Phase Change Energy Storage Elastic Fiber: A Simple Route to Personal Thermal Management
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
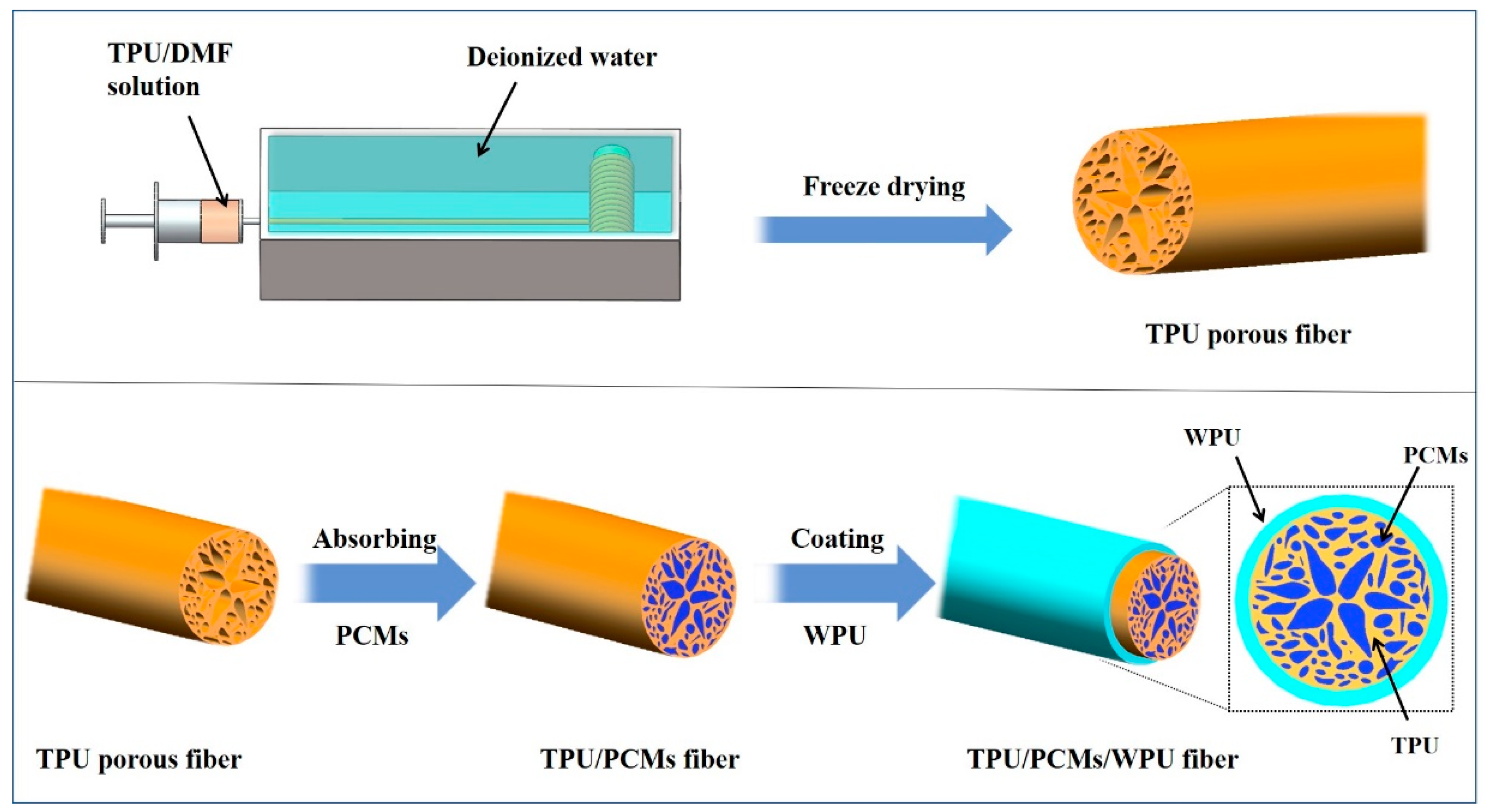
2.2. Preparation of PCFs
2.3. Characterization
3. Results and Discussion
3.1. Structure and Morphology
3.2. Characterization of PCFs
3.2.1. FT-IR
3.2.2. XRD
3.3. Thermal Behaviors and Properties
3.3.1. Thermal Storage Capability
3.3.2. Thermal Durability
3.3.3. Encapsulation Stability
3.3.4. Thermal Insulation Performance of Fabric
3.4. Thermal Behaviors and Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akpinar, E.K.; Koçyiğit, F. Energy and exergy analysis of a new flat-plate solar air heater having different obstacles on absorber plates. Appl. Energy 2010, 87, 3438–3450. [Google Scholar] [CrossRef]
- Nong, D.; Simshauser, P.; Nguyen, D.B. Greenhouse gas emissions vs. CO2 emissions: Comparative analysis of a global carbon tax. Appl. Energy 2021, 298, 117223. [Google Scholar] [CrossRef]
- Zhang, P.; Ma, F.; Xiao, X. Thermal energy storage and retrieval characteristics of a molten-salt latent heat thermal energy storage system. Appl. Energy 2016, 173, 255–271. [Google Scholar] [CrossRef]
- Alva, G.; Lin, Y.; Fang, G. An overview of thermal energy storage systems. Energy 2018, 144, 341–378. [Google Scholar] [CrossRef]
- Ibrahim, H.; Ilinca, A.; Perron, J. Energy storage systems—Characteristics and comparisons. Renew. Sustain. Energy Rev. 2008, 12, 1221–1250. [Google Scholar] [CrossRef]
- Li, Z.; Lu, Y.; Huang, R.; Chang, J.; Yu, X.; Jiang, R.; Yu, X.; Roskilly, A.P. Applications and technological challenges for heat recovery, storage and utilisation with latent thermal energy storage. Appl. Energy 2020, 283, 116277. [Google Scholar] [CrossRef]
- Pasupathy, A.; Velraj, R.; Seeniraj, R. Phase change material-based building architecture for thermal management in residential and commercial establishments. Renew. Sustain. Energy Rev. 2008, 12, 39–64. [Google Scholar] [CrossRef]
- Dheep, G.R.; Sreekumar, A. Influence of nanomaterials on properties of latent heat solar thermal energy storage materials–A review. Energy Convers. Manag. 2014, 83, 133–148. [Google Scholar] [CrossRef]
- Chen, X.; Gao, H.; Tang, Z.; Dong, W.; Li, A.; Wang, G. Optimization strategies of composite phase change materials for thermal energy storage, transfer, conversion and utilization. Energy Environ. Sci. 2020, 13, 4498–4535. [Google Scholar] [CrossRef]
- Kuznik, F.; David, D.; Johannes, K.; Roux, J.-J. A review on phase change materials integrated in building walls. Renew. Sustain. Energy Rev. 2011, 15, 379–391. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Hong, G.; Dong, D.; Song, W.; Zhang, X. Multiresponsive graphene-aerogel-directed phase-change smart fibers. Adv. Mater. 2018, 30, 1801754. [Google Scholar] [CrossRef]
- Rao, Z.; Wang, Q.; Huang, C. Investigation of the thermal performance of phase change material/mini-channel coupled battery thermal management system. Appl. Energy 2016, 164, 659–669. [Google Scholar] [CrossRef]
- Lyu, B.; Kim, M.; Jing, H.; Kang, J.; Qian, C.; Lee, S.; Cho, J.H. Large-area MXene electrode array for flexible electronics. ACS Nano 2019, 13, 11392–11400. [Google Scholar] [CrossRef] [PubMed]
- Aftab, W.; Usman, A.; Shi, J.; Yuan, K.; Qin, M.; Zou, R. Phase change material-integrated latent heat storage systems for sustainable energy solutions. Energy Environ. Sci. 2021, 14, 4268–4291. [Google Scholar] [CrossRef]
- Feng, H.; Liu, X.; He, S.; Wu, K.; Zhang, J. Studies on solid–solid phase transitions of polyols by infrared spectroscopy. Thermochim. Acta 2000, 348, 175–179. [Google Scholar] [CrossRef]
- Kou, Y.; Sun, K.; Luo, J.; Zhou, F.; Huang, H.; Wu, Z.-S.; Shi, Q. An intrinsically flexible phase change film for wearable thermal managements. Energy Storage Mater. 2021, 34, 508–514. [Google Scholar] [CrossRef]
- Cheng, P.; Gao, H.; Chen, X.; Chen, Y.; Han, M.; Xing, L.; Liu, P.; Wang, G. Flexible monolithic phase change material based on carbon nanotubes/chitosan/poly (vinyl alcohol). Chem. Eng. J. 2020, 397, 125330. [Google Scholar] [CrossRef]
- Yi, L.; Ming, Y.; Ma, Q.; Yue, Q.; Ge, W. Introduction of an organic acid phase changing material into metal–organic frameworks and the study of its thermal properties. J. Mater. Chem. A 2016, 4, 7641–7649. [Google Scholar]
- Guo, J.; Xiang, H.X.; Gong, X.Y.; Wang, Q.Q. Preparation and Properties of Polyacrylonitrile / Binary of Fatty Acids Composites as Phase Change Fibers. Adv. Mater. Res. 2011, 239, 1199–1202. [Google Scholar] [CrossRef]
- Ng, D.Q.; Tseng, Y.L.; Shih, Y.F.; Lian, H.Y.; Yu, Y.H. Synthesis of novel phase change material microcapsule and its application. Polymer 2017, 133, 250–262. [Google Scholar] [CrossRef]
- Zhang, Z.; Shi, G.; Wang, S.; Fang, X.; Liu, X. Thermal energy storage cement mortar containing n-octadecane/expanded graphite composite phase change material. Renew. Energy 2013, 50, 670–675. [Google Scholar] [CrossRef]
- Cai, Y.; Ke, H.; Lin, L.; Fei, X.; Wei, Q.; Song, L.; Hu, Y.; Fong, H. Preparation, morphology and thermal properties of electrospun fatty acid eutectics/polyethylene terephthalate form-stable phase change ultrafine composite fibers for thermal energy storage. Energy Convers. Manag. 2012, 64, 245–255. [Google Scholar] [CrossRef]
- Qian, T.; Li, J.; Min, X.; Deng, Y.; Guan, W.; Ning, L. Diatomite: A promising natural candidate as carrier material for low, middle and high temperature phase change material. Energy Convers. Manag. 2015, 98, 34–45. [Google Scholar] [CrossRef]
- Yan, Y.; Li, W.; Zhu, R.; Lin, C.; Hufenus, R. Flexible Phase Change Material Fiber: A Simple Route to Thermal Energy Control Textiles. Materials 2021, 14, 401. [Google Scholar] [CrossRef]
- McCann, J.T.; Marquez, M.; Xia, Y. Melt coaxial electrospinning: A versatile method for the encapsulation of solid materials and fabrication of phase change nanofibers. Nano Lett. 2006, 6, 2868–2872. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Yan, Y.; Liu, Z.; Ke, H.; Qiu, Z.; Hufenus, R. Flexible phase change filament with ionic liquid core. J. Appl. Polym. Sci. 2019, 136, 47830. [Google Scholar] [CrossRef]
- Xia, W.; Xiang, H.; Zhou, Z.; Fei, X.; Zhu, M. Hybridizing rational designed hydrophobic PEG-based derivatives into nanoporous F–SiO2 as form-stable phase change materials for melt-spun PA6 phase change fibers with a superior washing durability. Compos. Commun. 2021, 24, 100633. [Google Scholar] [CrossRef]
- Hu, L.; Li, X.; Ding, L.; Chen, L.; Zhu, X.; Mao, Z.; Feng, X.; Sui, X.; Wang, B. Flexible textiles with polypyrrole deposited phase change microcapsules for efficient photothermal energy conversion and storage. Sol. Energy Mater. Sol. Cells 2021, 224, 110985. [Google Scholar] [CrossRef]
- Chen, L.; Zou, R.; Xia, W.; Liu, Z.; Shang, Y.; Zhu, J.; Wang, Y.; Lin, J.; Xia, D.; Cao, A. Electro-and photodriven phase change composites based on wax-infiltrated carbon nanotube sponges. ACS Nano 2012, 6, 10884–10892. [Google Scholar] [CrossRef]
- Qi, G.Q.; Yang, J.; Bao, R.Y.; Liu, Z.Y.; Yang, W.; Xie, B.H.; Yang, M.B. Enhanced comprehensive performance of polyethylene glycol based phase change material with hybrid graphene nanomaterials for thermal energy storage. Carbon 2015, 88, 196–205. [Google Scholar] [CrossRef]
- Yi, H.; Zhan, W.; Zhao, Y.; Qu, S.; Wang, W.; Chen, P.; Song, S. A novel core-shell structural montmorillonite nanosheets/stearic acid composite PCM for great promotion of thermal energy storage properties. Sol. Energy Mater. Sol. Cells 2018, 192, 57–64. [Google Scholar] [CrossRef]
- Li, C.; Yu, H.; Song, Y.; Wang, M.; Liu, Z. A N-octadecane/hierarchically Porous TiO2 Form-Stable PCM for Thermal Energy Storage. Renew. Energy 2020, 145, 1465–1473. [Google Scholar] [CrossRef]
- Qian, T.; Li, J.; Min, X. Enhanced thermal conductivity of PEG/diatomite shape-stabilized phase change materials with Ag nanoparticles for thermal energy storage. J. Mater. Chem. A 2015, 3, 8526–8536. [Google Scholar] [CrossRef]
- Cai, Y.; Ke, H.; Dong, J.; Wei, Q.; Lin, J.; Zhao, Y.; Song, L.; Hu, Y.; Huang, F.; Gao, W. Effects of nano-SiO2 on morphology, thermal energy storage, thermal stability, and combustion properties of electrospun lauric acid/PET ultrafine composite fibers as form-stable phase change materials. Appl. Energy 2011, 88, 2106–2112. [Google Scholar] [CrossRef]
- Lin, C.; Li, W.; Yan, Y.; Ke, H.; Liu, Z.; Deng, L.; Qiu, Z. Ultrafine electrospun fiber based on ionic liquid/AlN/copolyamide composite as novel form-stable phase change material for thermal energy storage. Sol. Energy Mater. Sol. Cells 2021, 223, 110953. [Google Scholar] [CrossRef]
- Kadoono, T.; Ogura, M. Heat storage properties of organic phase-change materials confined in the nanospace of mesoporous SBA-15 and CMK-3. Phys. Chem. Chem. Phys. 2014, 16, 5495–5498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, L.; Zhao, W.; Zheng, J.; Frisco, S.; Song, P.; Li, X. The shape-stabilized phase change materials composed of polyethylene glycol and various mesoporous matrices (AC, SBA-15 and MCM-41). Sol. Energy Mater. Sol. Cells 2011, 95, 3550–3556. [Google Scholar] [CrossRef]
- Li, X.; Chen, X.; Zhang, S.; Yin, Y.; Wang, C. UV-resistant transparent lignin-based polyurethane elastomer with repeatable processing performance. Eur. Polym. J. 2021, 159, 110763. [Google Scholar] [CrossRef]
- Moon, J.; Kwak, S.B.; Lee, J.Y.; Kim, D.; Ha, J.U.; Oh, J.S. Recycling of bio-polyurethane foam using high power ultrasound. Polymer 2020, 186, 122072. [Google Scholar] [CrossRef]
- Gao, J.; Wang, X.; Zhai, W.; Liu, H.; Zheng, G.; Dai, K.; Mi, L.; Liu, C.; Shen, C. Ultrastretchable Multilayered Fiber with a Hollow-Monolith Structure for High-Performance Strain Sensor. ACS Appl. Mater. Interfaces 2018, 10, 34592–34603. [Google Scholar] [CrossRef]
- Goitandia, A.M.; Beobide, G.; Aranzabe, E.; Aranzabe, A. Development of content-stable phase change composites by infiltration into inorganic porous supports. Sol. Energy Mater. Sol. Cells 2015, 134, 318–328. [Google Scholar] [CrossRef]
- Nomura, T.; Zhu, C.Y.; Sheng, N.; Tabuchi, K.; Sagara, A.; Akiyama, T. Shape-stabilized phase change composite by impregnation of octadecane into mesoporous SiO2. Sol. Energy Mater. Sol. Cells 2015, 143, 424–429. [Google Scholar] [CrossRef] [Green Version]
- Graziella, T.; Sanches, E.A.; Neto, S.C.; Mascarenhas, Y.P.; Chierice, G.O. Characterization of polyurethane resins by FTIR, TGA, and XRD. J. Appl. Polym. Sci. 2010, 115, 263–268. [Google Scholar]
- Du, X.; Fang, Y.; Cheng, X.; Du, Z.; Zhou, M.; Wang, H. Fabrication and characterization of flame-retardant nanoencapsulated n-octadecane with melamine–formaldehyde shell for thermal energy storage. ACS Sustain. Chem. Eng. 2018, 6, 15541–15549. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, Y.; Wang, D.; Zhao, Y.; Weng, S.; Xu, D.; Wu, J. FT-IR spectroscopic investigation on the interaction between nylon 66 and lithium salts. J. Appl. Polym. Sci. 2004, 91, 2869–2875. [Google Scholar] [CrossRef]
- Mitran, R.A.; Berger, D.; Munteanu, C.; Matei, C. Evaluation of Different Mesoporous Silica Supports for Energy Storage in Shape-Stabilized Phase Change Materials with Dual Thermal Responses. J. Phys. Chem. C 2015, 119, 15177–15184. [Google Scholar] [CrossRef]
- Yu, S.; Wang, X.; Wu, D. Microencapsulation of n-octadecane phase change material with calcium carbonate shell for enhancement of thermal conductivity and serving durability: Synthesis, microstructure, and performance evaluation. Appl. Energy 2014, 114, 632–643. [Google Scholar] [CrossRef]
- Sen, S.K.; Paul, T.C.; Dutta, S.; Hossain, M.N.; Mia, M.N.H. XRD peak profile and optical properties analysis of Ag-doped h-MoO3 nanorods synthesized via hydrothermal method. J. Mater. Sci. Mater. Electron. 2020, 31, 1768–1786. [Google Scholar] [CrossRef]
- Heu, C.S.; Kim, S.W.; Lee, K.S.; Kim, D.R. Fabrication of three-dimensional metal-graphene network phase change composite for high thermal conductivity and suppressed subcooling phenomena. Energy Convers. Manag. 2017, 149, 608–615. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, W.; Gao, C.; Yin, D. Shape stabilization of phase change material by polymerized high internal phase emulsion for thermal energy storage. Int. J. Energy Res. 2021, 45, 5263–5271. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Tao, S.; An, Y.; Meng, C.; Hu, T. Phase change in modified hierarchically porous monolith: An extra energy increase. Microporous Mesoporous Mater. 2014, 193, 69–76. [Google Scholar] [CrossRef]
- Qian, T.; Li, J.; Min, X.; Fan, B. Integration of pore confinement and hydrogen-bond influence on the crystallization behavior of C18 PCMs in mesoporous silica for form-stable phase change materials. Acs Sustain. Chem. Eng. 2018, 6, 897–908. [Google Scholar] [CrossRef]
- Liang, S.; Li, Q.; Zhu, Y.; Chen, K.; Tian, C.; Wang, J.; Bai, R. Nanoencapsulation of n-octadecane phase change material with silica shell through interfacial hydrolysis and polycondensation in miniemulsion. Energy 2015, 93, 1684–1692. [Google Scholar] [CrossRef]
- Syamak, F.; Rebecca, V.A.; Sepidar, S.; Shafei, S.; Fay, C.D.; Beirne, S.; Javadi, M.; Wang, X.; Innis, P.C.; Paull, B. Processable Thermally Conductive Polyurethane Composite Fibers. Macromol. Mater. Eng. 2019, 304, 1800542. [Google Scholar]
- Ahn, Y.-H.; DeWitt, S.J.; McGuire, S.; Lively, R.P. Incorporation of Phase Change Materials into Fibers for Sustainable Thermal Energy Storage. Ind. Eng. Chem. Res. 2021, 60, 3374–3384. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Tao, X.; Yick, K.L. Energy storage polymer/MicroPCMs blended chips and thermo-regulated fibers. J. Mater. Sci. 2005, 40, 3729–3734. [Google Scholar] [CrossRef]
- Zhang, X.-X.; Wang, X.-C.; Tao, X.-M.; Yick, K.-L. Structures and properties of wet spun thermo-regulated polyacrylonitrile-vinylidene chloride fibers. Text. Res. J. 2006, 76, 351–359. [Google Scholar] [CrossRef]
- Iqbal, K.; Sun, D. Development of thermo-regulating polypropylene fibre containing microencapsulated phase change materials. Renew. Energy 2014, 71, 473–479. [Google Scholar] [CrossRef]
- Jiang, M.; Song, X.; Ye, G.; Xu, J. Preparation of PVA/paraffin thermal regulating fiber by in situ microencapsulation. Compos. Sci. Technol. 2008, 68, 2231–2237. [Google Scholar] [CrossRef]
- Wen, G.-Q.; Xie, R.; Liang, W.-G.; He, X.-H.; Wang, W.; Ju, X.-J.; Chu, L.-Y. Microfluidic fabrication and thermal characteristics of core–shell phase change microfibers with high paraffin content. Appl. Therm. Eng. 2015, 87, 471–480. [Google Scholar] [CrossRef]
- Zdraveva, E.; Fang, J.; Mijovic, B.; Lin, T. Electrospun poly (vinyl alcohol)/phase change material fibers: Morphology, heat properties, and stability. Ind. Eng. Chem. Res. 2015, 54, 8706–8712. [Google Scholar] [CrossRef]
- MG, A.; Chatterjee, T.; Naskar, K. Assessing thermomechanical properties of a reactive maleic anhydride grafted styrene-ethylene-butylene-styrene/thermoplastic polyurethane blend with temperature scanning stress relaxation method. J. Appl. Polym. Sci. 2020, 137, 49598. [Google Scholar]
- Yang, Z.; Zhai, Z.; Song, Z.; Wu, Y.; Liang, J.; Shan, Y.; Zheng, J.; Liang, H.; Jiang, H. Conductive and Elastic 3D Helical Fibers for Use in Washable and Wearable Electronics. Adv. Mater. 2020, 32, 1907495. [Google Scholar] [CrossRef] [PubMed]













| Fiber | <5 μm/% | 5~50 μm/% | >50 μm/% |
|---|---|---|---|
| TPU-0.20 | 6.83 | 29.82 | 63.35 |
| TPU-0.28 | 13.44 | 31.83 | 54.73 |
| TPU-0.40 | 46.86 | 19.23 | 33.91 |
| PCMs | Before/nm | After/nm |
|---|---|---|
| OCC | 39.11 | 25.15 |
| HEO | 46.89 | 19.32 |
| SA | 41.46 | 24.23 |
| Sample | Tmo (°C) | Tmp (°C) | ΔHm (J/g) | Tco (°C) | Tcp (°C) | ΔHc (J/g) | E (%) |
|---|---|---|---|---|---|---|---|
| OCC | 26.4 | 31.4 | 226.0 | 25.4 | 21.6 | 230.1 | - |
| OCC/TPU-0.20 | 25.8 | 37.2 | 176.1 | 24.1 | 17.1 | 174.6 | 76.9 |
| OCC/TPU-0.28 | 26.4 | 35.0 | 154.2 | 25.0 | 17.9 | 157.8 | 68.4 |
| OCC/TPU-0.40 | 26.3 | 33.7 | 127.7 | 24.8 | 18.7 | 128.8 | 56.2 |
| PCMs | φ/% | ||
|---|---|---|---|
| 6.83/TPU-0.20 | 13.44/TPU-0.28 | 46.86/TPU-0.40 | |
| OCC | 99.83 | 98.08 | 97.83 |
| HEO | 97.24 | 96.37 | 94.81 |
| SA | 98.75 | 96.97 | 96.64 |
| Sample | Number of Thermal Cycles | ΔHm (J/g) | ΔHc (J/g) | η (%) |
|---|---|---|---|---|
| OCC/TPU-0.28 | 1 | 154.2 | 157.8 | 96.7 |
| 50 | 150.2 | 151.6 | ||
| HEO/TPU-0.28 | 1 | 177.8 | 185.1 | 94.3 |
| 50 | 174.5 | 182.3 | ||
| SA/TPU-0.28 | 1 | 157.1 | 161.4 | 98.3 |
| 50 | 150.3 | 157.9 |
| PCMs | Matrix | Strategy for PCMs Loading | ΔHm (J/g) | E (%) | Breaking Elongation (%) | Reference |
|---|---|---|---|---|---|---|
| Paraffin | Cellulose | Microcapsule and wet spun | 69.01 | 48.06 | 2.0 | [55] |
| Paraffin | Cellulose | Microcapsule and wet spun | 130.34 | 77.28 | 1.2 | [55] |
| Octadecane | Polypropylene | microcapsule and melt spun | 11.00 | 20.00 | 30.2 | [56] |
| Octadecane | Polyacrylonitrile-vinylidene chloride | Microcapsule and wet spun | 30.00 | 30.00 | 7.0 | [57] |
| Octadecane | Polypropylene | Microcapsule and melt spun | 9.20 | 12.00 | - | [58] |
| Paraffin | Polyvinyl alcohol | Microcapsule and wet spun | 23.70 | 25.10 | 14.9 | [59] |
| Paraffin | Polyvinyl butyral | Coaxial spun | 128.20 | 50.00 | 110 | [60] |
| Mixture of plant oils | Polyvinyl alcohol | Electro spin | 84.72 | 70.00 | 83.77 | [61] |
| Polyethylene glycol | Graphene aerogel fiber | Porous impregnation | 188.40 | 84.20 | 2.5 | [11] |
| Octadecane | Thermoplastic polyurethane fiber | Porous impregnation | 176.10 | 76.90 | 364 | This study |
| Stearic acid | Polyurethane fiber | Porous impregnation | 174.00 | 84.20 | 253.4 | This study |
| Stearic acid | Polyurethane fiber | Porous impregnation | 148.30 | 72.00 | 498 | This study |
| Sample | Loading/(J/m) | Unloading/(J/m) | Hysteresis Loop/(J/m) | Hysteresis Loop/% |
|---|---|---|---|---|
| Elongation 10% TPU-0.28 | 0.619 | 0.552 | 0.066 | 15.763 |
| Elongation 30% TPU-0.28 | 6.820 | 5.511 | 1.30 | 19.194 |
| Elongation 50% TPU-0.28 | 17.535 | 12.033 | 5.502 | 31.377 |
| Elongation 30% Liquid HEO/TPU-0.28 | 9.035 | 7.500 | 1.535 | 16.989 |
| Elongation 30% Solid HEO/TPU-0.28 | 30.886 | 20.500 | 10.386 | 33.627 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Xu, L.; Wang, X.; Zhu, R.; Yan, Y. Phase Change Energy Storage Elastic Fiber: A Simple Route to Personal Thermal Management. Polymers 2022, 14, 53. https://doi.org/10.3390/polym14010053
Li W, Xu L, Wang X, Zhu R, Yan Y. Phase Change Energy Storage Elastic Fiber: A Simple Route to Personal Thermal Management. Polymers. 2022; 14(1):53. https://doi.org/10.3390/polym14010053
Chicago/Turabian StyleLi, Weipei, Liqing Xu, Xiangqin Wang, Ruitian Zhu, and Yurong Yan. 2022. "Phase Change Energy Storage Elastic Fiber: A Simple Route to Personal Thermal Management" Polymers 14, no. 1: 53. https://doi.org/10.3390/polym14010053
APA StyleLi, W., Xu, L., Wang, X., Zhu, R., & Yan, Y. (2022). Phase Change Energy Storage Elastic Fiber: A Simple Route to Personal Thermal Management. Polymers, 14(1), 53. https://doi.org/10.3390/polym14010053






