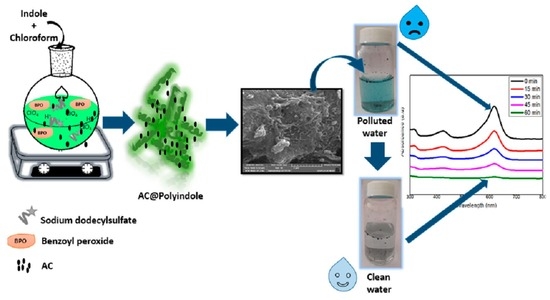
Preparation and Characterization of a Novel Activated Carbon@Polyindole Composite for the Effective Removal of Ionic Dye from Water
Abstract
:1. Introduction
2. Materials and Methods
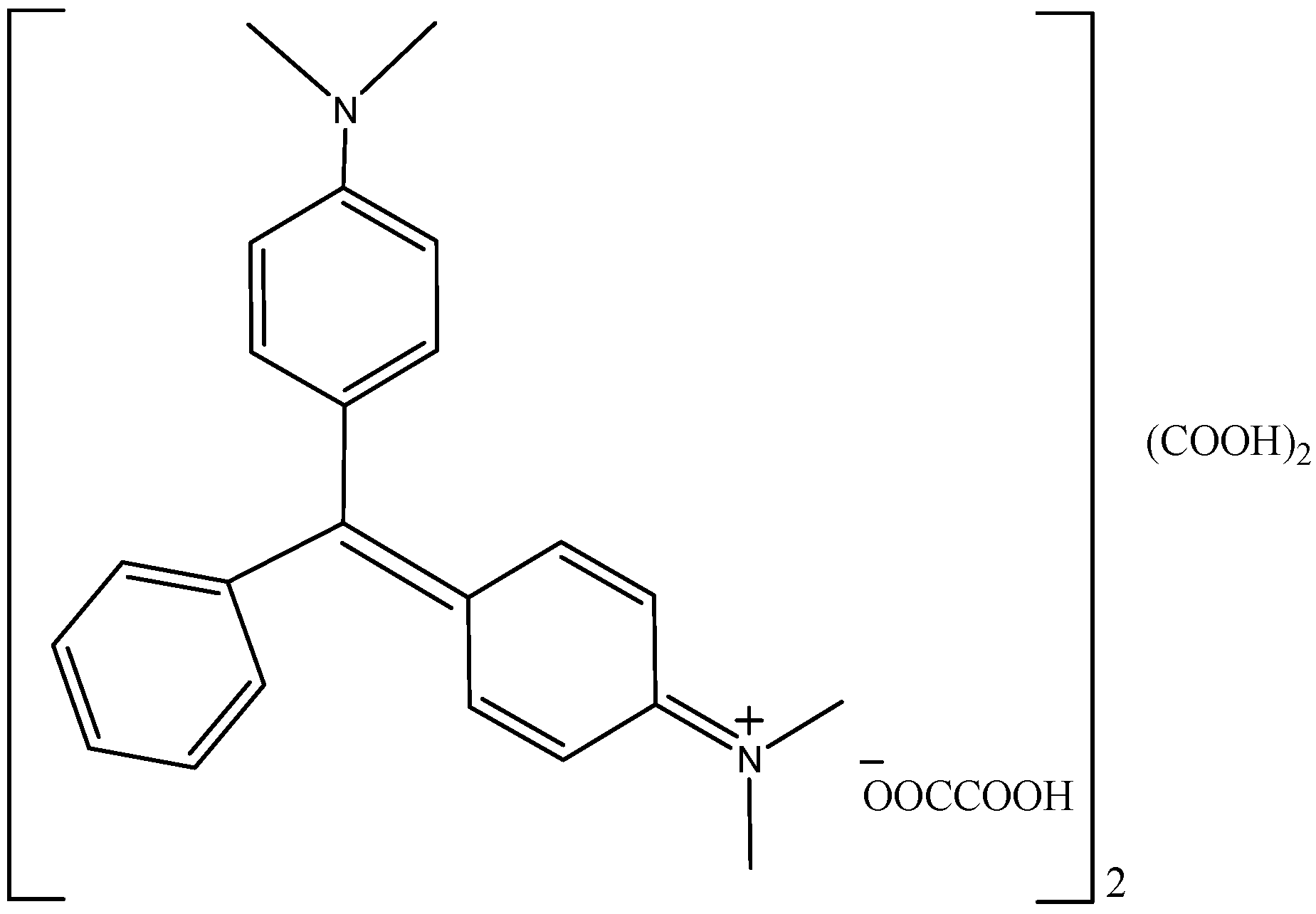
2.1. Chemical and Reagents
2.2. Chemical Activation of AC
2.3. Synthesis of AC@PIN Composite
2.4. Characterizations
2.5. Adsorption Studies
3. Results and Discussion
3.1. Chemical Structure Analysis
3.2. Surface Morphology Analysis
3.3. EDX Analysis
3.4. X-ray Diffraction Analysis
3.5. UV-Visible Spectroscopy
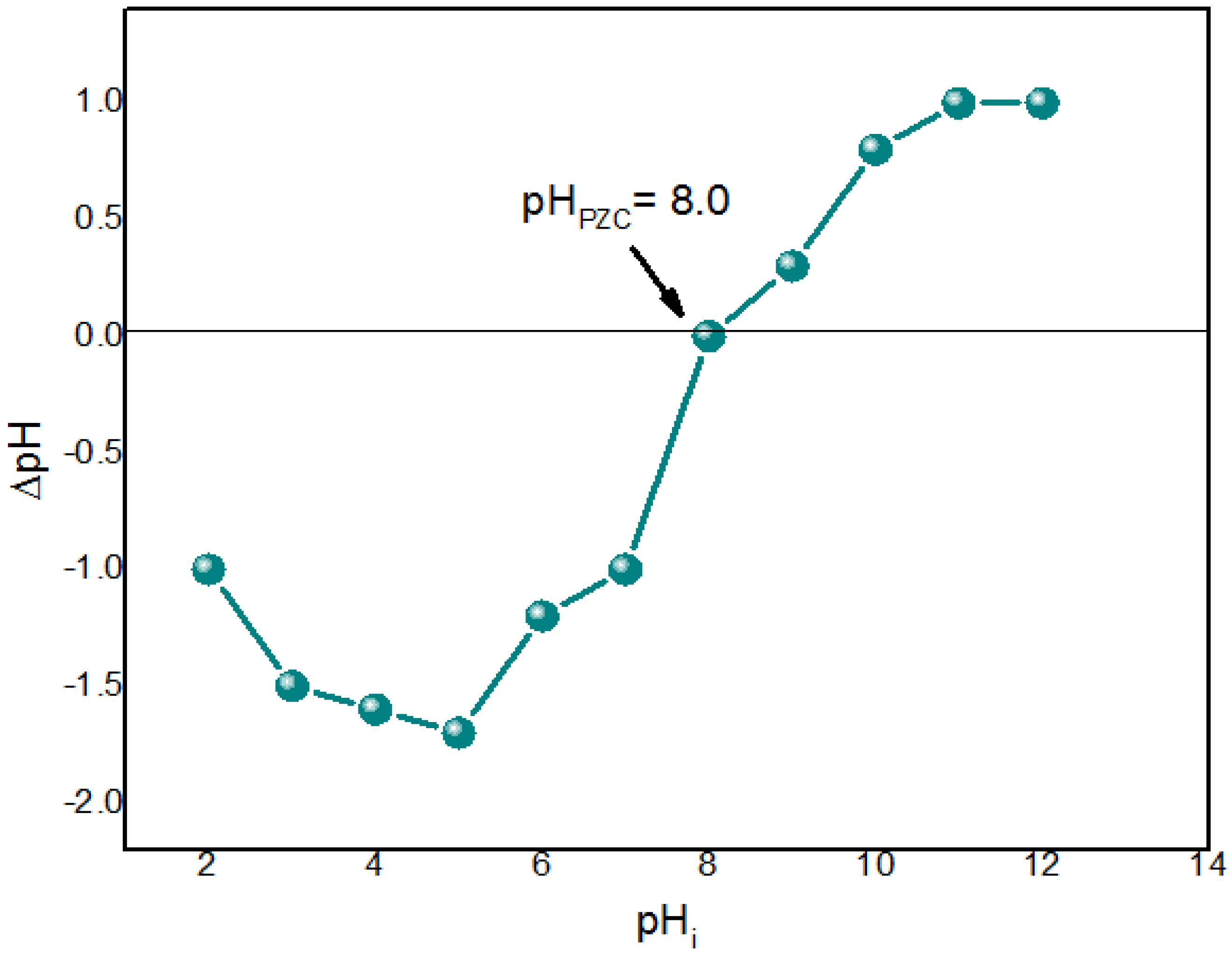
3.6. Point of Zero Charge (pHPZC)
3.7. Adsorption Studies
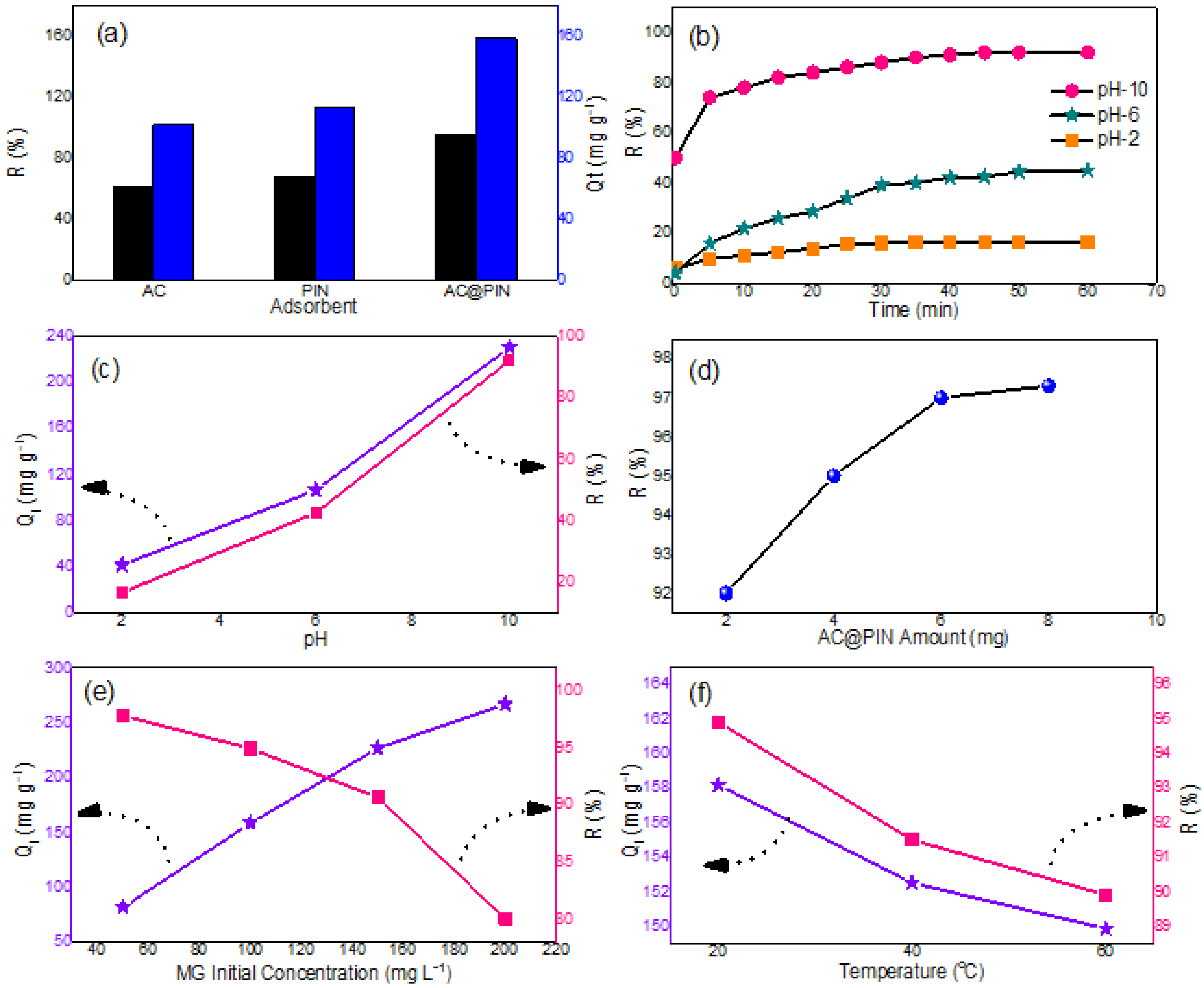
3.7.1. Adsorption Efficiency of Prepared PIN, AC, and AC@PIN
3.7.2. Influence of pH and Contact Time
3.7.3. Influence of AC@PIN Dosage
3.7.4. Influence of MG Concentration
3.7.5. Influence of Temperature
3.7.6. Adsorption Kinetics
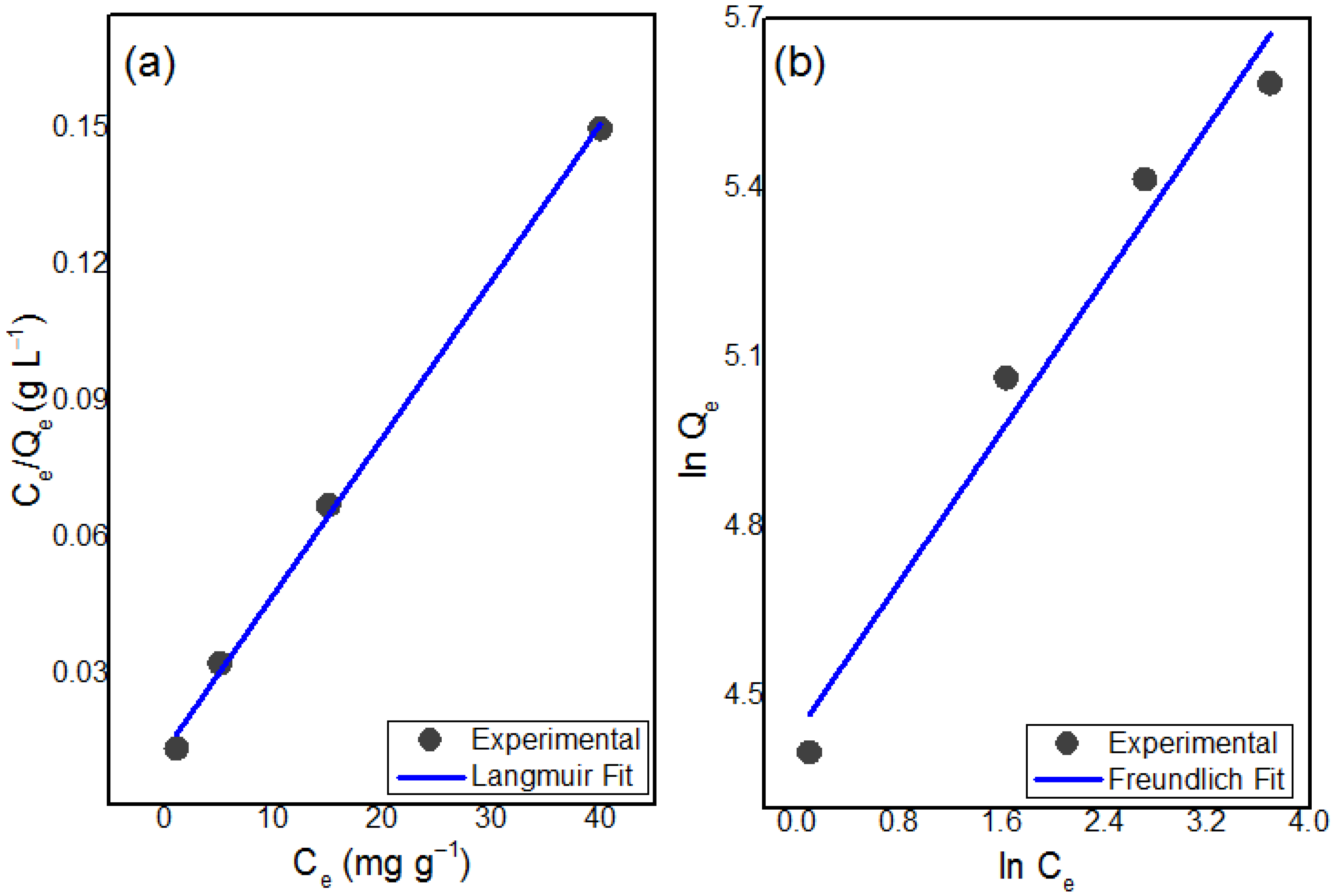
3.7.7. Adsorption Isotherms
3.7.8. Thermodynamic Study
3.7.9. Reusability Study
3.7.10. Probable Mechanism of Adsorption
4. Conclusions
Author Contributions
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Data Availability Statement
Conflicts of Interest
References
- Abdelghani, H.; Abdelilah, E.; Nouh, A.; Mohamed, L.; Abdelaziz, A.A.; Rajae, L.; Abdallah, A. Elaboration of novel polyaniline@almond shell biocomposite for effective removal of hexavalent chromium ions and orange G dye from aqueous solutions. Environ. Sci. Pollut. Res. 2020, 27, 15245–15258. [Google Scholar]
- Sabarish, R.; Jasila, K.; Aswathy, J.; Jyotishkumar, P.; Suchart, S. An efficient removal of malachite green dye from aqueous environment using ZSM-5 zeolite/polyvinyl alcohol/carboxymethyl cellulose/sodium alginate bio composite. J. Polym. Environ. 2021, 29, 2126–2139. [Google Scholar]
- Shilpi, A.; Inderjeet, T.; Vinod, K.G.; Fariba, G.; Ahmad, N.G.; Omid, M. Synthesis and characteristics of polyaniline/zirconium oxide conductive nanocomposite for dye adsorption application. J. Mol. Liq. 2016, 218, 494–498. [Google Scholar] [CrossRef]
- Sabarish, R.; Jasila, K.; Aswathy, J.; Jyotishkumar, P.; Suchart, S. Removal of anionic dye congo red from aqueous environment using polyvinyl alcohol/sodium alginate/ZSM-5 zeolite membrane. Sci. Rep. 2020, 10, 15452–15466. [Google Scholar]
- Sudhindra, P.; Radhika, A.K.; Libi, T. Polyaniline-multiwalled carbon nanotubes (PANI-MWCNTs) composite revisited: An efficient and reusable material for methyl orange dye removal. Diam. Relat. Mater. 2021, 117, 108455–108460. [Google Scholar] [CrossRef]
- Khaoula, L.; Sonia, F.; Mostafa, S.; Julia, G.A. Adsorption of an anionic dye (Congo red) from aqueous solutions by pine bark. Sci. Rep. 2019, 9, 16530–16540. [Google Scholar]
- Mudassir, H.; MdMamoon, R.; Muhammad, M.H.; Mohammed, K.A.M.; Muhammad, A.; Mohd, D.; Moonyong, L.; Atef, E.J.; Narendra, K. Fabrication of polyaniline/activated carbon composite and its testing for methyl orange removal: Optimization, equilibrium, isotherm, and kinetic study. Polym. Test. 2019, 77, 105909–105917. [Google Scholar] [CrossRef]
- Sarojini, G.; Babu, S.V.; Rajamohan, N.; Rajasimman, M. Performance evaluation of polymer-marine biomass based bionanocomposite for the adsorptive removal of malachite green from synthetic wastewater. Environ. Res. 2022, 204, 112132. [Google Scholar] [CrossRef] [PubMed]
- Saima, N.; Haq, N.B.; Munawar, I.; Fida, H.; Fazli, M.S. Chitosan, starch, polyaniline and polypyrrole biocomposite with sugarcane bagasse for the efficient removal of acid black dye. Inter. J. Biol. Macromol. 2020, 147, 439–452. [Google Scholar] [CrossRef]
- Cai, Z.; Yang, G. Synthesis of polyindole and its evaluation for Li-ion battery applications. Synth. Met. 2010, 160, 1902–1905. [Google Scholar] [CrossRef]
- Mudila, H.; Prasher, P.; Kumar, M.; Kumar, A.; Zaidi, M.G.H.; Kumar, A. Critical analysis of polyindole and its composites in supercapacitor application. Mater. Renew. Sustain. Energy 2019, 8, 9. [Google Scholar] [CrossRef] [Green Version]
- Zhijiang, C.; Xianyou, S.; Qing, Z.; Tingting, Z. Electrospun polyindole nanofibers as a nano-adsorbent for heavy metal ions adsorption for wastewater treatment. Fibers Polym. 2017, 18, 502–513. [Google Scholar] [CrossRef]
- Cai, Z.; Jia, J.; Zhang, Q.; Yang, H. Preparation of amidoxime surface-functionalized polyindole (ASFPI) nanofibers for Pb(II) and Cd(II) adsorption from aqueous solutions. RSC Adv. 2015, 5, 82310–82323. [Google Scholar] [CrossRef]
- Cai, Z.; Song, X.; Zhang, Q.; Liu, Y. Amidoxime surface modification of polyindole nanofiber membrane for effective removal of Cr(VI) from aqueous solution. J. Mater. Sc. 2017, 52, 5417–5434. [Google Scholar]
- Halit, Y.; Serife, T.; Cengis, S. Preparation of polyacrylonitrile/polyindole conducting polymer composite and its use for solid phase extraction of copper in a certified reference material. Spectrochim. Part A Molecul. Biomolecul. Spect. 2021, 244, 118826–118834. [Google Scholar] [CrossRef]
- Dedhila, D.; Baiju, V.; Deepa, J.P.; Raveendran, R. The novel polyindole base ZnO/MgO nanocomposite adsorbent for the removal of heavy metal ions from industrial effluents. Nanosyst. Phys. Chem. Math. 2020, 11, 666–671. [Google Scholar] [CrossRef]
- Venkata, S.M.; Hsin-Yu, W.; Jet-Chau, W.; Yuvaraja, G.; Zhong, T.; Guda, M.R.; Jarem, R.G. Anionic congo red dye removal from aqueous medium using Turkey tail (Trametes versicolor) fungal biomass: Adsorption kinetic, isotherms, thermodynamics, reusability, and characterization. J. Dispers. Sci. Technol. 2021, 42, 1785–1798. [Google Scholar] [CrossRef]
- Cai, Z.-J.; Zhang, Q.; Song, X.-Y. Improved electrochemical performance of polyindole/carbon nanotubes composite as electrode material for supercapacitors. Electron. Mater. Lett. 2016, 12, 830–840. [Google Scholar] [CrossRef]
- Goel, S.; Mazumdar, N.A.; Gupta, A. Fabrication of polyindene and polyindole nanostructures. Appl. Surf. Sci. 2010, 256, 4426–4433. [Google Scholar] [CrossRef]
- Abdollahi-Alibeik, M.; Poorirani, S. Perchloric acid-doped polyaniline as an efficient and reusable catalyst for the synthesis of 2-subsituted benzothiazoles. Phosphorus Sulfur Silicon 2009, 184, 3182–3190. [Google Scholar] [CrossRef]
- Eraldemir, O.; Sari, B.; Gok, A.; Ibrahim, U.H. Synthesis and characterization of polyindole/poly(vinyl acetate) conducting composites. J. Macromol. Sci. Part A 2008, 45, 205–211. [Google Scholar] [CrossRef]
- Phasuksom, K.; Sirivat, A. Synthesis of nano-sized polyindole via emulsion polymerization and doping. Synth. Met. 2016, 219, 142–153. [Google Scholar] [CrossRef]
- Anisuzzaman, S.M.; Joseph, C.G.; Daud, W.M.A.B.W.; Krishnaiah, D.; Yee, H.S. Preparation and characterization of activated carbon rom Typhaorientalis leaves. Int. J. Ind. Chem. 2015, 6, 9–21. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Wu, L.; Wan, W.; Xu, Q.; Li, Z. Preparation of activated carbons from walnut shell by fast activation with H3PO4: Influence of fluidization of particles. Int. J. Chem. React. Eng. 2017, 16, 20170074–20170083. [Google Scholar] [CrossRef]
- Yakout, S.M.; EI-Deen, G.S. Characterization of activated carbon prepared by phosphoric acid activation of olive stones. Arab. J. Chem. 2016, 9, S1155–S1162. [Google Scholar] [CrossRef] [Green Version]
- Saptarshi, D.; Pallab, B.; Goutam, H.; Sumanta, S.; Das, C.K. Transition Metal-Doped Polyaniline/Single Walled Carbon Nanotubes Nanocomposites: Efficient Electrode Material for High Performance Supercapacitor. ACS Sustain. Chem. Eng. 2014, 2, 1114–11127. [Google Scholar]
- Leela, J.; Arun, K.S.; Rajiv, P. Polyindole/carboxylated-multiwall carbon nanotube composites produced by in-situ and interfacial polymerization. Mater. Chem. Phys. 2012, 135, 80–87. [Google Scholar]
- Karim, M.R.; Lee, C.J.; Lee, M.S. Synthesis and characterization of conducting polyaniline-activated carbon nanocomposites. J. Appl. Polym. Sci. 2007, 103, 1973–1977. [Google Scholar] [CrossRef]
- Patil, D.S.; Pawar, S.A.; Devan, R.S.; Ma, Y.R.; Bae, W.R.; Kim, J.H.; Patil, P.S. Improved electrochemical performance of activated carbon/polyaniline composite electrode. Mater. Lett. 2014, 117, 248–251. [Google Scholar] [CrossRef]
- Olad, A.; Gharekhani, H. Preparation and Electrochemical investigation of the polyaniline/activated carbon nanocomposite for supercapacitor applications. Prog. Organ. Coat. 2015, 81, 19–26. [Google Scholar] [CrossRef]
- Lopamudra, G.; Papita, D.; Avijit, B.; Chiranjib, B. Treatment of malachite green dye containing solution using bio-degradable sodium alginate/NaOH treated activated carbon sugarcane bagasse charcoal beads: Batch, optimization using response surface methodology continuous fixed bed column study. J. Environ. Manag. 2020, 276, 111272–111283. [Google Scholar]
- Nirva, P.R.; Prapti, U.S.; Nisha, K.S. Nanoparticles loaded biopolymer as effective adsorbent for adsorptive removal of malachite green from aqueous solution. Water Conserv. Sci. Eng. 2016, 1, 169–181. [Google Scholar]
- Khamngoen, K.; Paradee, N.; Sirivat, A. Chemical oxidation polymerization and Characterization of polyortho-anisidinenanoparticles. J. Polym. Res. 2016, 23, 172. [Google Scholar] [CrossRef]
- Palaniappan, S.; Srinivas, P. Nano fiber polyaniline containing long chain and small molecule dopants and carbon composites for supercapacitor. Electrochim. Acta 2013, 95, 251–259. [Google Scholar]
- Zengin, H.; Kalaci, G. Synthesis and characterization of polyaniline/activated Carbon composites and preparation of conductive films. Mater. Chem. Phys. 2010, 120, 46–53. [Google Scholar] [CrossRef]
- Khalil, H.P.S.; Jawaid, M.; Firoozian, P.; Rashid, U.; Islam, A.; Akil, H.M. Activated carbon from various agricultural wastes by chemical activation with KOH: Preparation and characterization. J. Biobased Mater. Bioenergy 2013, 7, 708–714. [Google Scholar] [CrossRef]
- Xu, J.; Chen, L.; Qu, H.; Jiao, Y.; Xie, J.; Xing, G. Preparation and characterization of activated caron from reedy grass leaves by chemical activation with H3PO4. Appl. Surf. Sci. 2014, 320, 674–680. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhu, D.; Ma, X.; Xu, J.; Zhou, W.; Zhao, F. High-performance Capacitive behavior of layered reduce graphene oxide and polyindole nanocomposite materials. RSC Adv. 2016, 6, 29840–29847. [Google Scholar] [CrossRef]
- Ramesan, M.T. Fabrication and characterization of conducting nanomaterials composed of copper sulfide and polyindole. Polym. Compos. 2012, 33, 2169–2176. [Google Scholar] [CrossRef]
- Majumder, M.; Choudhary, R.B.; Koiry, S.P.; Thakur, A.K.; Kumar, U. Gravimetric and volumetric capacitive performance of polyindole/carbon black/MoS2 hybrid electrode material for supercapacitor applications. Electrochim. Acta 2017, 248, 98–111. [Google Scholar] [CrossRef]
- Jayakrishnan, K.; Joseph, A.; Bhattathiripad, J.; Ramesan, M.T.; Chandrasekharan, K.; Narendran, N.S. Reverse saturable absorption studies in polymerized indole-Effect of polymerization in the phenomenal enhancement of third order optical nonlinearity. Optic. Mater. 2016, 54, 252–261. [Google Scholar] [CrossRef]
- Mishra, R.; Nirala, N.R.; Pandey, R.K.; Ojha, R.P.; Prakash, R. Homogeneous Dispersion of MoS2 Nanosheets in Polyindole Matrix at Air-Water interface Assisted by Langmuir Technique. Langmuir 2017, 33, 13572–13580. [Google Scholar] [CrossRef]
- Joshi, L.; Prakash, R. One-pot synthesis of polyindole-Au nanocomposites and its nanoscale electrical properties. Mater. Lett. 2011, 65, 3016–3019. [Google Scholar] [CrossRef]
- Mike, T.; Xingping, W.; Ifra, M.; Pan, D.; Shengyuan, Y.; Meifang, Z. Green approach to fabricate polyindole composite nanofibers for energy and sensor applications. Mater. Lett. 2017, 209, 400–403. [Google Scholar]
- Vighnesha, K.M.; Sangeetha, D.N.; Selvakumar, M. Synthesis and characterization of activated carbon/conducting polymer composite electrode for supercapacitor applications. J. Mater. Sci. Mater. Electron. 2018, 29, 914–921. [Google Scholar] [CrossRef]
- Rahmat, A.; Tahira, M.; Salah, U.D.; Abdul, N.; Madeeha, A.; Muhammad, F. Efficient removal of hazardous malachite green dye from aqueous solutions using H2O2 modified activated carbon as potential low-cost adsorbent: Kinetic, equillibrium, and thermodynamic studies. Desalin. Water Treat. 2019, 151, 167–182. [Google Scholar]
- Mahto, T.K.; Chowdhuri, A.R.; Sahu, S.K. Polyaniline-functionalized magnetic nanoparticles for the removal of toxic dye from wastewate. J. Appl. Poly. Sci. 2014, 131, 1–9. [Google Scholar] [CrossRef]
- Patil, M.R.; Shrivastava, V.S. Adsorption of malachite green by polyaniline-ferrite magnetic nanocomposite: An isotherm and kinetic study. Appl. Nanosci. 2015, 5, 809–816. [Google Scholar] [CrossRef] [Green Version]
- Rajabi, M.; Mahanpoor, K.; Moradi, O. Preparation of PMMA/GO and PMMA/GO-Fe3O4 nanocomposites for malachite green dye adsorption: Kinetic and thermodynamic studies. Compos. Part B Eng. 2019, 167, 544–555. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Doulabi, M.; Doroudian, M. Adsorption characteristics of malachite green dye onto novel kappa-carrageenan-g-polyacrylic acid/TiO2-NH2 hydrogel nanocomposite. J. Iran. Chem. Soc. 2014, 11, 1057–1065. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Zhang, S.; Ye, Z. Preparation and characterization of new foam adsorbents of poly(vinyl alcohol)/chitosan composites and their removal for dye and heavy metal from aqueous solution. Chem. Eng. J. 2012, 183, 88–97. [Google Scholar] [CrossRef]






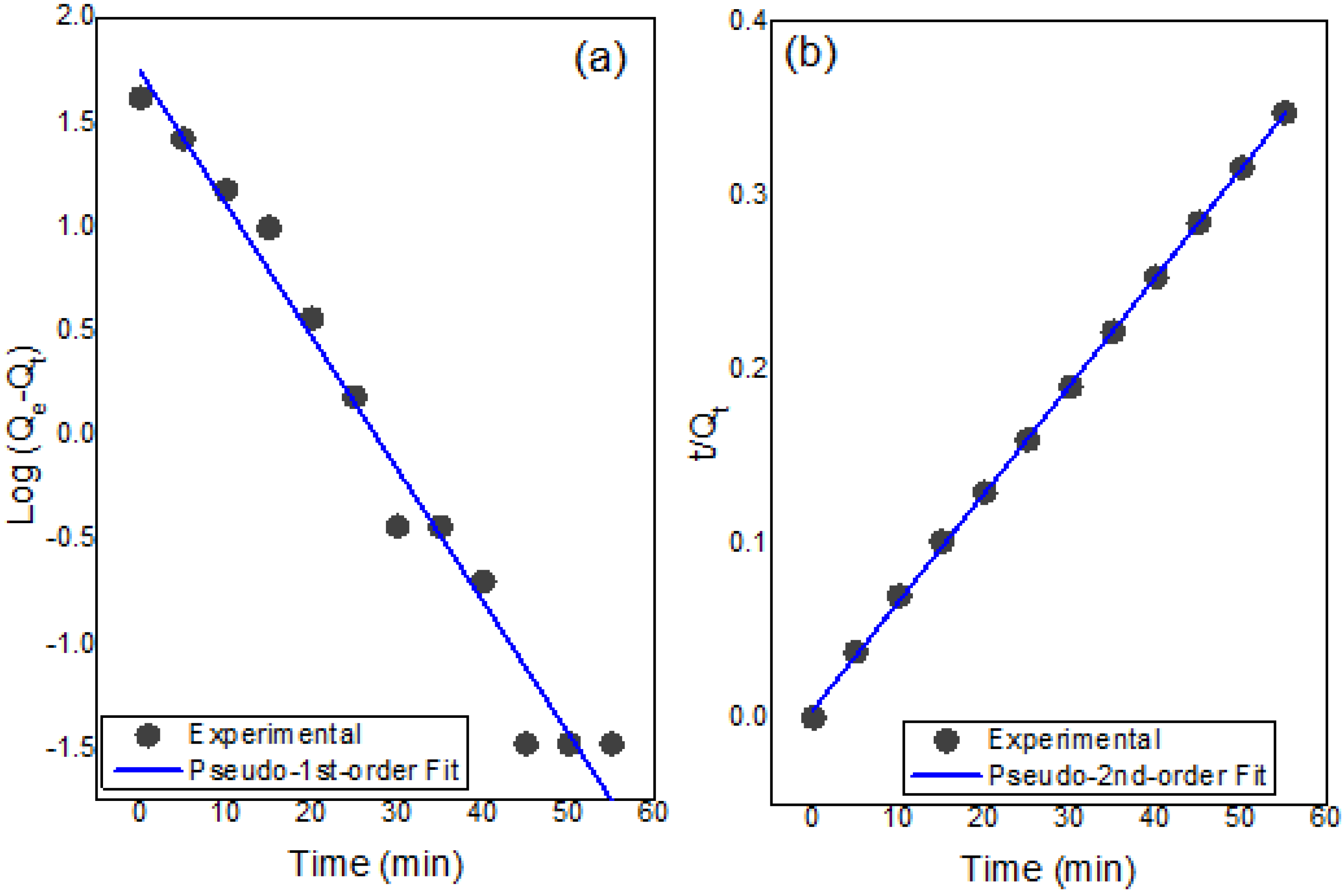


| Experimental | Pseudo-1st-Order | Pseudo-2nd-Order | ||||
|---|---|---|---|---|---|---|
| Qe (mg g−1) | Qe (mg g−1) | K1 (min−1) | R2 | Qe (mg g−1) | K2 (g mg−1 min−1) | R2 |
| 226.5 | 51.12 | −1.46 | 0.97 | 250 | 0.003 | 0.99 |
| Experimental | Langmuir Isotherm Model | Freundlich Isotherm Model | ||||
|---|---|---|---|---|---|---|
| Qe (mg g−1) | Qe (mg g−1) | KL | R2 | KF (mg g−1) | n | R2 |
| 226.5 | 289.01 | 0.271 | 0.99 | 26915 | 2.9 | 0.95 |
| Temperature (K) | ∆G° (kJ mol−1) | ∆H° (kJ mol−1) | ∆S° (kJ mol−1 K−1) | R2 |
|---|---|---|---|---|
| 293 | −27.23 | −17.15 | −0.034 | 0.95 |
| 313 | −27.92 | |||
| 328 | −28.43 |
| Adsorbents | Qe (mg g−1) | Equilibrium Time (min) | Adsorbent Dosage (g) | Kinetic Study | Isotherm Study | Thermodynamic Study | Ref |
|---|---|---|---|---|---|---|---|
| PVA/CMC/SA/ZSM a | 5.95 | 220 | 5.0 | Pseudo-2nd-order | Freundlich | - | [2] |
| Fe2O3@PANI | 240.0 | 240 | 0.01 | Pseudo-2nd-order | Langmuir | exothermic | [47] |
| PANI-nickel ferrite | 4.09 | 210 | 5.0 | Pseudo-2nd-order | Langmuir | - | [48] |
| Poly(methyl methacrylate)/graphene oxide-Fe3O | 3.5 | 35 | 0.005 | Pseudo-2nd-order | - | exothermic | [49] |
| Kappa-carrageenan-g-polyacrylic acid/TiO2-NH2 | 666.66 | 180 | 0.05 | Pseudo-2nd-order | Langmuir | exothermic | [50] |
| Poly(vinyl alcohol)-chitosan | 380.6 | 540 | 0.1 | Pseudo-2nd-order | Freundlich | endothermic | [51] |
| AC@PIN | 226.5 | 60 | 0.006 | Pseudo-2nd-order | Langmuir | exothermic | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Begum, B.; Ijaz, S.; Khattak, R.; Qazi, R.A.; Khan, M.S.; Mahmoud, K.H. Preparation and Characterization of a Novel Activated Carbon@Polyindole Composite for the Effective Removal of Ionic Dye from Water. Polymers 2022, 14, 3. https://doi.org/10.3390/polym14010003
Begum B, Ijaz S, Khattak R, Qazi RA, Khan MS, Mahmoud KH. Preparation and Characterization of a Novel Activated Carbon@Polyindole Composite for the Effective Removal of Ionic Dye from Water. Polymers. 2022; 14(1):3. https://doi.org/10.3390/polym14010003
Chicago/Turabian StyleBegum, Bushra, Saba Ijaz, Rozina Khattak, Raina Aman Qazi, Muhammad Sufaid Khan, and Khaled H. Mahmoud. 2022. "Preparation and Characterization of a Novel Activated Carbon@Polyindole Composite for the Effective Removal of Ionic Dye from Water" Polymers 14, no. 1: 3. https://doi.org/10.3390/polym14010003
APA StyleBegum, B., Ijaz, S., Khattak, R., Qazi, R. A., Khan, M. S., & Mahmoud, K. H. (2022). Preparation and Characterization of a Novel Activated Carbon@Polyindole Composite for the Effective Removal of Ionic Dye from Water. Polymers, 14(1), 3. https://doi.org/10.3390/polym14010003