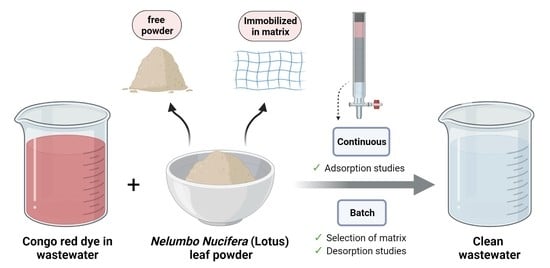
Continuous Fixed-Bed Column Studies on Congo Red Dye Adsorption-Desorption Using Free and Immobilized Nelumbo nucifera Leaf Adsorbent
Abstract
:1. Introduction
2. Materials and Methods
2.1. N. nucifera Leaf Powder Preparation, Chemical Reagents and Analytical Methods
2.2. Immobilization of NNLP Adsorbent for CR Dye Adsorption
2.2.1. Immobilization of the NNLP Adsorbent in Calcium Alginate
2.2.2. Immobilization of the NNLP Adsorbent in Polyvinyl Alcohol
2.2.3. Immobilization of the NNLP Adsorbent in Polysulfone
2.2.4. Immobilization of the NNLP Adsorbent in Sodium Silicate
2.3. Batch Experiments with Synthetic CR Dye Wastewater Using Various Immobilized NNLP Adsorbents
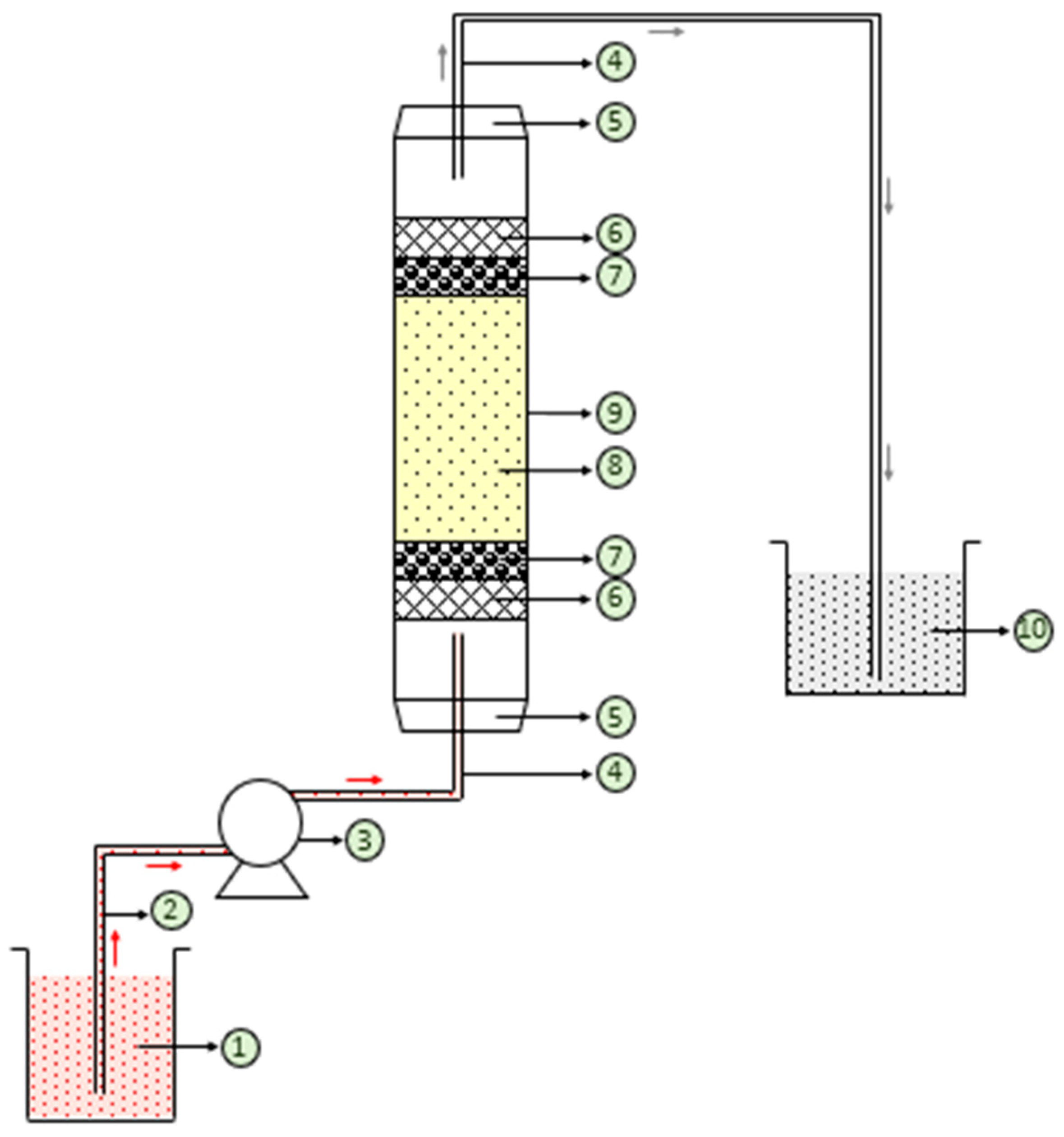
2.4. Column Experiments for Removal of Color from Synthetic Dye Wastewater Using Free and Immobilized NNLP Adsorbent in Various Runs
2.5. Reusability of Free and Immobilized NNLP Adsorbents for CR Dye Adsorption in Column Studies
2.6. Mathematical Description of Adsorption in a Continuous Fixed-Bed Column Study
2.7. Mathematical Models Used for the Breakthrough Curve in Fixed-Bed Column Studies
2.7.1. Adams–Bohart Model
2.7.2. Bed Depth Service Time (BDST) Model
2.7.3. Thomas Model
2.7.4. Yoon–Nelson Model
2.7.5. Wolborska Model
2.8. Physicochemical Characteristics of Textile Industrial Dye Effluent
3. Results and Discussions
3.1. Evaluation of a Suitable Matrix for Immobilization of the NNLP Adsorbent in Batch Studies
3.2. Inference from Desorption Studies and Reusability of Free and Sodium Silicate Gel Immobilized NNLP Adsorbent in Batch Studies
3.3. Reusability of Free and Sodium Silicate Gel-Immobilized NNLP Adsorbents for CR Dye Adsorption in Column Studies
3.4. Analysis of BTCs for CR Dye Adsorption in Various Runs and Estimation of Kinetic Model Parameters in Different Models
3.4.1. Adams–Bohart Model
3.4.2. Bed Depth Service Time Model
3.4.3. Thomas Model
3.4.4. Yoon–Nelson Model
3.4.5. Wolborska Model
3.5. Physicochemical Analysis of Textile Industrial CR Dye Effluent in Batch Studies
3.6. Analysis of Column Experiments with Textile Industrial CR Dye Effluent Using Free and Sodium Silicate Gel-Immobilized NNLP Adsorbents
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taher, T.; Rohendi, D.; Mohadi, R.; Lesbani, A. Congo red dye removal from aqueous solution by acid-activated bentonite from sarolangun: Kinetic, equilibrium, and thermodynamic studies. Arab J. Basic Appl. Sci. 2019, 26, 125–136. [Google Scholar] [CrossRef]
- Jhilirani, M.; Banashree, D.; Soumen, D. Highly porous iron-zirconium binary oxide for efficient removal of Congo red from water. Desalin. Water Treat. 2020, 189, 227–242. [Google Scholar] [CrossRef]
- Vairavel, P.; Murty, V.R. Residence time distribution studies and modeling of rotating biological contactor reactor for decolorization of Congo red from synthetic dye wastewater. Desalin. Water Treat. 2021, 220, 380–391. [Google Scholar] [CrossRef]
- Rahimi, R.; Kerdari, H.; Rabbani, M.; Shafiee, M. Synthesis, characterization and adsorbing properties of hollow Zn-Fe2O4 nanospheres on removal of Congo red from aqueous solution. Desalination 2011, 280, 412–418. [Google Scholar] [CrossRef]
- Islam, M.T.; Aimone, F.; Ferri, A.; Rovero, G. Use of N-methylformanilide as swelling agent for meta-aramid fibers dyeing: Kinetics and equilibrium adsorption of Basic blue 41. Dyes Pigment. 2015, 113, 554–561. [Google Scholar] [CrossRef]
- Vairavel, P.; Murty, V.R. Decolorization of Congo red dye in a continuously operated rotating biological contactor reactor. Desalin. Water Treat. 2020, 196, 299–314. [Google Scholar] [CrossRef]
- Zahir, A.; Aslam, Z.; Kamal, M.S.; Ahmad, W.; Abbas, A.; Shawabkeh, R.A. Development of novel cross-linked chitosan for the removal of anionic Congo red dye. J. Mol. Liq. 2017, 244, 211–218. [Google Scholar] [CrossRef]
- Vahidhabanu, S.; Swathika, I.; Adeogun, A.I.; Roshni, R.; Babu, B.R. Biomass-derived magnetically tuned carbon modified Sepiolite for effective removal of Congo red from aqueous solution. Desalin. Water Treat. 2020, 184, 326–339. [Google Scholar] [CrossRef]
- Srilakshmi, C.; Saraf, R. Ag-doped hydroxyapatite as efficient adsorbent for removal of Congo red dye from aqueous solutions: Synthesis, kinetic and equilibrium adsorption isotherm analysis. Microporous Mesoporous Mater. 2016, 219, 134–144. [Google Scholar] [CrossRef]
- Jiao, C.; Liu, D.; Wei, N.; Gao, J.; Fu, F.; Liu, T.; Wang, J. Efficient Congo red removal using porous cellulose/gelatin/sepiolite gel Beads: Assembly, characterization, and adsorption mechanism. Polymers 2021, 13, 3890. [Google Scholar] [CrossRef]
- Dawood, S.; Sen, T.K. Removal of anionic dye Congo red from aqueous solution by raw pine and acid-treated pine cone powder as adsorbent: Equilibrium, thermodynamic, kinetics, mechanism and process design. Water Res. 2012, 46, 1933–1946. [Google Scholar] [CrossRef]
- Kittappa, S.; Jais, F.M.; Ramalingam, M.; Mohd, N.S.; Ibrahim, S. Functionalized magnetic mesoporous palm shell activated carbon for enhanced removal of azo dyes. J. Environ. Chem. Eng. 2020, 8, 104081. [Google Scholar] [CrossRef]
- Lakshmipathy, R.; Sarada, N.C. Methylene blue adsorption onto native watermelon rind: Batch and fixed bed column studies. Desalin. Water Treat. 2016, 57, 10632–10645. [Google Scholar] [CrossRef]
- Bulgariu, L.; Escudero, L.B.; Bello, O.S.; Iqbal, M.; Nisar, J.; Adegoke, K.A.; Alakhras, F.; Kornaros, M.; Anastopoulos, I. The utilization of leaf-based adsorbents for dyes removal: A review. J. Mol. Liq. 2019, 276, 728–747. [Google Scholar] [CrossRef] [Green Version]
- Lim, L.B.L.; Priyantha, N.; Latip, S.A.A.; Lu, Y.C.; Mahadi, A.H. Converting Hylocereus undatus (white dragon fruit) peel waste into a useful potential adsorbent for the removal of toxic Congo red dye. Desalin. Water Treat. 2020, 185, 307–317. [Google Scholar] [CrossRef]
- Dai, H.; Huang, Y.; Zhang, H.; Ma, L.; Huang, H.; Wu, J.; Zhang, Y. Direct fabrication of hierarchically processed pineapple peel hydrogels for efficient Congo red adsorption. Carbohydr. Polym. 2020, 230, 115599. [Google Scholar] [CrossRef] [PubMed]
- Vairavel, P.; Murty, V.R.; Nethaji, S. Removal of Congo red dye from aqueous solutions by adsorption onto a dual adsorbent (Neurospora crassa dead biomass and wheat bran): Optimization, isotherm, and kinetics studies. Desalin. Water Treat. 2017, 68, 274–292. [Google Scholar] [CrossRef]
- Garba, Z.N.; Bello, I.; Galadima, A.; Lawal, A.Y. Optimization of adsorption conditions using central composite design for the removal of copper(II) and lead(II) by defatted papaya seed. Karbala Int. J. Mod. Sci. 2016, 2, 20–28. [Google Scholar] [CrossRef] [Green Version]
- Song, J.Y.; Zou, W.H.; Bian, Y.Y.; Su, F.Y.; Han, R.P. Adsorption characteristics of methylene blue by peanut husk in batch and column modes. Desalination 2011, 265, 119–125. [Google Scholar] [CrossRef]
- Vairavel, P.; Rampal, N.; Jeppu, G. Adsorption of toxic Congo red dye from aqueous solution using untreated coffee husks: Kinetics, equilibrium, thermodynamics and desorption study. Int. J. Environ. Anal. Chem. 2021, in press. [Google Scholar] [CrossRef]
- Debamita, C.; Nakul, R.; Gautham, J.P.; Vairavel, P. Process optimization, isotherm, kinetics, and thermodynamics studies for removal of Remazol Brilliant Blue—R dye from contaminated water using adsorption on guava leaf powder. Desalin. Water Treat. 2020, 185, 318–343. [Google Scholar] [CrossRef]
- Divya, J.M.; Palak, K.; Vairavel, P. Optimization, kinetics, equilibrium isotherms, and thermodynamics studies of Coomassie violet dye adsorption using Azadirachta indica (neem) leaf adsorbent. Desalin. Water Treat. 2020, 190, 353–382. [Google Scholar] [CrossRef]
- Romzia, A.A.; Lim, L.B.L.; Chan, C.M.; Priyantha, N. Application of Dimocarpus longan ssp. malesianus leaves in the sequestration of toxic brilliant green dye. Desalin. Water Treat. 2020, 189, 428–439. [Google Scholar] [CrossRef]
- Meghana, C.; Juhi, B.; Rampal, N.; Vairavel, P. Isotherm, kinetics, process optimization and thermodynamics studies for removal of Congo red dye from aqueous solutions using Nelumbo nucifera (lotus) leaf adsorbent. Desalin. Water Treat. 2020, 207, 373–397. [Google Scholar] [CrossRef]
- Aksu, Z.; Gonen, F. Biosorption of phenol by immobilized activated sludge in a continuous packed bed: Prediction of breakthrough curves. Process Biochem. 2004, 39, 599–613. [Google Scholar] [CrossRef]
- Bai, R.S.; Abraham, T.E. Studies on chromium(VI) adsorption-desorption using immobilized fungal biomass. Bioresour. Technol. 2003, 87, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Vairavel, P.; Murty, V.R. Continuous fixed-bed adsorption of Congo red dye by dual adsorbent (Neurospora crassa dead fungal biomass and wheat bran): Experimental and theoretical breakthrough curves, immobilization and reusability studies. Desalin. Water Treat. 2017, 98, 276–293. [Google Scholar] [CrossRef]
- Yang, Y.; Lin, E.; Tao, X.; Hu, K. High efficiency removal of Pb(II) by modified spent compost of Hypsizygus marmoreus in a fixed-bed column. Desalin. Water Treat. 2018, 102, 220–228. [Google Scholar] [CrossRef]
- Aksu, Z.; Cagatay, S.S.; Gonen, F. Continuous fixed bed biosorption of reactive dyes by dried Rhizopus arrhizus: Determination of column capacity. J. Hazard. Mater. 2007, 143, 362–371. [Google Scholar] [CrossRef]
- Han, X.; Yuan, J.; Ma, X. Adsorption of malachite green from aqueous solutions onto lotus leaf: Equilibrium, kinetic, and thermodynamic studies. Desalin. Water Treat. 2014, 52, 5563–5574. [Google Scholar] [CrossRef]
- Vairavel, P.; Murty, V.R. Optimization of batch process parameters for Congo red color removal by Neurospora crassa live fungal biomass with wheat bran dual adsorbent using response surface methodology. Desalin. Water Treat. 2018, 103, 84–101. [Google Scholar] [CrossRef]
- Boyaci, E.; Eroglu, A.E.; Shahwan, T. Sorption of As(V) from waters using chitosan and chitosan-immobilized sodium silicate prior to atomic spectrometric determination. Talanta 2010, 80, 1452–1460. [Google Scholar] [CrossRef]
- Rangsayatorn, N.; Pokethitiyook, P.; Upatham, E.S.; Lanza, G.R. Cadmium biosorption by cells of Spirulina platensis TISTR 8217 immobilized in alginate and silica gel. Environ. Int. 2004, 30, 57–63. [Google Scholar] [CrossRef]
- Vairavel, P.; Murty, V.R. Optimization, kinetics, equilibrium isotherms and thermodynamics studies for Congo red dye adsorption using calcium alginate beads immobilized with dual adsorbent (Neurospora crassa dead fungal biomass and wheat bran). Desalin. Water Treat. 2017, 97, 338–362. [Google Scholar] [CrossRef]
- Vairavel, P.; Rampal, N. Continuous fixed-bed column study for removal of Congo red dye from aqueous solutions using Nelumbo nucifera leaf adsorbent. Int. J. Environ. Anal. Chem. 2021, in press. [Google Scholar] [CrossRef]
- Padmesh, T.V.N.; Vijayaraghavan, K.; Sekaran, G.; Velan, M. Biosorption of acid blue 15 using water macroalga Azolla filiculoides: Batch and column studies. Dyes Pigment. 2006, 71, 77–82. [Google Scholar] [CrossRef]
- Lim, A.P.; Aris, A.Z. Continuous fixed-bed column study and adsorption modeling: Removal of cadmium (II) and lead (II) ions in aqueous solution by dead calcareous skeletons. Biochem. Eng. J. 2014, 87, 50–61. [Google Scholar] [CrossRef]
- Alardhi, S.M.; Albayati, T.M.; Alrubaye, J.M. Adsorption of the methyl green dye pollutant from aqueous solution using mesoporous materials MCM-41 in a fixed-bed column. Heliyon 2020, 6, e03253. [Google Scholar] [CrossRef] [Green Version]
- Sadaf, S.; Bhatti, H.N. Batch and fixed bed column studies for the removal of Indosol Yellow BG dye by peanut husk. J. Taiwan Inst. Chem. Eng. 2014, 45, 541–553. [Google Scholar] [CrossRef]
- Padmesh, T.V.N.; Vijayaraghavan, K.; Sekaran, G.; Velan, M. Batch and column studies on biosorption of acid dyes on fresh water macro alga Azolla filiculoides. J. Hazard. Mater. 2005, B125, 121–129. [Google Scholar] [CrossRef]
- Vishali, S.; Karthikeyan, R.; Prabhakar, S. Utilization of seafood processing waste as an adsorbent in the treatment of paint industry effluent in a fixed-bed column. Desalin. Water Treat. 2017, 66, 149–157. [Google Scholar] [CrossRef]
- Adhami, L.; Mirzaei, M. Removal of copper (II) from aqueous solution using granular sodium alginate/activated carbon hydrogel in a fixed-bed column. Desalin. Water Treat. 2018, 103, 208–215. [Google Scholar] [CrossRef]
- Kishor, B.; Rawal, N. Column adsorption studies on copper(II) ion removal from aqueous solution using natural biogenic iron oxide. Desalin. Water Treat. 2019, 153, 216–225. [Google Scholar] [CrossRef]
- Jain, S.N.; Gogate, P.R. Fixed bed column study for the removal of Acid blue 25 dye using NaOH-treated fallen leaves of Ficus racemose. Desalin. Water Treat. 2017, 85, 215–225. [Google Scholar] [CrossRef]
- Olivares, J.C.; Alonso, C.P.; Diaz, C.B.; Nunez, F.U.; Mercado, M.C.C.; Bilyeue, B. Modeling of lead (II) biosorption by residue of allspice in a fixed-bed column. Chem. Eng. J. 2013, 228, 21–27. [Google Scholar] [CrossRef]
- Omar, W.; Dwairi, R.A.; Hamatteh, Z.S.A.; Jabarin, N. Investigation of natural Jordanian zeolite tuff (JZT) as adsorbent for TOC removal from industrial wastewater in a continuous fixed bed column: Study of the influence of particle size. Desalin. Water Treat. 2019, 152, 26–32. [Google Scholar] [CrossRef]
- APHA. Standard Methods for Examination of Water and Wastewater, 22nd ed.; American Public Health Association: Washington DC, USA, 2012. [Google Scholar]
- Tchobanoglous, G.; Burton, F.L.; Stensel, H.D. Metcalf & Eddy Wastewater Engineering: Treatment and Reuse, 4th ed.; Tata McGraw-Hill Publishing Company Limited: New York, NY, USA, 2003. [Google Scholar]
- ASTM International. Annual Book of ASTM Standards; v. 11.0; Water and Environmental Technology: Montgomery, PA, USA, 2003. [Google Scholar]
- APHA. Standard Methods for Examination of Water and Wastewater, 19th ed.; American Public Health Association: Washington DC, USA, 1995. [Google Scholar]
- Trivedy, R.K.; Goel, P.K. Chemical and Biological Methods for Water Pollution Studies; Environmental Publication: Varanasi, India, 1984. [Google Scholar]
- Spinti, M.; Zhuang, H.; Trujillo, E.M. Evaluation of immobilized biomass beads for removing heavy metals from waste water. Water Environ. Res. 1995, 67, 943–952. [Google Scholar] [CrossRef]
- Patel, R.; Suresh, S. Kinetic and equilibrium studies on the biosorption of Reactive black 5 dye by Aspergillus foetidus. Bioresour. Technol. 2008, 99, 51–58. [Google Scholar] [CrossRef]
- Purkait, M.K.; Maiti, A.; Gupta, S.D.; De, S. Removal of Congo red using activated carbon and its regeneration. J. Hazard. Mater. 2007, 145, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.N.; Gogate, P.R. Adsorptive removal of azo dye in a continuous column operation using biosorbent based on NaOH and surfactant activation of Prunus dulcis leaves. Desalin. Water Treat. 2019, 141, 331–341. [Google Scholar] [CrossRef]
- Nethaji, S.; Sivasamy, A.; Vimal Kumar, R.; Mandal, A.B. Preparation of char from lotus seed biomass and the exploration of its dye removal capacity through batch and column adsorption studies. Environ. Sci. Pollut. Res. 2013, 20, 3670–3678. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Lee, M.W.; Yun, Y.S. A new approach to study the decolorization of complex reactive dye bath effluent by biosorption technique. Bioresour. Technol. 2008, 99, 5778–5785. [Google Scholar] [CrossRef]
- Gupta, V.K.; Mittal, A.; Krishnan, L.; Gajbe, V. Adsorption kinetics and column operations for the removal and recovery of Malachite green from wastewater using bottom ash. Sep. Purif. Technol. 2004, 40, 87–96. [Google Scholar] [CrossRef]
- Vieira, M.L.G.; Martinez, M.S.; Santos, G.B.; Dotto, G.L.; Pinto, L.A.A. Azo dyes adsorption in fixed bed column packed with different deacetylation degrees chitosan coated glass beads. J. Environ. Chem. Eng. 2018, 6, 3233–3241. [Google Scholar] [CrossRef]
- Yoon, Y.H.; Nelson, J.H. Application of gas adsorption kinetics I. A theoretical model for respirator cartridge service life. Am. Ind. Hyg. Assoc. J. 1984, 45, 509–516. [Google Scholar] [CrossRef]
- Malkoc, E.; Nuhoglu, Y.; Abali, Y. Cr(VI) adsorption by waste acron of Querus ithaburensis in fixed beds: Prediction of breakthrough curves. Chem. Eng. J. 2006, 119, 61–68. [Google Scholar] [CrossRef]
- Yaseen, D.A.; Scholz, M. Textile dye wastewater characteristics and constituents of synthetic effluents: A critical review. Int. J. Environ. Sci. Technol. 2019, 16, 1193–1226. [Google Scholar] [CrossRef] [Green Version]
- Koprivanac, N.; Bosanac, G.; Grabaric, Z.; Papic, S. Treatment of wastewaters from dye industry. Environ. Technol. 1993, 14, 385–390. [Google Scholar] [CrossRef]
- Holkar, C.R.; Jadhav, A.J.; Pinjari, D.V.; Mahamuni, N.M.; Pandit, A.B. A critical review on textile wastewater treatments: Possible approaches. J. Environ. Manag. 2016, 182, 351–366. [Google Scholar] [CrossRef]
- Yusuff, R.O.; Sonibare, J.A. Characterization of textile industries effluents in Kaduna, Nigeria and pollution implications. Glob. Nest Int. J. 2004, 6, 212–221. [Google Scholar] [CrossRef]









| Sl. No | Physicochemical Parameters | Method/Instrument |
|---|---|---|
| 1 | pH | Digital pH meter, Systronics |
| 2 | Turbidity, NTU | Nephelometric turbidimeter, Systronics |
| 3 | Total suspended solids, mg L−1 | Gravimetric method, oven-drying at 378 K |
| 4 | Total dissolved solids, mg L−1 | Gravimetric method, oven-drying at 378 K |
| 5 | Biological oxygen demand, mg L−1 | Incubating the sample at 303 K for 5 days followed by titration |
| 6 | Chemical oxygen demand, mg L−1 | Closed reflux method |
| 7 | Total alkalinity, mg L−1 | Acid-base titration |
| 8 | Total hardness, mg L−1 | Complexometric titration |
| 9 | Dissolved oxygen concentration, mg L−1 | Dissolved oxygen meter, Systronics |
| 10 | Electrical conductivity, mS cm−1 | Conductivity meter, Digisun |
| 11 | Sulfates, mg L−1 | Titrimetric method |
| 12 | Chlorides, mg L−1 | Argentometric titration |
| Various Polymeric Matrices | NNLP Adsorbent Optimum Loading % (w/v) | Immobilized Adsorbent Characteristics | NNLP Adsorbent Maximum Loading % (w/v) | Immobilized Adsorbent Characteristics | ||
|---|---|---|---|---|---|---|
| BET Surface Area (m2 g−1) | Pore Volume (mm3 g−1) | BET Surface Area (m2 g−1) | Pore Volume (mm3 g−1) | |||
| Calcium alginate | 5 | 0.65 | 0.82 | 10 | 0.27 | 0.35 |
| Polyvinyl alcohol | 4 | 0.38 | 0.53 | 10 | 0.13 | 0.16 |
| Polysulfone | 6 | 0.72 | 0.94 | 10 | 0.34 | 0.48 |
| Sodium silicate | 3 | 1.84 | 2.18 | 10 | 1.16 | 1.65 |
| Adsorbent | Column Parameters | First Run | Second Run | Third Run |
|---|---|---|---|---|
| Free NNLP adsorbent | Z (cm) | 2.50 | 2.25 | 2.10 |
| W (g) | 3.72 | 3.35 | 3.07 | |
| tb (h) | 8.75 | 5.25 | 3.00 | |
| tE (h) | 17.50 | 12.00 | 7.50 | |
| mad (mg) | 12.04 | 7.053 | 3.622 | |
| mtotal (mg) | 15.75 | 10.80 | 6.75 | |
| Veff (L) | 1.05 | 0.72 | 0.45 | |
| qe (mg g−1) | 3.236 | 2.106 | 1.180 | |
| % color removal | 76.43 | 65.31 | 53.66 | |
| Sodium silicate gel-immobilized NNLP adsorbent | Z (cm) | 2.50 | 2.10 | 1.75 |
| W (g) | 3.12 | 2.69 | 2.24 | |
| tb (h) | 6.50 | 3.50 | 1.25 | |
| tE (h) | 13.5 | 9.00 | 4.50 | |
| mad (mg) | 8.178 | 4.596 | 1.755 | |
| mtotal (mg) | 12.15 | 8.10 | 4.05 | |
| Veff (L) | 0.81 | 0.54 | 0.27 | |
| qe (mg g−1) | 2.621 | 1.708 | 0.783 | |
| % color removal | 67.30 | 56.74 | 43.33 |
| Free NNLP Adsorbent | Model Parameters | |||
|---|---|---|---|---|
| First Run | Second Run | Third Run | ||
| Adams–Bohart model | KAB (L mg−1min−1) | 4.98 × 10−4 | 3.09 × 10−4 | 1.34 × 10−4 |
| No (mg L−1) | 1624.1 | 1226.7 | 779.92 | |
| R2 | 0.85 | 0.88 | 0.78 | |
| BDST model | K (L mg−1min−1) | 1.142 × 10−3 | 8.943 × 10−4 | 6.152 × 10−4 |
| No (mg L−1) | 1329.8 | 966.03 | 560.18 | |
| R2 | 0.96 | 0.95 | 0.96 | |
| Thomas model | KTH (mL mg−1 min−1) | 1.069 × 10−3 | 8.264 × 10−4 | 5.839 × 10−4 |
| qoTH (mg g−1) | 3.236 | 2.244 | 1.309 | |
| R2 | 0.98 | 0.98 | 0.99 | |
| Yoon–Nelson model | KYN (min−1) | 0.0164 | 0.0085 | 0.0057 |
| τ (min) | 766.9 | 501.3 | 268.0 | |
| qoYN (mg g−1) | 3.092 | 2.244 | 1.309 | |
| R2 | 0.98 | 0.98 | 0.98 | |
| Wolborska model | βa (min−1) | 0.809 | 0.773 | 0.658 |
| No (mg L−1) | 1624.1 | 1226.7 | 779.92 | |
| R2 | 0.85 | 0.88 | 0.78 | |
| Immobilized NNLP Adsorbent | Model Parameters | |||
|---|---|---|---|---|
| First Run | Second Run | Third Run | ||
| Adams–Bohart model | KAB (L mg−1min−1) | 4.17 × 10−4 | 2.65 × 10−4 | 1.09 × 10−4 |
| No (mg L−1) | 1239.2 | 938.88 | 538.04 | |
| R2 | 0.83 | 0.80 | 0.72 | |
| BDST model | K (L mg−1min−1) | 1.086 × 10−3 | 6.754 × 10−4 | 4.563 × 10−4 |
| No (mg L−1) | 994.68 | 678.16 | 339.04 | |
| R2 | 0.95 | 0.95 | 0.94 | |
| Thomas model | KTH (mL mg−1 min−1) | 9.542 × 10−4 | 5.483 × 10−4 | 2.256 × 10−4 |
| qoTH (mg g−1) | 2.754 | 1.822 | 0.944 | |
| R2 | 0.98 | 0.99 | 0.98 | |
| Yoon–Nelson model | KYN (min−1) | 0.0118 | 0.0062 | 0.0024 |
| τ (min) | 572.9 | 326.8 | 132.3 | |
| qoYN (mg g−1) | 2.754 | 1.822 | 0.886 | |
| R2 | 0.98 | 0.98 | 0.99 | |
| Wolborska model | βa (min−1) | 0.765 | 0.642 | 0.573 |
| No (mg L−1) | 1239.2 | 938.88 | 538.04 | |
| R2 | 0.83 | 0.80 | 0.72 | |
| Sl. No | Physicochemical Parameters | Raw Effluent Value | Treated Effluent Value | CPCB Standard | |
|---|---|---|---|---|---|
| Free NNLP Adsorbent | Sodium Silicate-Immobilized NNLP Adsorbent | ||||
| 1 | pH | 8.56 | 8.50 | 8.52 | 6–9 |
| 2 | Turbidity, NTU | 150 | 18 | 24 | <10 |
| 3 | Total suspended solids, mg L−1 | 134 | 26 | 45 | <100 |
| 4 | Total dissolved solids, mg L−1 | 3824 | 965 | 1012 | <2000 |
| 5 | Biological oxygen demand, mg L−1 | 622 | 42 | 58 | <30 |
| 6 | Chemical oxygen demand, mg L−1 | 1845 | 238 | 246 | <250 |
| 7 | Total alkalinity, mg L−1 | 418 | 87 | 102 | <200 |
| 8 | Total hardness, mg L−1 | 756 | 415 | 420 | <300 |
| 9 | Dissolved oxygen concentration, mg L−1 | 1.12 | 1.06 | 1.08 | <4.00 |
| 10 | Electrical conductivity, mS cm−1 | 5.34 | 4.92 | 5.13 | <2.25 |
| 11 | Sulfates, mg L−1 | 560 | 138 | 165 | <250 |
| 12 | Chlorides, mg L−1 | 1524 | 236 | 274 | <500 |
| Adsorbent | W (g) | tb (h) | tE (h) | mad (mg) | mtotal (mg) | Veff (L) | qe (mg g−1) | % Color Removal |
|---|---|---|---|---|---|---|---|---|
| Free NNLP adsorbent | 3.72 | 0.375 | 3.00 | 29.783 | 39.06 | 0.180 | 8.00 | 76.25 |
| Immobilized NNLP adsorbent | 3.12 | 0.20 | 2.25 | 18.215 | 29.295 | 0.135 | 5.84 | 62.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parimelazhagan, V.; Jeppu, G.; Rampal, N. Continuous Fixed-Bed Column Studies on Congo Red Dye Adsorption-Desorption Using Free and Immobilized Nelumbo nucifera Leaf Adsorbent. Polymers 2022, 14, 54. https://doi.org/10.3390/polym14010054
Parimelazhagan V, Jeppu G, Rampal N. Continuous Fixed-Bed Column Studies on Congo Red Dye Adsorption-Desorption Using Free and Immobilized Nelumbo nucifera Leaf Adsorbent. Polymers. 2022; 14(1):54. https://doi.org/10.3390/polym14010054
Chicago/Turabian StyleParimelazhagan, Vairavel, Gautham Jeppu, and Nakul Rampal. 2022. "Continuous Fixed-Bed Column Studies on Congo Red Dye Adsorption-Desorption Using Free and Immobilized Nelumbo nucifera Leaf Adsorbent" Polymers 14, no. 1: 54. https://doi.org/10.3390/polym14010054
APA StyleParimelazhagan, V., Jeppu, G., & Rampal, N. (2022). Continuous Fixed-Bed Column Studies on Congo Red Dye Adsorption-Desorption Using Free and Immobilized Nelumbo nucifera Leaf Adsorbent. Polymers, 14(1), 54. https://doi.org/10.3390/polym14010054