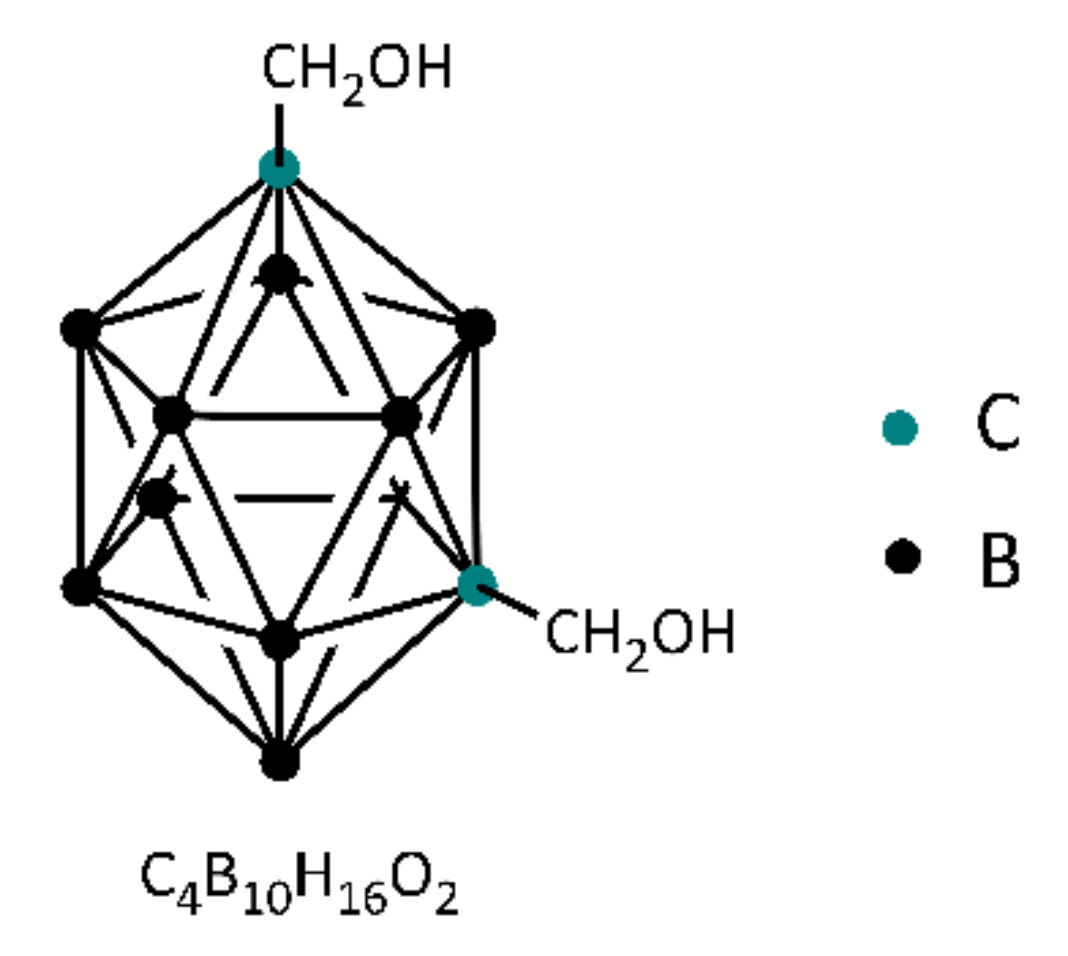
2.2. Synthesis of BPh-Q Prepolymer
2.3. Synthesis of BPh-B Prepolymer
2.4. Preparation of Thermosets
2.5. Characterization
2.6. Molecular Simulation
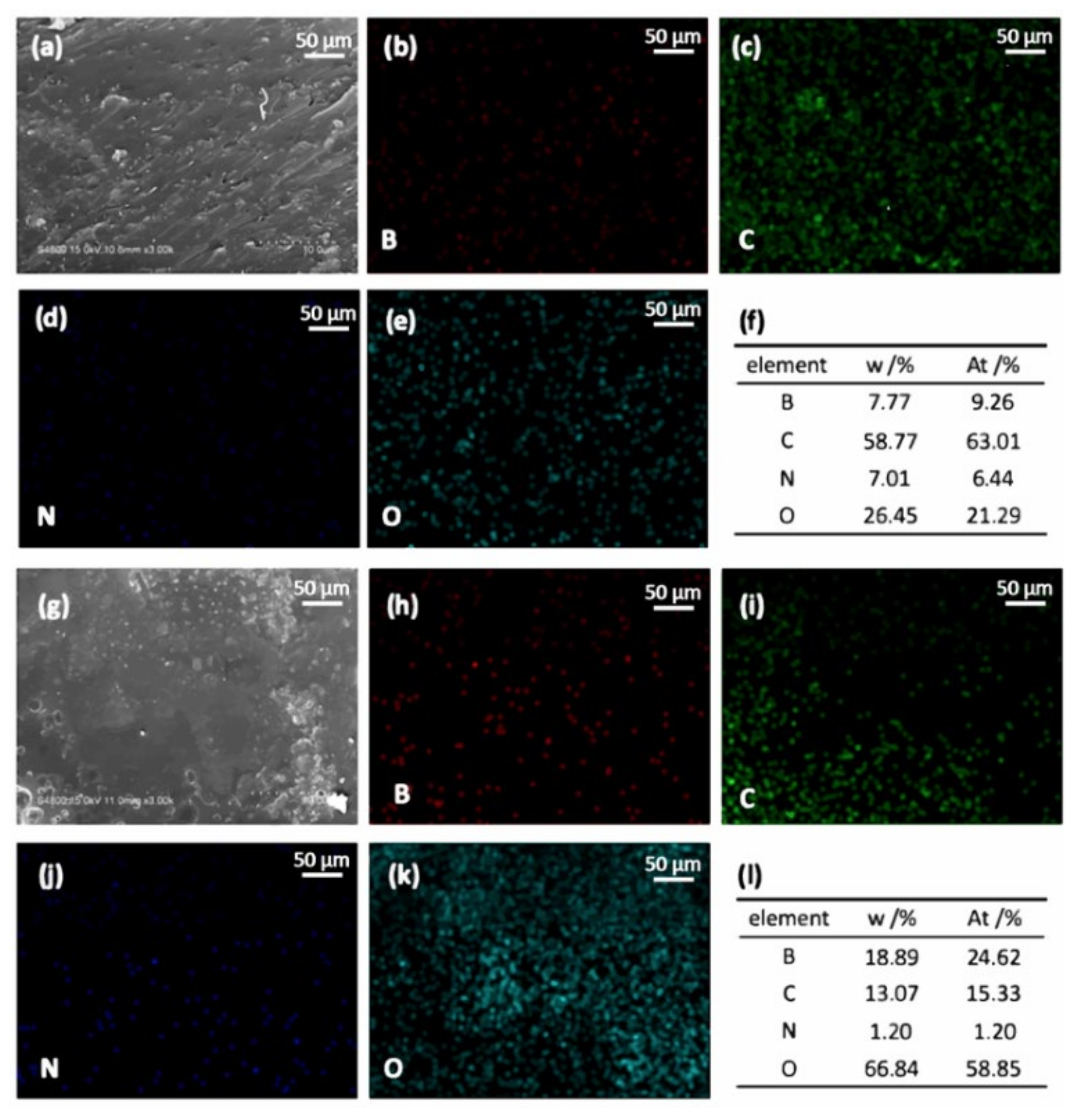
3. Results
3.1. Solubility Investigation of BPh-Q and BPh-B Prepolymers
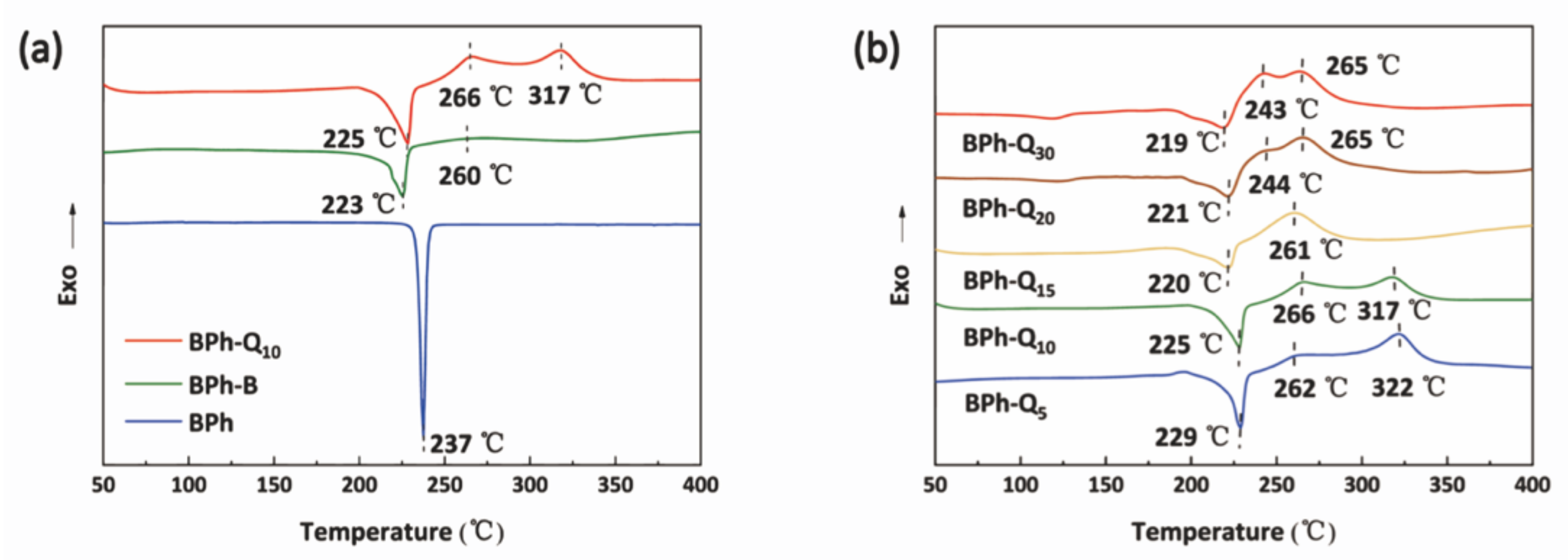
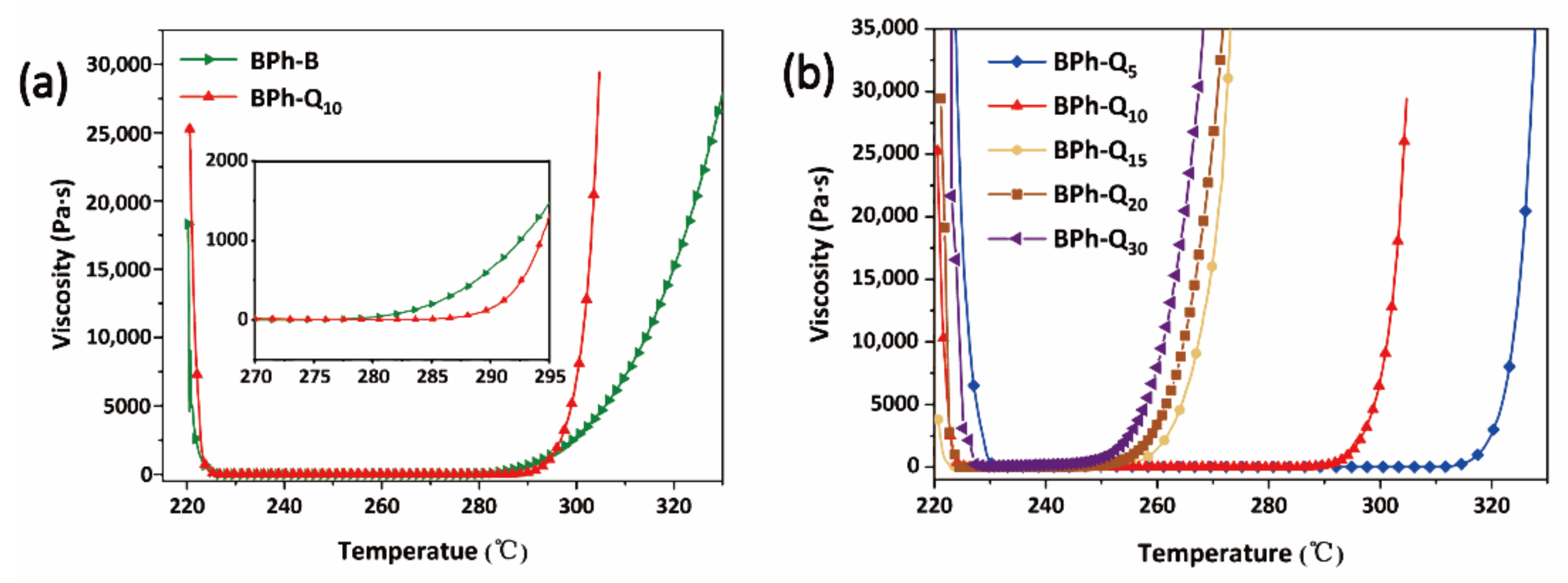
3.2. Curing Behavior of BPh-Q and BPh-B Prepolymers
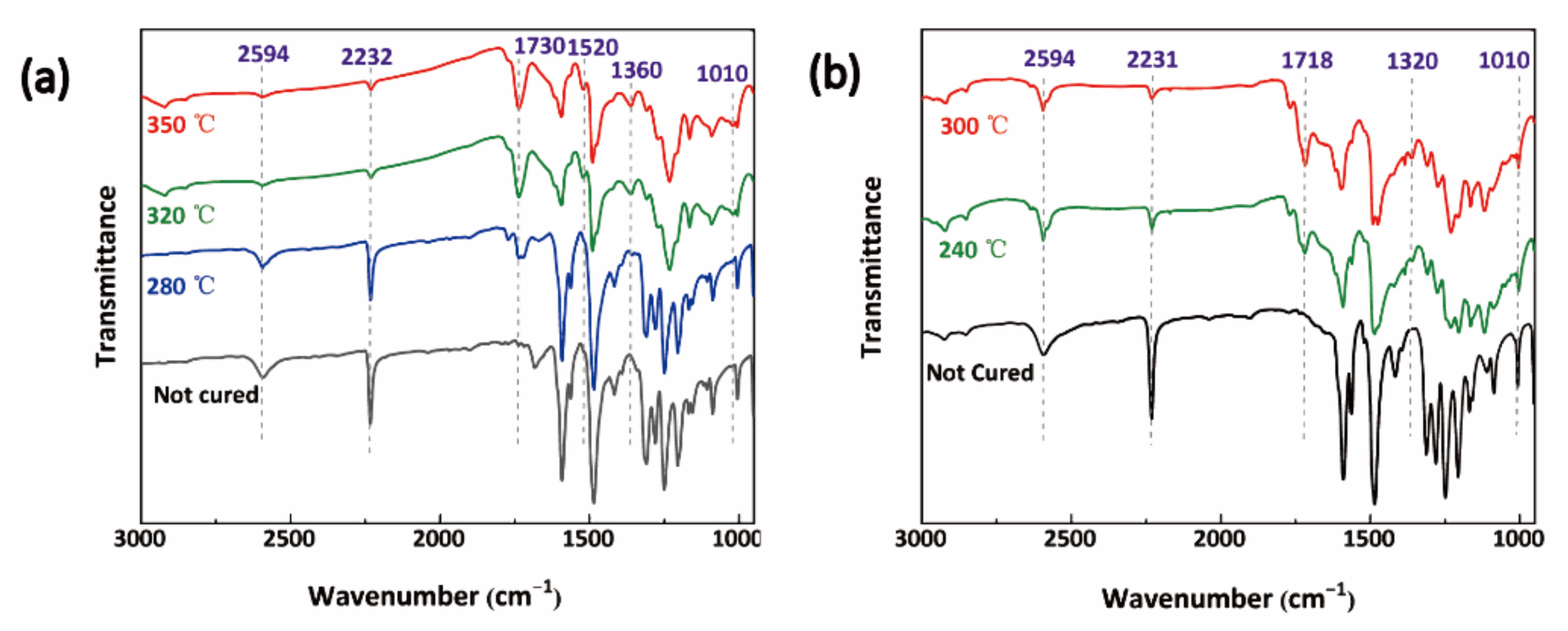
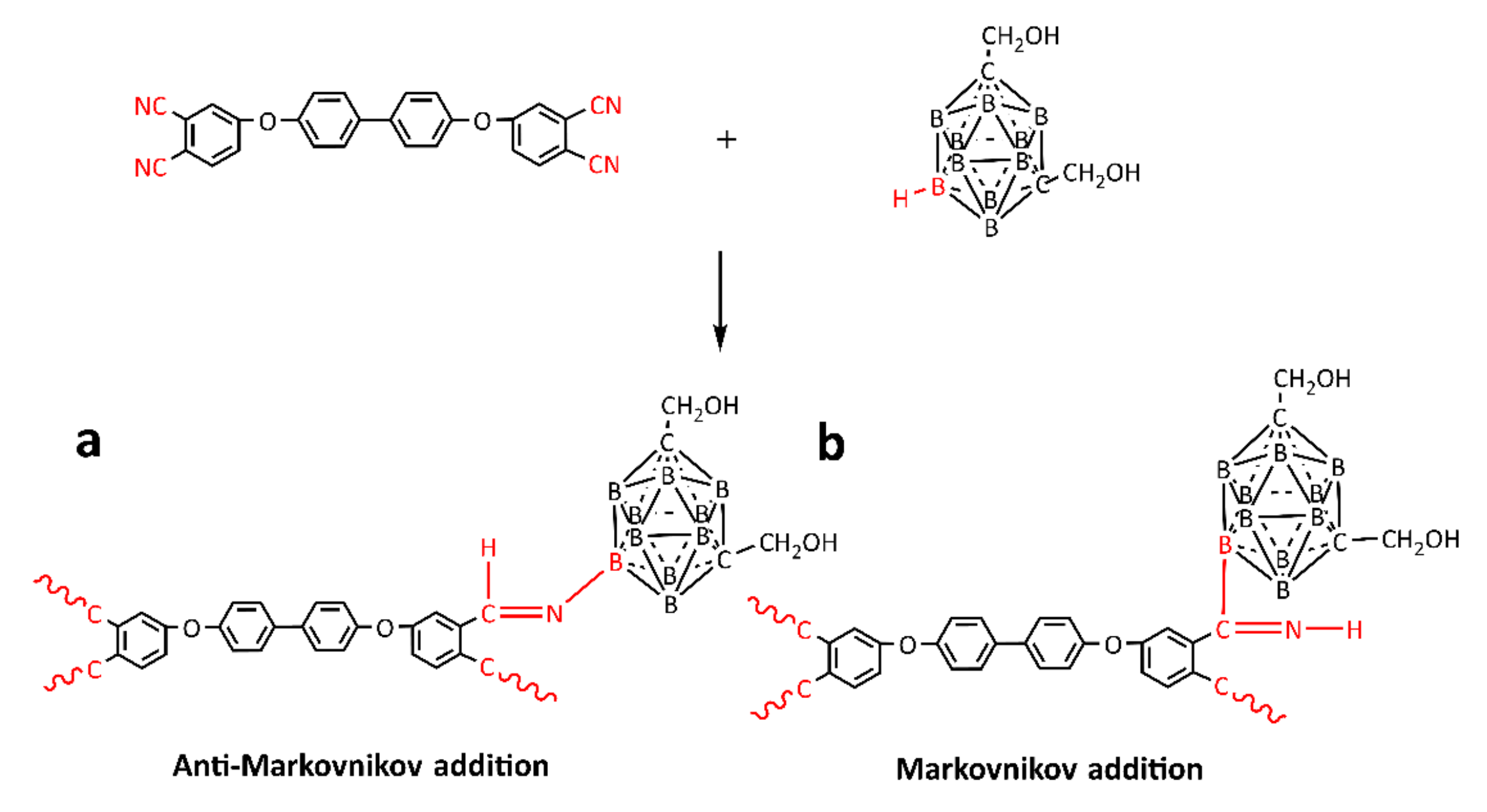
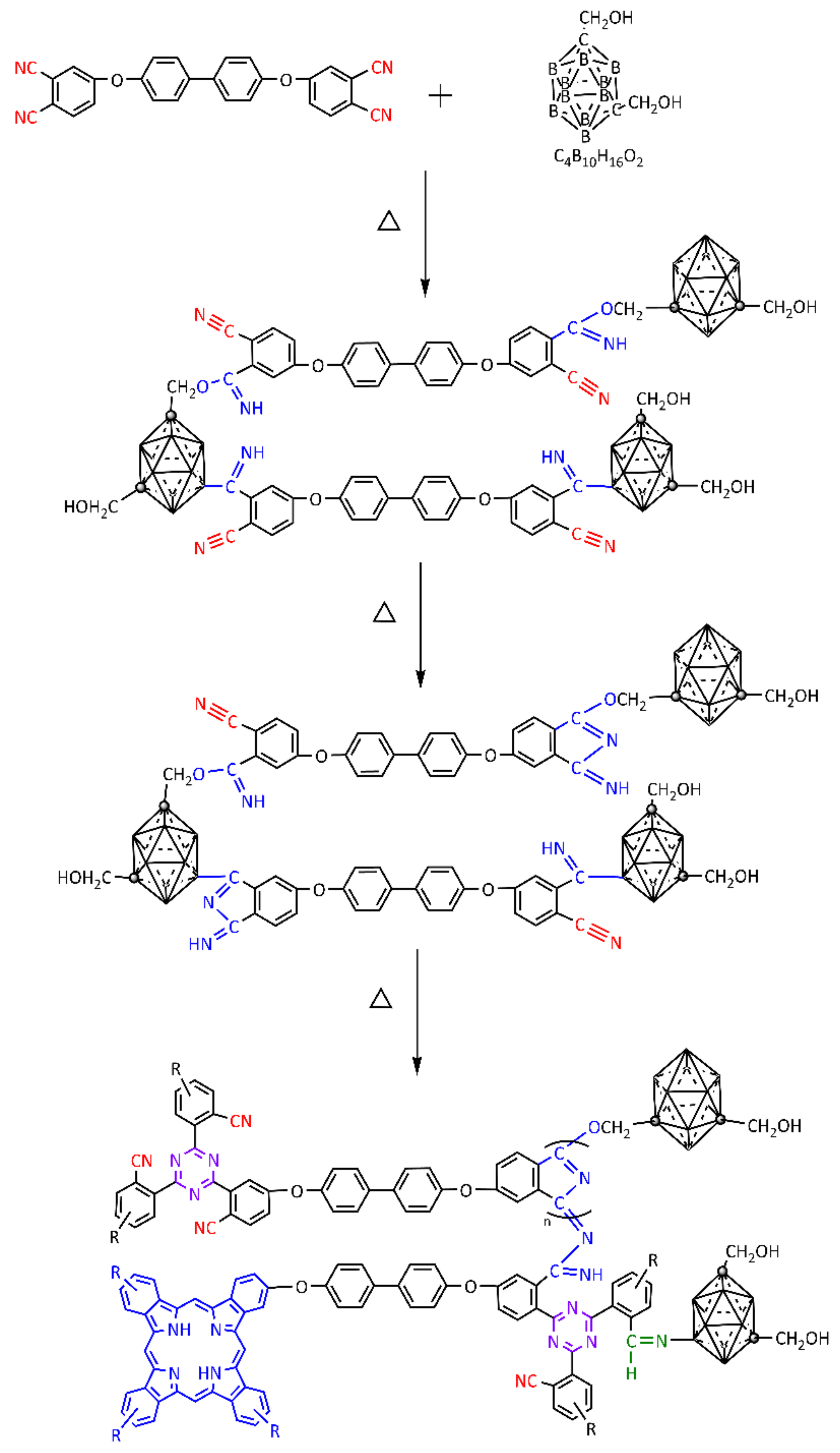
3.3. Curing Mechanism of BPh-Q Prepolymer
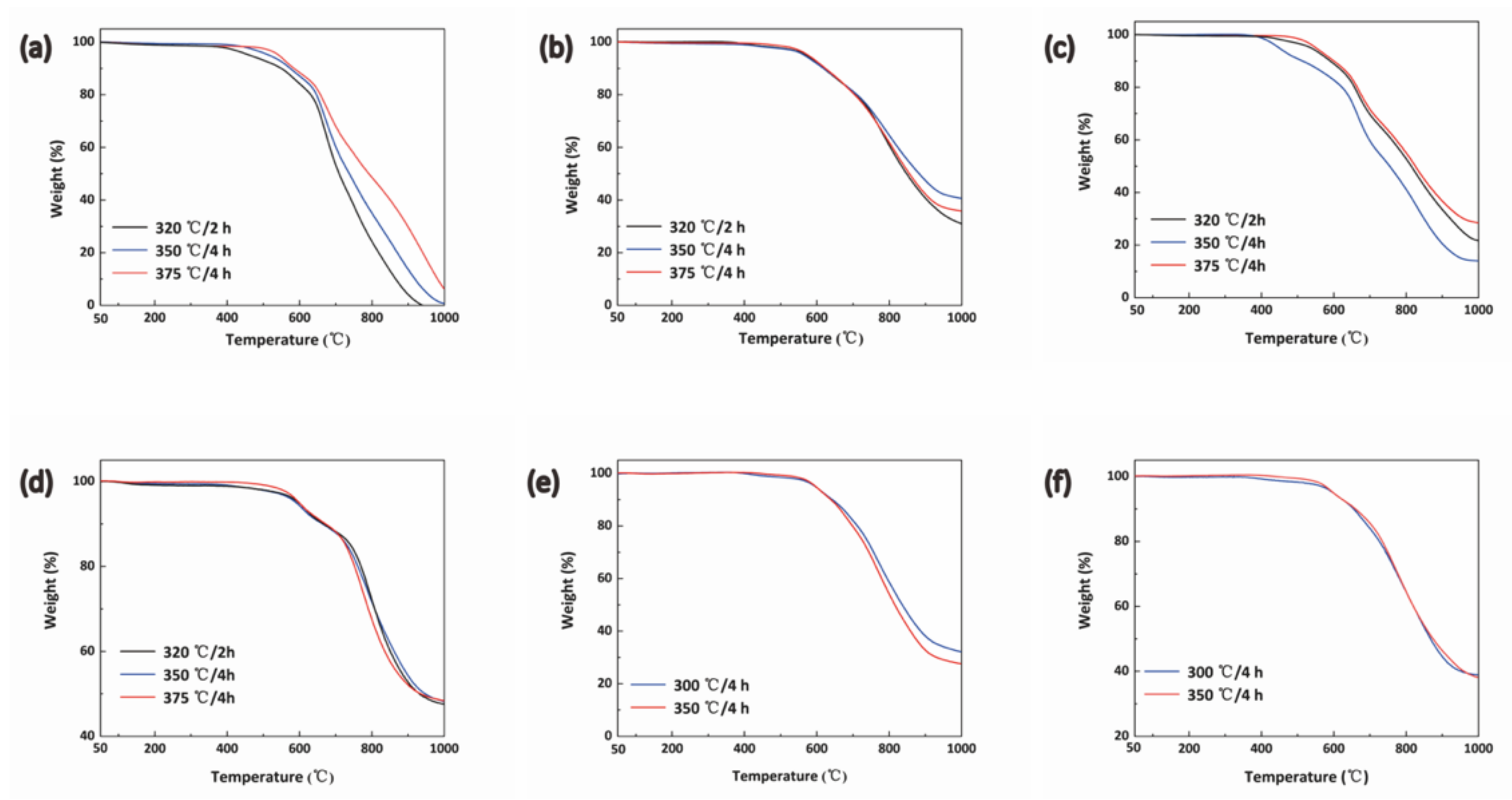
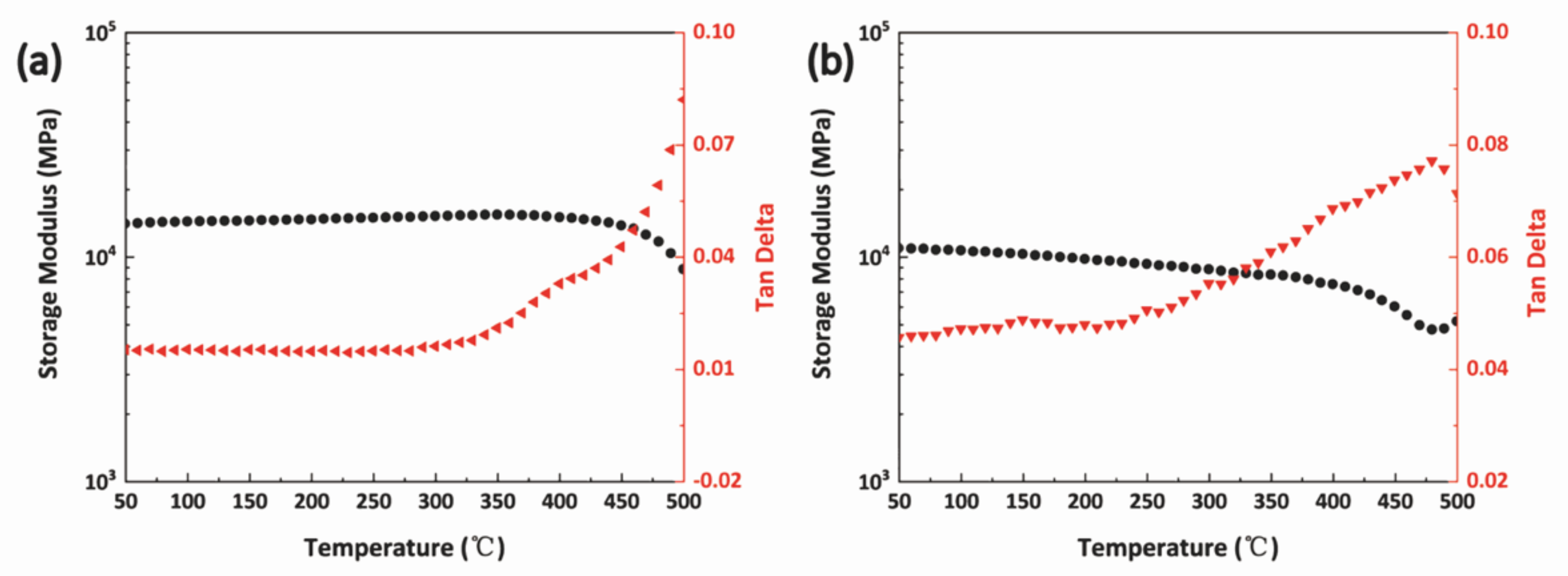
3.4. Thermal and ThermoOoxidative Stability of BPh-Q and BPh-B Resins
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wang, J.; Hu, J.; Zeng, K.; Yang, G. Preparation of self-promoted hydroxy-containing phthalonitrile resins by an in situ reaction. RSC Adv. 2015, 5, 105038–105046. [Google Scholar] [CrossRef]
- Wu, Z.; Han, J.; Nan, L.; Weng, Z.; Jian, X. Improving the curing process and thermal stability of phthalonitrile resin via novel mixed curing agents. Polym. Int. 2017, 66, 876–881. [Google Scholar] [CrossRef]
- Derradji, M.; Wang, J.; Liu, W.-B. High performance ceramic-based phthalonitrile micro and nanocomposites. Mater. Lett. 2016, 182, 380–385. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, J.; Zheng, P.; Feng, M.; Chen, J.; Liu, X. Phthalonitrile end-capped sulfonated polyarylene ether nitriles for low-swelling proton exchange membranes. J. Polym. Res. 2016, 23, 256. [Google Scholar] [CrossRef]
- Sezer, E.; Ustamehmetoğlu, B.; Bayır, Z.A.; Çoban, K.; Kalkan, A. Corrosion Inhibition Effect of 4-(2-Diethylamino-Ethylsulfonyl)-Phthalonitrile and 4,5-Bis(Hexylsulfonyl)-Phthalonitrile. Int. J. Electrochem. 2011, 2011, 235360. [Google Scholar] [CrossRef] [Green Version]
- Xu, M.; Ren, D.; Chen, L.; Li, K.; Liu, X. Understanding of the polymerization mechanism of the phthalonitrile-based resins containing benzoxazine and their thermal stability. Polymer 2018, 143, 28–39. [Google Scholar] [CrossRef]
- Dominguez, D.D.; Jones, H.N.; Keller, T.M. The effect of curing additive on the mechanical properties of phthalonitrile-carbon fiber composites. Polym. Compos. 2004, 25, 554–561. [Google Scholar] [CrossRef]
- Keller, T.M. Synthesis and Polymerization of Multiple Aromatic Ether Phthalonitriles. Chem. Mater. 1994, 6, 302–305. [Google Scholar] [CrossRef]
- Keller, T.M.; Price, T.R. Amine-Cured Bisphenol-Linked Phthalonitrile Resins. J. Macromol. Sci. Part A Chem. 2006, 18, 931–937. [Google Scholar] [CrossRef]
- Sastri, S.B.; Keller, T.M. Phthalonitrile polymers: Cure behavior and properties. J. Polym. Sci. Part A Polym. Chem. 1999, 37, 2105–2111. [Google Scholar] [CrossRef]
- Hu, J.; Liu, Y.; Jiao, Y.; Ji, S.; Sun, R.; Yuan, P.; Zeng, K.; Pu, X.; Yang, G. Self-promoted phthalimide-containing phthalonitrile resins with sluggish curing process and excellent thermal stability. RSC Adv. 2015, 5, 16199–16206. [Google Scholar] [CrossRef]
- Laskoski, M.; Neal, A.; Keller, T.M.; Dominguez, D.; And, C.; Saab, A.P. Improved synthesis of oligomeric phthalonitriles and studies designed for low temperature cure. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 1662–1668. [Google Scholar] [CrossRef]
- Ke, Z.; Zhou, S.; Hong, H.; Zhou, H.; Wang, Y.; Miao, P.; Gang, Y. Studies on self-promoted cure behaviors of hydroxy-containing phthalonitrile model compounds. Eur. Polym. J. 2009, 45, 1328–1335. [Google Scholar]
- Zhou, S.; Hong, H.; Zeng, K.; Miao, P.; Zhou, H.; Wang, Y.; Liu, T.; Zhao, C.; Xu, G.; Yang, G. Synthesis, characterization and self-promoted cure behaviors of a new phthalonitrile derivative 4-(4-(3, 5-diaminobenzoyl) phenoxy) phthalonitrile. Polym. Bull. 2009, 62, 581. [Google Scholar] [CrossRef]
- Zeng, K.; Zhou, K.; Tang, W.R.; Tang, Y.; Zhou, H.F.; Liu, T.; Wang, Y.P.; Zhou, H.B.; Yang, G. Synthesis and curing of a novel amino-containing phthalonitrile derivative. Chin. Chem. Lett. 2007, 18, 523–526. [Google Scholar] [CrossRef]
- Amir, B.; Zhou, H.; Liu, F.; Aurangzeb, H. Synthesis and characterization of self-catalyzed imide-containing pthalonitrile resins. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 5916–5920. [Google Scholar] [CrossRef]
- Xu, M.; Hu, J.; Zou, X.; Liu, M.; Dong, S.; Zou, Y.; Liu, X. Mechanical and thermal enhancements of benzoxazine-based GF composite laminated by in situ reaction with carboxyl functionalized CNTs. J. Appl. Polym. Sci. 2013, 129, 2629–2637. [Google Scholar] [CrossRef]
- Xu, M.; Yang, X.; Rui, Z.; Liu, X. Copolymerizing behavior and processability of benzoxazine/epoxy systems and their applications for glass fiber composite laminates. J. Appl. Polym. Sci. 2013, 128, 1176–1184. [Google Scholar] [CrossRef]
- Zou, X.; Xu, M.; Jia, K.; Liu, X. Synthesis, polymerization, and properties of the allyl-functional phthalonitrile. J. Appl. Polym. Sci. 2014, 131, 41203. [Google Scholar] [CrossRef]
- Guo, H.; Lei, Y.; Zhao, X.; Yang, X.; Zhao, R.; Liu, X. Curing behaviors and properties of novolac/bisphthalonitrile blends. J. Appl. Polym. Sci. 2012, 125, 649–656. [Google Scholar] [CrossRef]
- Jiang, M.; Xu, M.; Jia, K.; Liu, X. Copolymerization of self-catalyzed phthalonitrile with bismaleimide toward high-temperature-resistant polymers with improved processability. High Perform. Polym. 2016, 28, 895–907. [Google Scholar] [CrossRef]
- Zou, X.; Xu, M.; Jia, K.; Liu, X. Copolymerizing behavior and processability of allyl-functional bisphthalonitrile/bismaleimide system. Polym. Compos. 2017, 38, 1591–1599. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, B.; Wang, Y.; Zhou, H. Novolac/Phenol-Containing Phthalonitrile Blends: Curing Characteristics and Composite Mechanical Properties. Polymers 2020, 12, 126. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Zong, L.; Bao, F.; Li, G.; Wu, Z. Reduced curing kinetic energy and enhanced thermal resistance of phthalonitrile resins modified with inorganic particles. Polym. Adv. Technol. 2018, 29, 1922–1929. [Google Scholar] [CrossRef]
- Suvorova, O.N.; Verle, D.; Bazyakina, N.L.; Kutyreva, V.V.; Makarov, S.G.; Shchupak, E.A. Reactions of ferrocene with phthalonitrile on the surface of oxide powders. Russ. J. Gen. Chem. 2006, 76, 649–653. [Google Scholar] [CrossRef]
- Bregadze, V.I. Dicarba-closo-dodecaboranes C2B10H12 and their derivatives. Cheminform 1992, 92, 209–223. [Google Scholar] [CrossRef]
- Li, N.; Zeng, F.; Qu, D.; Zhang, J.; Shao, L.; Bai, Y. Synthesis and characterization of carborane-containing polyester with excellent thermal and ultrahigh char yield. J. Appl. Polym. Sci. 2016, 133, 44202. [Google Scholar] [CrossRef]
- Zhao, W.; He, J.; Lu, C. Development of Carborane Chemistry. Univ. Chem. 2019, 34, 39–47. [Google Scholar] [CrossRef]
- Keller, T.M. Phthalonitrile-based high temperature resin. J. Polym. Sci. Part A Polym. Chem. 1988, 26, 3199–3212. [Google Scholar] [CrossRef]
- Ditchfield, R. Self-Consistent Molecular-Orbital Methods. IX. An Extended Gaussian-Type Basis for Molecular-Orbital Studies of Organic Molecules. J. Chem. Phys. 1971, 54, 724–728. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy into a function of the electron density. Phys. Rev. B Condens. Matter 1988, 37, 785–789. [Google Scholar] [CrossRef] [Green Version]
- Weigend, F. Accurate Coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef]
- Yan, Z.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 119, 215–241. [Google Scholar]
- Frisch, M.; Trucks, W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A. Gaussian 09, Revision A.1; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Liao, S.; Wu, H.; He, X.; Hu, J.; Li, R.; Liu, Y.; Lv, J.; Liu, Y.; Liu, Z.; Zeng, K.; et al. Promoting effect of methyne/methylene moiety of bisphenol E/F on phthalonitrile resin curing: Expanding the structural design route of phthalonitrile resin. Polymer 2020, 210, 123001. [Google Scholar] [CrossRef]
- Jiang, F.; Drummer, D. Curing Kinetic Analysis of Acrylate Photopolymer for Additive Manufacturing by Photo-DSC. Polymers 2020, 12, 1080. [Google Scholar] [CrossRef]
- Jung, Y.; Son, Y.H.; Lee, J.K.; Phuoc, T.X.; Soong, Y.; Chyu, M.K. Rheological behavior of clay-nanoparticle hybrid-added bentonite suspensions: Specific role of hybrid additives on the gelation of clay-based fluids. ACS Appl. Mater. Interfaces 2011, 3, 3515–3522. [Google Scholar] [CrossRef]
- Balogh-Hergovich, É.; Speier, G.; Réglier, M.; Giorgi, M.; Kuzmann, E.; Vértes, A. Synthesis, structure and spectral properties of a novel stable homoleptic iron(II) complex of 1,3-bis(2′-pyridylimino)isoindoline, Fe(ind) 2. Inorg. Chem. Commun. 2005, 8, 457–459. [Google Scholar] [CrossRef]
- Gagne, R.R.; Marks, D.N. Ruthenium complexes of 1,3-bis(2-pyridylimino)isoindolines as alcohol oxidation catalysts. Inorg. Chem. 1984, 23, 65–74. [Google Scholar] [CrossRef]
- Pan, L.; Jia, K.; Shou, H.; Zhou, X.; Wang, P.; Liu, X. Unification of molecular NIR fluorescence and aggregation-induced blue emission via novel dendritic zinc phthalocyanines. J. Mater. Sci. 2017, 52, 3402–3418. [Google Scholar] [CrossRef]
- Zeng, K.; Yang, G. Phthalonitrile matrix resins and composites. In Wiley Encyclopedia of Composites; Wiley & Sons: New York, NY, USA, 2012; Volume 1, pp. 2073–2086. [Google Scholar]
- Carey, J.S.; Laffan, D.; Thomson, C.; Williams, M.T. Analysis of the reactions used for the preparation of drug candidate molecules. Cheminform 2006, 4, 2337–2347. [Google Scholar]
- Vries, J.D.; Mršić, N. Organocatalytic asymmetric transfer hydrogenation of imines. Catal. Sci. Technol. 2011, 1, 727–735. [Google Scholar] [CrossRef] [Green Version]
- Westcott, S.A.; Vogels, C.M. Recent Advances in Organic Synthesis Using Transition Metal-Catalyzed Hydroborations. Curr. Org. Chem. 2010, 9, 687–699. [Google Scholar]
- Yu, Z.; Jin, W.; Jiang, Q. Bronsted Acid Activation Strategy in Transition-Metal Catalyzed Asymmetric Hydrogenation of N-Unprotected Imines, Enamines, and N-Heteroaromatic Compounds. Angew. Chem.-Int. Ed. 2012, 51, 6060–6072. [Google Scholar] [CrossRef]
- Wang, J.; Feng, Y.Q.; Xie, J.Y.; Li, G.; Li, X.G. Dispersion of phthalocyanine green G in nonaqueous medium using hyperdispersants and application in E-ink. J. Dispers. Sci. Technol. 2006, 27, 975–981. [Google Scholar] [CrossRef]
- Zhang, M.X.; Xu, H.L. Possible B-C bonding in the hydroboration of benzonitrile by an external electric field. Phys. Chem. Chem. Phys. 2018, 21, 18–21. [Google Scholar] [CrossRef]
- BurchilL, P.J. On the Formation and Properties of a High-Temperature Resin from a Bisphthalonitrile. J. Polym. Sci. 1994, 32, 1–8. [Google Scholar] [CrossRef]
- Choi, S.-S.; Ha, S.H.; Woo, C. Thermal Aging Behaviors of Resole-Cured Rubber Composites. Elastomers Compos. 2005, 40, 284–289. [Google Scholar]



| Samples | Solubility a | |||||
|---|---|---|---|---|---|---|
| NMP b | DMF | DMAc | THF | Acetone | CHCl3 | |
| BPh-Q5 | ++ | ++ | ++ | ++ | ++ | ++ |
| BPh-Q10 | ++ | ++ | ++ | ++ | ++ | ++ |
| BPh-Q15 | ++ | ++ | ++ | ++ | ++ | ++ |
| BPh-Q20 | ++ | ++ | ++ | ++ | ++ | ++ |
| BPh-Q30 | ++ | ++ | ++ | ++ | ++ | ++ |
| BPh-B | ++ | ++ | ++ | +- | +− | + |
| BPh | ++ | + | + | − | − | + |
| Samples | ΔH (J/g) | Curing Degree (%) | |
|---|---|---|---|
| ΔHtotal | ΔHres | ||
| BPh-Q5 | 455.25 | 10.88 | 97.61 |
| BPh-Q10 | 427.83 | 6.59 | 98.46 |
| BPh-Q15 | 567.69 | 6.53 | 98.85 |
| BPh-Q20 | 525.55 | 9.09 | 98.27 |
| BPh-Q30 | 462.93 | 7.73 | 98.33 |
| No. | Crack Products at 800 °C Volatile Products | Content (%) |
|---|---|---|
| 1 |  | 19.00 |
| 2 |  | 16.59 |
| 3 | 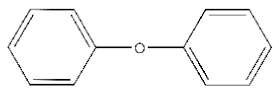 | 13.94 |
| 4 |  | 7.28 |
| 5 | 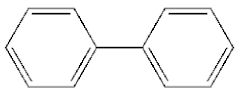 | 4.72 |
| 6 |  | 3.14 |
| Sample | Curing Conditions | In N2 | In Air | ||
|---|---|---|---|---|---|
| Td5 (°C) | Char Yield (%) | Td5 (°C) | Char Yield (%) | ||
| BPh-B | 320 °C/2 h | 487 | 67.41 | 462 | 0.00 |
| 350 °C/4 h | 492 | 72.60 | 516 | 0.80 | |
| 375 °C/4 h | 538 | 75.80 | 542 | 6.40 | |
| BPh-Q5 | 320 °C/2 h | 492 | 73.53 | 460 | 13.97 |
| 350 °C/4 h | 534 | 74.79 | 537 | 21.78 | |
| 375 °C/4 h | 535 | 73.86 | 547 | 28.45 | |
| BPh-Q10 | 320 °C/2 h | 570 | 79.30 | 568 | 31.10 |
| 350 °C/4 h | 580 | 80.30 | 565 | 40.60 | |
| 375 °C/4 h | 578 | 79.50 | 575 | 35.90 | |
| BPh-Q15 | 320 °C/2 h | 590 | 84.21 | 591 | 48.22 |
| 350 °C/4 h | 596 | 82.09 | 597 | 47.61 | |
| 375 °C/4 h | 609 | 84.20 | 597 | 48.40 | |
| BPh-Q20 | 320 °C/4 h | 594 | 83.31 | 595 | 32.20 |
| 375 °C/4 h | 592 | 83.23 | 596 | 28.64 | |
| BPh-Q30 | 320 °C/4 h | 613 | 84.87 | 598 | 38.93 |
| 375 °C/4 h | 615 | 86.52 | 598 | 38.17 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, Y.; Bu, X.; Dong, J.; Zhou, Q.; Liu, M.; Wang, F.; Wang, M. Catalytic Polymerization of Phthalonitrile Resins by Carborane with Enhanced Thermal Oxidation Resistance: Experimental and Molecular Simulation. Polymers 2022, 14, 219. https://doi.org/10.3390/polym14010219
Jia Y, Bu X, Dong J, Zhou Q, Liu M, Wang F, Wang M. Catalytic Polymerization of Phthalonitrile Resins by Carborane with Enhanced Thermal Oxidation Resistance: Experimental and Molecular Simulation. Polymers. 2022; 14(1):219. https://doi.org/10.3390/polym14010219
Chicago/Turabian StyleJia, Yuxiang, Xiaojun Bu, Junyu Dong, Quan Zhou, Min Liu, Fang Wang, and Maoyuan Wang. 2022. "Catalytic Polymerization of Phthalonitrile Resins by Carborane with Enhanced Thermal Oxidation Resistance: Experimental and Molecular Simulation" Polymers 14, no. 1: 219. https://doi.org/10.3390/polym14010219