Bisphenol A Release from Dental Composites and Resin-Modified Glass Ionomers under Two Polymerization Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Preparation and Extraction
2.2. Uptake of Extraction Media and Mass Loss
2.3. Chromatographic Analysis
2.4. Statistical Analysis
3. Results
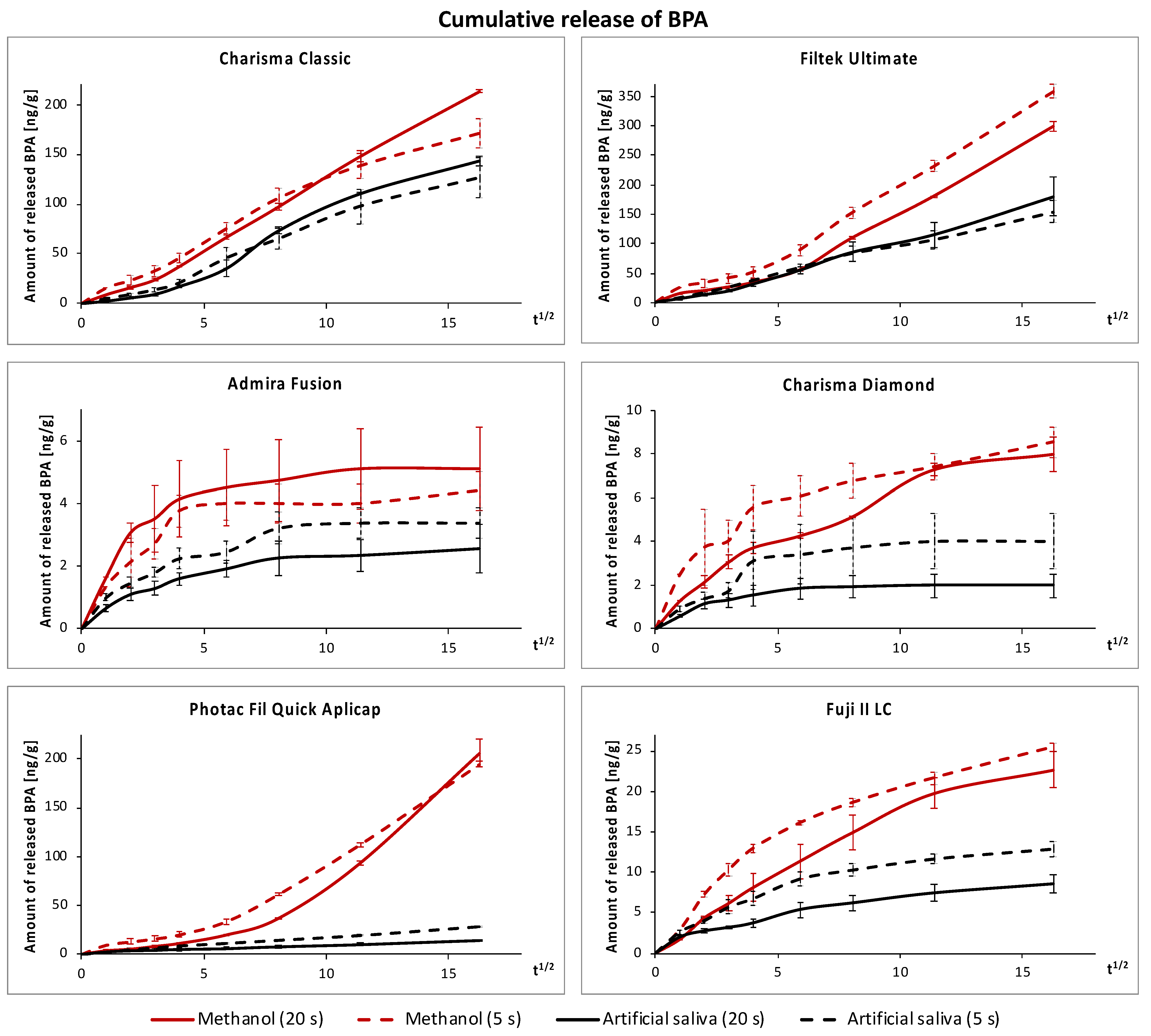
3.1. Release of BPA
3.2. Uptake of Extraction Media and Mass Loss
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vandenberg, L.N.; Maffini, M.V.; Sonnenschein, C.; Rubin, B.S.; Soto, A.M. Bisphenol-A and the great divide: A review of controversies in the field of endocrine disruption. Endocr. Rev. 2009, 30, 75–95. [Google Scholar] [CrossRef] [PubMed]
- Kolatorova Sosvorova, L.; Chlupacova, T.; Vitku, J.; Vlk, M.; Heracek, J.; Starka, L.; Saman, D.; Simkova, M.; Hampl, R. Determination of selected bisphenols, parabens and estrogens in human plasma using LC-MS/MS. Talanta 2017, 174, 21–28. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Bourguignon, J.-P.; Giudice, L.C.; Hauser, R.; Prins, G.S.; Soto, A.M.; Zoeller, R.T.; Gore, A.C. Endocrine-disrupting chemicals: An Endocrine Society scientific statement. Endocr. Rev. 2009, 30, 293–342. [Google Scholar] [CrossRef] [PubMed]
- Rochester, J.R. Bisphenol A and human health: A review of the literature. Reprod. Toxicol. 2013, 42, 132–155. [Google Scholar] [CrossRef] [PubMed]
- Kolatorova, L.; Duskova, M.; Vitku, J.; Starka, L. Prenatal exposure to bisphenols and parabens and impacts on human physiology. Physiol. Res. 2017, 66, S305–S315. [Google Scholar] [CrossRef]
- The United States Food and Drug Administration. Bisphenol A (BPA): Use in Food Contact Application. Available online: https://www.fda.gov/food/food-additives-petitions/bisphenol-bpa-use-food-contact-application (accessed on 22 July 2021).
- EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids. Scientific opinion on the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs. EFSA J. 2015, 13, 3978. [Google Scholar] [CrossRef]
- Vandenberg, L.N.; Colborn, T.; Hayes, T.B.; Heindel, J.J.; Jacobs, D.R., Jr.; Lee, D.H.; Shioda, T.; Soto, A.M.; vom Saal, F.S.; Welshons, W.V.; et al. Hormones and endocrine-disrupting chemicals: Low-dose effects and nonmonotonic dose responses. Endocr. Rev. 2012, 33, 378–455. [Google Scholar] [CrossRef]
- Birnbaum, L.S. Environmental chemicals: Evaluating low-dose effects. Environ. Health Perspect 2012, 120, a143–a144. [Google Scholar] [CrossRef]
- vom Saal, F.S.; Hughes, C. An extensive new literature concerning low-dose effects of bisphenol A shows the need for a new risk assessment. Environ. Health Perspect 2005, 113, 926–933. [Google Scholar] [CrossRef]
- Vogel, S.A. The politics of plastics: The making and unmaking of bisphenol a “safety”. Am. J. Public Health 2009, 99 (Suppl. S3), S559–S566. [Google Scholar] [CrossRef]
- Geens, T.; Aerts, D.; Berthot, C.; Bourguignon, J.-P.; Goeyens, L.; Lecomte, P.; Maghuin-Rogister, G.; Pironnet, A.-M.; Pussemier, L.; Scippo, M.-L.; et al. A review of dietary and non-dietary exposure to bisphenol-A. Food Chem. Toxicol. 2012, 50, 3725–3740. [Google Scholar] [CrossRef]
- Fleisch, A.F.; Sheffield, P.E.; Chinn, C.; Edelstein, B.L.; Landrigan, P.J. Bisphenol A and related compounds in dental materials. Pediatrics 2010, 126, 760–768. [Google Scholar] [CrossRef] [PubMed]
- De Nys, S.; Duca, R.C.; Vervliet, P.; Covaci, A.; Boonen, I.; Elskens, M.; Vanoirbeek, J.; Godderis, L.; Van Meerbeek, B.; Van Landuyt, K.L. Bisphenol A as degradation product of monomers used in resin-based dental materials. Dent. Mater. 2021, 37, 1020–1029. [Google Scholar] [CrossRef]
- Soderholm, K.J.; Mariotti, A. BIS-GMA--based resins in dentistry: Are they safe? J. Am. Dent. Assoc. 1999, 130, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Olea, N.; Pulgar, R.; Pérez, P.; Olea-Serrano, F.; Rivas, A.; Novillo-Fertrell, A.; Pedraza, V.; Soto, A.M.; Sonnenschein, C. Estrogenicity of resin-based composites and sealants used in dentistry. Environ. Health Perspect 1996, 104, 298–305. [Google Scholar] [CrossRef]
- Habib, C.M.; Kugel, G. Estrogenicity of resin-based composites and sealants in dentistry. Environ. Health Perspect 1996, 104, 808. [Google Scholar] [CrossRef][Green Version]
- Imai, Y. Comments on “Determination of bisphenol A and related aromatic compounds released from bis-GMA-based composites and sealants by high performance liquid chromatography”. Environ. Health Perspect 2000, 108, a545–546. [Google Scholar] [CrossRef] [PubMed]
- Kechagias, K.; Anastasaki, P.; Kyriakidou, M.; Dedi, K.D. Bisphenol A in dentistry. Eur. J. Prosthodont. Restor. Dent. 2020, 28, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Löfroth, M.; Ghasemimehr, M.; Falk, A.; Vult von Steyern, P. Bisphenol A in dental materials—Existence, leakage and biological effects. Heliyon 2019, 5, e01711. [Google Scholar] [CrossRef]
- Testai, E.; Epstein, M.; Emri, I.; Hartemann, P.; Hoet, P.; Leitgeb, N.; Martínez Martinez, L.; Proykova, A.; Rizzo, L.; Rodriguez-Farré, E.; et al. The safety of the use of bisphenol A in medical devices. Regul. Toxicol. Pharmacol. 2016, 79, 106–107. [Google Scholar] [CrossRef]
- Van Landuyt, K.L.; Nawrot, T.; Geebelen, B.; De Munck, J.; Snauwaert, J.; Yoshihara, K.; Scheers, H.; Godderis, L.; Hoet, P.; Van Meerbeek, B. How much do resin-based dental materials release? A meta-analytical approach. Dent. Mater. 2011, 27, 723–747. [Google Scholar] [CrossRef] [PubMed]
- Manabe, A.; Kaneko, S.; Numazawa, S.; Itoh, K.; Inoue, M.; Hisamitsu, H.; Sasa, R.; Yoshida, T. Detection of bisphenol-A in dental materials by gas chromatography-mass spectrometry. Dent. Mater. J. 2000, 19, 75–86. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kwon, H.J.; Oh, Y.J.; Jang, J.H.; Park, J.E.; Hwang, K.S.; Park, Y.D. The effect of polymerization conditions on the amounts of unreacted monomer and bisphenol A in dental composite resins. Dent. Mater. J. 2015, 34, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Polydorou, O.; König, A.; Hellwig, E.; Kümmerer, K. Long-term release of monomers from modern dental-composite materials. Eur. J. Oral Sci. 2009, 117, 68–75. [Google Scholar] [CrossRef]
- Simkova, M.; Tichy, A.; Duskova, M.; Bradna, P. Dental composites—A low-dose source of bisphenol A? Phys. Res. 2020, 69, S295–S304. [Google Scholar] [CrossRef]
- Becher, R.; Wellendorf, H.; Sakhi, A.K.; Samuelsen, J.T.; Thomsen, C.; Bølling, A.K.; Kopperud, H.M. Presence and leaching of bisphenol A (BPA) from dental materials. Acta Biomater. Odontol. Scand. 2018, 4, 56–62. [Google Scholar] [CrossRef]
- De Nys, S.; Putzeys, E.; Duca, R.C.; Vervliet, P.; Covaci, A.; Boonen, I.; Elskens, M.; Vanoirbeek, J.; Godderis, L.; Van Meerbeek, B.; et al. Long-term elution of bisphenol A from dental composites. Dent. Mater. 2021, 37, 1561–1568. [Google Scholar] [CrossRef] [PubMed]
- Rogalewicz, R.; Batko, K.; Voelkel, A. Identification of organic extractables from commercial resin-modified glass-ionomers using HPLC-MS. J. Environ. Monit. 2006, 8, 750–758. [Google Scholar] [CrossRef]
- Mazzaoui, S.A.; Burrow, M.F.; Tyas, M.J.; Rooney, F.R.; Capon, R.J. Long-term quantification of the release of monomers from dental resin composites and a resin-modified glass ionomer cement. J. Biomed. Mater. Res. 2002, 63, 299–305. [Google Scholar] [CrossRef]
- Tichy, A.; Bradna, P. Applicability of exposure reciprocity law for fast polymerization of restorative composites containing various photoinitiating systems. Oper Dent. 2021, 46, 406–418. [Google Scholar] [CrossRef]
- Bradna, P.; Vrbova, R.; Fialova, V.; Housova, D.; Gojisova, E. Formation of protective deposits by anti-erosive toothpastes—A microscopic study on enamel with artificial defects. Scanning 2016, 38, 380–388. [Google Scholar] [CrossRef][Green Version]
- Vitku, J.; Chlupacova, T.; Sosvorova, L.; Hampl, R.; Hill, M.; Heracek, J.; Bicikova, M.; Starka, L. Development and validation of LC-MS/MS method for quantification of bisphenol A and estrogens in human plasma and seminal fluid. Talanta 2015, 140, 62–67. [Google Scholar] [CrossRef]
- Arenholt-Bindslev, D.; Breinholt, V.; Preiss, A.; Schmalz, G. Time-related bisphenol-A content and estrogenic activity in saliva samples collected in relation to placement of fissure sealants. Clin. Oral Investig. 1999, 3, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Fung, E.Y.K.; Ewoldsen, N.O.; St. Germain, H.A.; Marx, D.B.; Miaw, C.-L.; Siew, C.; Chou, H.-N.; Gruninger, S.E.; Meyer, D.M. Pharmacokinetics of bisphenol A released from a dental sealant. J. Am. Dent. Assoc. 2000, 131, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Yi, S.-K.; Kim, S.-Y.; Kim, J.-S.; Son, S.-A.; Jeong, S.-H.; Kim, J.-B. Salivary bisphenol A levels and their association with composite resin restoration. Chemosphere 2017, 172, 46–51. [Google Scholar] [CrossRef]
- Zimmerman-Downs, J.M.; Shuman, D.; Stull, S.C.; Ratzlaff, R.E. Bisphenol A blood and saliva levels prior to and after dental sealant placement in adults. J. Dent. Hyg. 2010, 84, 145–150. [Google Scholar]
- Sasaki, N.; Okuda, K.; Kato, T.; Kakishima, H.; Okuma, H.; Abe, K.; Tachino, H.; Tuchida, K.; Kubono, K. Salivary bisphenol-A levels detected by ELISA after restoration with composite resin. J. Mater. Sci. Mater. Med. 2005, 16, 297–300. [Google Scholar] [CrossRef]
- Joskow, R.; Barr, D.B.; Barr, J.R.; Calafat, A.M.; Needham, L.L.; Rubin, C. Exposure to bisphenol A from bis-glycidyl dimethacrylate–based dental sealants. J. Am. Dent. Assoc. 2006, 137, 353–362. [Google Scholar] [CrossRef]
- Berge, T.L.L.; Lygre, G.B.; Lie, S.A.; Lindh, C.H.; Bjorkman, L. Bisphenol A in human saliva and urine before and after treatment with dental polymer-based restorative materials. Eur. J. Oral Sci. 2019, 127, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Price, R.B.; Murphy, D.G.; Derand, T. Light energy transmission through cured resin composite and human dentin. Quintessence Int. 2000, 31, 659–667. [Google Scholar]
- De Nys, S.; Putzeys, E.; Vervliet, P.; Covaci, A.; Boonen, I.; Elskens, M.; Vanoirbeek, J.; Godderis, L.; Van Meerbeek, B.; Van Landuyt, K.L.; et al. A novel high sensitivity UPLC-MS/MS method for the evaluation of bisphenol A leaching from dental materials. Sci. Rep. 2018, 8, 6981. [Google Scholar] [CrossRef] [PubMed]
- Imai, Y.; Komabayashi, T. Elution of bisphenol A from composite resin: A model experiment. Dent. Mater. J. 2000, 19, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Pilo, R.; Cardash, H.S. Post-irradiation polymerization of different anterior and posterior visible light-activated resin composites. Dent. Mater. 1992, 8, 299–304. [Google Scholar] [CrossRef]
- Leung, R.L.; Fan, P.L.; Johnston, W.M. Post-irradiation polymerization of visible light-activated composite resin. J. Dent. Res. 1983, 62, 363–365. [Google Scholar] [CrossRef]

| Material (Abbreviation) | Manufacturer (Batch Number) | Composition |
|---|---|---|
| Charisma Classic A2 (CC) | Kulzer, Hanau, Germany (K010733) | Bis-GMA, TEGDMA, Ba-Al-F glass fillers, pre-polymerized filler, pyrogenic silica, initiator |
| Filtek Ultimate Universal Restorative A2 Dentin (FU) | 3M, St. Paul, MN, USA (N985020) | Bis-GMA, Bis-EMA, UDMA, TEGDMA, PEGDMA, non-agglomerated/non-aggregated silica and zirconia filler, aggregated zirconia/silica cluster filler, initiator |
| Charisma Diamond A2 (CD) | Kulzer, Hanau, Germany (K010073) | TCD-DI-HEA, UDMA, TEGDMA, Ba-Al-F glass fillers, pyrogenic silica, initiator |
| Admira Fusion A2 (AF) | Voco, Cuxhaven, Germany (1919447) | no conventional methacrylate monomers, “organically modified ceramics” resin, glass ceramic filler, nano filler, initiator |
| Photac Fil Quick Aplicap A2 (PF) | 3M, St. Paul, MN, USA (4587570) | Na-Ca-Al-La-F silicate glass, HEMA, difunctional monomers, activator (amine), copolymer of acrylic acid and maleic acid, camphorquinone; stabilizers |
| GC Fuji II LC Capsule A2 (F2) | GC, Tokyo, Japan (190219A) | Al-F silicate glass, polyacrylic acid, HEMA, 2,2,4-trimethyl hexamethylene dicarbonate, TEGDMA |
| Material | Polymer Conditions | 1 Day (Day 1) | 4 Days (Days 2–4) | 9 Days (Days 5–9) | 16 Days (Days 10–16) | 35 Days (Days 17–35) | 65 Days (Days 36–65) | 130 Days (Days 66–130) | 260 Days (Days 130–260) |
|---|---|---|---|---|---|---|---|---|---|
| Charisma Classic | 20 s, 1300 mW/cm2 | 2.43 ± 0.19 Aa | 1.11 ± 0.12 Abc | 0.73 ± 0.02 Acd | 1.11 ± 0.20 Abc | 0.95 ± 0.38 Abcd | 1.27 ± 0.19 Bb | 0.57 ± 0.05 Ade | 0.25 ± 0.04 Ae |
| 5 s, 3000 mW/cm2 | 5.09 ± 0.79 Ba | 1.36 ± 0.11 Ab | 0.97 ± 0.19 Abc | 0.99 ± 0.18 Abc | 1.31 ± 0.40 Ab | 0.64 ± 0.09 Acd | 0.50 ± 0.10 Acd | 0.21 ± 0.03 Ad | |
| Filtek Ultimate Universal Restorative | 20 s, 1300 mW/cm2 | 6.24 ± 0.84 Aa | 2.23 ± 0.09 Ab | 1.28 ± 0.12 Acd | 1.71 ± 0.31 Abc | 1.25 ± 0.14 Acd | 1.03 ± 0.39 Acd | 0.46 ± 0.07 Ad | 0.48 ± 0.27 Ad |
| 5 s, 3000 mW/cm2 | 7.87 ± 1.37 Ba | 2.82 ± 0.10 Bb | 1.95 ± 0.26 Ac | 1.62 ± 0.14 Acd | 1.18 ± 0.18 Acd | 0.80 ± 0.21 Ade | 0.35 ± 0.04 Ae | 0.35 ± 0.03 Ae | |
| Charisma Diamond | 20 s, 1300 mW/cm2 | 0.58 ± 0.06 Aa | 0.19 ± 0.08 Ab | 0.03 ± 0.03 Ac | 0.05 ± 0.01 Ac | 0.02 ± 0.00 Ac | 0.00 ± 0.00 Ac | 0.00 ± 0.00 Ac | 0.00 ± 0.00 Ac |
| 5 s, 3000 mW/cm2 | 0.93 ± 0.12 Ba | 0.15 ± 0.06 Ab | 0.07 ± 0.01 Abc | 0.10 ± 0.01 Abc | 0.01 ± 0.00 Ac | 0.01 ± 0.01 Ac | 0.00 ± 0.00 Ac | 0.00 ± 0.00 Ac | |
| Admira Fusion | 20 s, 1300 mW/cm2 | 0.66 ± 0.11 Aa | 0.14 ± 0.04 Ab | 0.04 ± 0.01 Ac | 0.04 ± 0.01 Ac | 0.02 ± 0.00 Ac | 0.01 ± 0.01 Ac | 0.00 ± 0.00 Ac | 0.00 ± 0.00 Ac |
| 5 s, 3000 mW/cm2 | 1.00 ± 0.10 Ba | 0.15 ± 0.02 Ab | 0.07 ± 0.02 Ac | 0.06 ± 0.03 Ac | 0.01 ± 0.00 Ac | 0.03 ± 0.02 Ac | 0.00 ± 0.00 Ac | 0.00 ± 0.00 Ac | |
| Photac Fil Quick Aplicap | 20 s, 1300 mW/cm2 | 2.40 ± 1.07 Aa | 0.37 ± 0.04 Ab | 0.09 ± 0.01 Ab | 0.13 ± 0.02 Ab | 0.06 ± 0.01 Ab | 0.06 ± 0.02 Ab | 0.03 ± 0.00 Ab | 0.03 ± 0.00 Ab |
| 5 s, 3000 mW/cm2 | 2.02 ± 0.12 Aa | 0.84 ± 0.28 Bb | 0.37 ± 0.02 Bbc | 0.28 ± 0.01 Bbc | 0.14 ± 0.01 Bc | 0.10 ± 0.01 Ac | 0.07 ± 0.01 Bc | 0.07 ± 0.01 Bc | |
| GC Fuji II LC Capsule | 20 s, 1300 mW/cm2 | 2.01 ± 0.19 Aa | 0.25 ± 0.02 Ab | 0.09 ± 0.01 Abc | 0.12 ± 0.04 Abc | 0.09 ± 0.02 Abc | 0.03 ± 0.00 Ac | 0.02 ± 0.00 Ac | 0.01 ± 0.00 Ac |
| 5 s, 3000 mW/cm2 | 2.62 ± 0.25 Ba | 0.46 ± 0.03 Bb | 0.34 ± 0.12 Bb | 0.15 ± 0.02 Ac | 0.13 ± 0.04 Ac | 0.04 ± 0.01 Ac | 0.02 ± 0.00 Ac | 0.01 ± 0.00 Ac |
| Material | Polymer. Conditions | 1 Day (Day 1) | 4 Days (Days 2–4) | 9 Days (Days 5–9) | 16 Days (Days 10–16) | 35 Days (Days 17–35) | 65 Days (Days 36–65) | 130 Days (Days 66–130) | 260 Days (Days 130–260) |
|---|---|---|---|---|---|---|---|---|---|
| Charisma Classic | 20 s, 1300 mW/cm2 | 9.01 ± 1.23 Aa | 2.36 ± 0.19 Ab | 1.57 ± 0.17 Ac | 1.90 ± 0.44 Abc | 1.54 ± 0.07 Acd | 1.03 ± 0.07 Ad | 0.78 ± 0.06 Be | 0.48 ± 0.02 Bf |
| 5 s, 3000 mW/cm2 | 15.7 ± 4.56 Ba | 2.51 ± 0.24 Ab | 1.93 ± 0.07 Abc | 1.84 ± 0.34 Abc | 1.54 ± 0.25 Acd | 1.03 ± 0.17 Ad | 0.50 ± 0.03 Ae | 0.24 ± 0.02 Af | |
| Filtek Ultimate Universal Restorative | 20 s, 1300 mW/cm2 | 15.0 ± 0.64 Aa | 1.66 ± 0.22 Ab | 1.21 ± 0.10 Abc | 1.12 ± 0.02 Ac | 1.14 ± 0.04 Ac | 1.82 ± 0.04 Ab | 1.09 ± 0.10 Ac | 0.88 ± 0.06 Ac |
| 5 s, 3000 mW/cm2 | 25.6 ± 7.03 Ba | 2.61 ± 0.52 Ab | 1.68 ± 0.31 Abc | 1.40 ± 0.30 Ac | 1.99 ± 0.04 Bb | 2.12 ± 0.19 Ab | 1.21 ± 0.05 Ac | 0.94 ± 0.03 Ad | |
| Charisma Diamond | 20 s, 1300 mW/cm2 | 1.28 ± 0.29 Aa | 0.28 ± 0.03 Ab | 0.18 ± 0.01 Ab | 0.09 ± 0.02 Ab | 0.06 ± 0.03 Ab | 0.03 ± 0.01 Ab | 0.03 ± 0.01 Ab | 0.01 ± 0.00 Ab |
| 5 s, 3000 mW/cm2 | 2.50 ± 1.74 Ba | 0.41 ± 0.31 Ab | 0.06 ± 0.02 Ab | 0.22 ± 0.08 Ab | 0.03 ± 0.02 Ab | 0.02 ± 0.01 Ab | 0.01 ± 0.01 Ab | 0.01 ± 0.00 Ab | |
| Admira Fusion | 20 s, 1300 mW/cm2 | 1.65 ± 0.29 Aa | 0.48 ± 0.43 Ab | 0.09 ± 0.06 Ab | 0.09 ± 0.02 Ab | 0.02 ± 0.01 Ab | 0.01 ± 0.00 Ab | 0.01 ± 0.00 Ab | 0.00 ± 0.00 Ab |
| 5 s, 3000 mW/cm2 | 1.35 ± 0.78 Aa | 0.26 ± 0.10 Ab | 0.12 ± 0.12 Ab | 0.15 ± 0.09 Ab | 0.01 ± 0.01 Ab | 0.00 ± 0.00 Ab | 0.00 ± 0.00 Ab | 0.00 ± 0.00 Ab | |
| Photac Fil Quick Aplicap | 20 s, 1300 mW/cm2 | 3.71 ± 0.47 Aa | 0.56 ± 0.12 Abc | 0.57 ± 0.03 Abc | 0.43 ± 0.07 Ac | 0.45 ± 0.02 Ac | 0.57 ± 0.06 Abc | 0.87 ± 0.21 Ab | 0.83 ± 0.01 Bb |
| 5 s, 3000 mW/cm2 | 9.27 ± 2.44 Ba | 1.11 ± 0.26 Ab | 0.62 ± 0.04 Ac | 0.60 ± 0.05 Ac | 0.70 ± 0.07 Bc | 0.94 ± 0.06 Bbc | 0.78 ± 0.07 Abc | 0.61 ± 0.06 Ac | |
| GC Fuji II LC Capsule | 20 s, 1300 mW/cm2 | 1.72 ± 0.11 Aa | 0.88 ± 0.29 Ab | 0.36 ± 0.18 Ac | 0.28 ± 0.06 Acd | 0.17 ± 0.01 Acd | 0.12 ± 0.01 Acd | 0.07 ± 0.01 Acd | 0.02 ± 0.00 Ad |
| 5 s, 3000 mW/cm2 | 2.91 ± 0.41 Ba | 1.46 ± 0.19 Bb | 0.61 ± 0.09 Ac | 0.38 ± 0.08 Acd | 0.17 ± 0.02 Ade | 0.08 ± 0.01 Ade | 0.05 ± 0.01 Ae | 0.03 ± 0.01 Ae |
| Extraction Medium | Polymerization Conditions | Charisma Classic | Filtek UltimateUniv. Restorative | Charisma Diamond | Admira Fusion | Photac Fil Quick Aplicap | GC Fuji II LC Capsule |
|---|---|---|---|---|---|---|---|
| Artificial saliva | 20 s, 1300 mW/cm2 | 143.7 ± 6.0 Aa | 180.4 ± 39.1 Aa | 2.09 ± 0.48 Ab | 2.54 ± 0.95 Ab | 14.1 ± 1.7 Ac | 8.84 ± 1.28 Ad |
| 5 s, 3000 mW/cm2 | 126.4 ± 24.7 Aa | 154.1 ± 23.1 Aa | 3.34 ± 0.42 Ab | 3.39 ± 0.59 ABb | 27.6 ± 1.1 Bc | 12.9 ± 1.2 Bd | |
| Methanol | 20 s, 1300 mW/cm2 | 213.1 ± 4.90 Ba | 299.0 ± 18.2 Bb | 7.98 ± 0.46 Bc | 5.11 ± 1.65 Bd | 206.1 ± 17.0 Ca | 22.7 ± 3.0 Ce |
| 5 s, 3000 mW/cm2 | 170.8 ± 19.0 Ca | 358.0 ± 16.3 Cb | 8.53 ± 0.82 Bc | 4.40 ± 1.03 ABd | 194.3 ± 7.8 Ca | 25.4 ± 2.2 Ce |
| Extraction Medium | Polymerization Conditions | Charisma Classic | Filtek UltimateUniv. Restorative | Charisma Diamond | Admira Fusion |
|---|---|---|---|---|---|
| Artificial saliva | 20 s, 1300 mW/cm2 | 0.73 ± 0.14 Aa | 1.24 ± 0.05 Ab | 0.61 ± 0.03 Aa | 0.64 ± 0.09 Aa |
| 5 s, 3000 mW/cm2 | 0.68 ± 0.17 Aa | 1.27 ± 0.01 Ab | 0.52 ± 0.02 Aa | 0.72 ± 0.004 Aa | |
| Methanol | 20 s, 1300 mW/cm2 | 3.39 ± 0.15 Ba | 1.37 ± 0.05 Bb | 3.37 ± 0.11 Ba | 2.29 ± 0.11 Bc |
| 5 s, 3000 mW/cm2 | 3.89 ± 0.17 Ca | 1.62 ± 0.17 Cb | 3.48 ± 0.01 Bc | 2.49 ± 0.07 Cd |
| Extraction Medium | Polymerization Conditions | Charisma Classic | Filtek UltimateUniv. Restorative | Charisma Diamond | Admira Fusion |
|---|---|---|---|---|---|
| Artificial saliva | 20 s, 1300 mW/cm2 | 0.05 ± 0.02 Aa | 0.04 ± 0.01 Aa | 0.06 ± 0.02 Aa | 0.12 ± 0.05 Ab |
| 5 s, 3000 mW/cm2 | 0.12 ± 0.07 Aa | 0.12 ± 0.03 Aa | 0.11 ± 0.03 Aa | 0.12 ± 0.01 Aa | |
| Methanol | 20 s, 1300 mW/cm2 | 2.27 ± 0.10 Ba | 0.38 ± 0.06 Bb | 2.04 ± 0.08 Bc | 1.13 ± 0.08 Bd |
| 5 s, 3000 mW/cm2 | 3.06 ± 0.19 Ca | 0.83 ± 0.13 Cb | 2.48 ± 0.05 Cc | 1.35 ± 0.08 Cd |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tichy, A.; Simkova, M.; Vrbova, R.; Roubickova, A.; Duskova, M.; Bradna, P. Bisphenol A Release from Dental Composites and Resin-Modified Glass Ionomers under Two Polymerization Conditions. Polymers 2022, 14, 46. https://doi.org/10.3390/polym14010046
Tichy A, Simkova M, Vrbova R, Roubickova A, Duskova M, Bradna P. Bisphenol A Release from Dental Composites and Resin-Modified Glass Ionomers under Two Polymerization Conditions. Polymers. 2022; 14(1):46. https://doi.org/10.3390/polym14010046
Chicago/Turabian StyleTichy, Antonin, Marketa Simkova, Radka Vrbova, Adela Roubickova, Michaela Duskova, and Pavel Bradna. 2022. "Bisphenol A Release from Dental Composites and Resin-Modified Glass Ionomers under Two Polymerization Conditions" Polymers 14, no. 1: 46. https://doi.org/10.3390/polym14010046
APA StyleTichy, A., Simkova, M., Vrbova, R., Roubickova, A., Duskova, M., & Bradna, P. (2022). Bisphenol A Release from Dental Composites and Resin-Modified Glass Ionomers under Two Polymerization Conditions. Polymers, 14(1), 46. https://doi.org/10.3390/polym14010046







