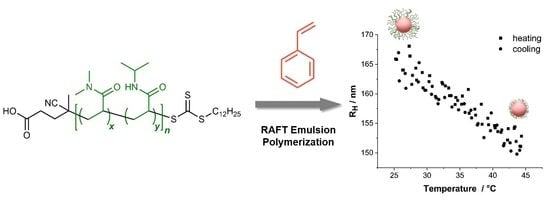
RAFT Emulsion Polymerization of Styrene Using a Poly((N,N-dimethyl acrylamide)-co-(N-isopropyl acrylamide)) mCTA: Synthesis and Thermosensitivity
Abstract
:1. Introduction
2. Materials and Methods
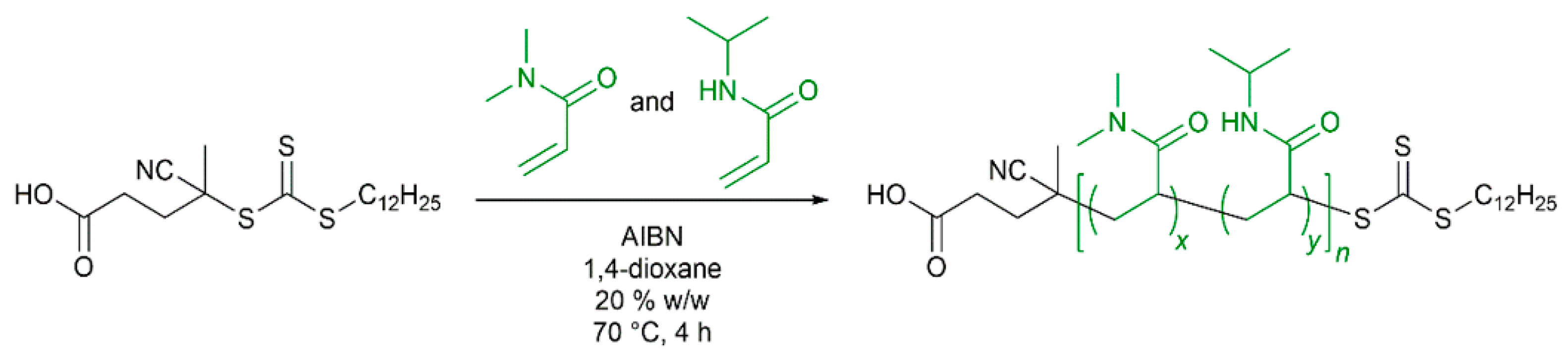
2.1. Synthesis of P(DMA-co-NIPAM) mCTA by RAFT Solution Copolymerization of DMA and NIPAM at 70 °C
2.2. Synthesis of P(DMA-co-NIPAM)-b-PS via RAFT Emulsion Polymerization of Styrene at 70 °C
2.3. Characterization
2.3.1. 1H NMR Spectroscopy
2.3.2. Gel Permeation Chromatography (GPC)
2.3.3. Transmission Electron Microscopy (TEM) on Polymer Films
2.3.4. Transmission Electron Microscopy on Polymerization Dispersions under Cryogenic Conditions (cryoTEM)
2.3.5. Transmission Electron Microscopy on Polymerization Dispersions at Room-Temperature
2.3.6. Visual Turbidimetry
2.3.7. Dynamic Light Scattering (DLS)
3. Results and Discussion
3.1. Random Copolymerization of DMA and NIPAM
3.2. Determination of Copolymerization Parameters According to the Method of Kelen–Tüdős
3.3. Emulsion Polymerization of Styrene
3.4. 1H NMR and GPC Characterization of the P(DMA-co-NIPAM)-b-PS Copolymers
3.5. Morphology of Diblock Copolymer Thin Films
3.6. Solubility Study of P(DMA-co-NIPAM) Random Copolymers and P(DMA-co-NIPAM)-b-PS Diblock Copolymers in Water
3.6.1. Cloud Point Measurement via Visual Turbidimetry
3.6.2. DLS Measurements
3.6.3. Effect of Additives
3.6.4. Aqueous Dispersions of the P(DMA-co-NIPAM)-b-PS Micelles
3.6.5. Influence of Salt on Micellar Assemblies
3.6.6. Morphological Study of the P(DMA-co-NIPAM)-b-PS Micelles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cao, Z.Q.; Wang, G.J. Multi-stimuli-responsive polymer materials: Particles, films, and bulk gels. Chem. Rec. 2016, 16, 1398–1435. [Google Scholar] [CrossRef] [PubMed]
- Lucht, N.; Eggers, S.; Abetz, V. Cononsolvency in the ‘drunken’ state: The thermoresponsiveness of a new acrylamide copolymer in water-alcohol mixtures. Polym. Chem. 2017, 8, 1196–1205. [Google Scholar] [CrossRef]
- Yin, X.; Hoffman, A.S.; Stayton, P.S. Poly (N-isopropylacrylamide-co-propylacrylic acid) copolymers that respond sharply to temperature and pH. Biomacromolecules 2006, 7, 1381–1385. [Google Scholar] [CrossRef] [PubMed]
- Valiaev, A.; Abu-Lail, N.I.; Lim, D.W.; Chilkoti, A.; Zauscher, S. Microcantilever sensing and actuation with end-grafted stimulus-responsive elastin-like polypeptides. Langmuir 2007, 23, 339–344. [Google Scholar] [CrossRef]
- Otake, K.; Karaki, R.; Ebina, T.; Yokoyama, C.; Takahashi, S. Pressure effects on the aggregation of poly (N-isopropylacrylamide) and poly (N-isopropylacrylamide-co-acrylic acid) in aqueous solutions. Macromolecules 1993, 26, 2194–2197. [Google Scholar] [CrossRef]
- Zheng, L.; Li, H.; Huang, W.; Lai, X.; Zeng, X. Light Stimuli-Responsive Superhydrophobic Films for Electric Switches and Water-Droplet Manipulation. ACS Appl. Mater. Interfaces 2021, 13, 36621–36631. [Google Scholar] [CrossRef]
- Andrade, F.; Roca-Melendres, M.M.; Durán-Lara, E.F.; Rafael, D.; Schwartz, S. Stimuli-Responsive Hydrogels for Cancer Treatment: The Role of pH, Light, Ionic Strength and Magnetic Field. Cancers 2021, 13, 1164. [Google Scholar] [CrossRef]
- Pietsch, C.; Hoogenboom, R.; Schubert, U.S. Soluble polymeric dual sensor for temperature and pH value. Angew. Chem. 2009, 121, 5763–5766. [Google Scholar] [CrossRef]
- Zhao, C.; Ma, Z.; Zhu, X. Rational design of thermoresponsive polymers in aqueous solutions: A thermodynamics map. Prog. Polym. Sci. 2019, 90, 269–291. [Google Scholar] [CrossRef]
- Kotsuchibashi, Y.; Ebara, M.; Aoyagi, T.; Narain, R. Recent advances in dual temperature responsive block copolymers and their potential as biomedical applications. Polymers 2016, 8, 380. [Google Scholar] [CrossRef] [Green Version]
- Eggers, S.; Fischer, B.; Abetz, V. Aqueous Solutions of Poly[2-(N-morpholino)ethyl methacrylate]: Learning about Macromolecular Aggregation Processes from a Peculiar Three-Step Thermoresponsive Behavior. Macromol. Chem. Phys. 2016, 217, 735–747. [Google Scholar] [CrossRef]
- Schild, H.G. Poly (N-isopropylacrylamide): Experiment, theory and application. Prog. Polym. Sci. 1992, 17, 163–249. [Google Scholar] [CrossRef]
- Okada, Y.; Tanaka, F. Cooperative hydration, chain collapse, and flat LCST behavior in aqueous poly (N-isopropylacrylamide) solutions. Macromolecules 2005, 38, 4465–4471. [Google Scholar] [CrossRef]
- Lazaridis, T.; Paulaitis, M.E. Entropy of hydrophobic hydration: A new statistical mechanical formulation. J. Phys. Chem. 1992, 96, 3847–3855. [Google Scholar] [CrossRef]
- Fujishige, S.; Kubota, K.; Ando, I. Phase transition of aqueous solutions of poly (N-isopropylacrylamide) and poly (N-isopropylmethacrylamide). J. Phys. Chem. 1989, 93, 3311–3313. [Google Scholar] [CrossRef]
- Ye, X.; Lu, Y.; Shen, L.; Ding, Y.; Liu, S.; Zhang, G.; Wu, C. How many stages in the coil-to-globule transition of linear homopolymer chains in a dilute solution? Macromolecules 2007, 40, 4750–4752. [Google Scholar] [CrossRef] [Green Version]
- Mulvenna, R.A.; Weidman, J.L.; Jing, B.; Pople, J.A.; Zhu, Y.; Boudouris, B.W.; Phillip, W.A. Tunable nanoporous membranes with chemically-tailored pore walls from triblock polymer templates. J. Membr. Sci. 2014, 470, 246–256. [Google Scholar] [CrossRef]
- Clodt, J.I.; Filiz, V.; Rangou, S.; Buhr, K.; Abetz, C.; Höche, D.; Hahn, J.; Jung, A.; Abetz, V. Double stimuli-responsive isoporous membranes via post-modification of pH-sensitive self-assembled diblock copolymer membranes. Adv. Funct. Mater. 2013, 23, 731–738. [Google Scholar] [CrossRef]
- Wandera, D.; Wickramasinghe, S.R.; Husson, S.M. Stimuli-responsive membranes. J. Membr. Sci. 2010, 357, 6–35. [Google Scholar] [CrossRef]
- Alexander, C.; Shakesheff, K.M. Responsive polymers at the biology/materials science interface. Adv. Mater. 2006, 18, 3321–3328. [Google Scholar] [CrossRef]
- Lee, W.-K.; Whitman, L.J.; Lee, J.; King, W.P.; Sheehan, P.E. The nanopatterning of a stimulus-responsive polymer by thermal dip-pen nanolithography. Soft Matter 2008, 4, 1844–1847. [Google Scholar] [CrossRef]
- Förster, S.; Zisenis, M.; Wenz, E.; Antonietti, M. Micellization of strongly segregated block copolymers. J. Chem. Phys. 1996, 104, 9956–9970. [Google Scholar] [CrossRef]
- Liu, X.-M.; Yang, Y.-Y.; Leong, K.W. Thermally responsive polymeric micellar nanoparticles self-assembled from cholesteryl end-capped random poly (N-isopropylacrylamide-co-N, N-dimethylacrylamide): Synthesis, temperature-sensitivity, and morphologies. J. Colloid Interface Sci. 2003, 266, 295–303. [Google Scholar] [CrossRef]
- He, L.; Vibhagool, S.; Zhao, H.; Hoven, V.; Theato, P. Photocaged PNIPAM: A light tunable thermal responsive polymer. Macromol. Chem. Phys. 2018, 219, 1800104. [Google Scholar] [CrossRef]
- Kono, K.; Nakai, R.; Morimoto, K.; Takagishi, T. Thermosensitive polymer-modified liposomes that release contents around physiological temperature. 1999, 1416, 239–250. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Yarin, A.L. Stimuli-responsive copolymers of n-isopropyl acrylamide with enhanced longevity in water for micro-and nanofluidics, drug delivery and non-woven applications. J. Mater. Chem. 2009, 19, 4732–4739. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Kohestanian, M.; Streb, C. pH and thermal dual-responsive poly (NIPAM-co-GMA)-coated magnetic nanoparticles via surface-initiated RAFT polymerization for controlled drug delivery. Mater. Sci. Eng. C 2020, 108, 110418. [Google Scholar] [CrossRef]
- Bastakoti, B.P.; Guragain, S.; Nakashima, K.; Yamauchi, Y. Stimuli-Induced Core–Corona Inversion of Micelle of Poly (acrylic acid)-block-Poly (N-isopropylacrylamide) and Its Application in Drug Delivery. Macromol. Chem. Phys. 2015, 216, 287–291. [Google Scholar] [CrossRef]
- Heinen, S.; Rackow, S.; Schäfer, A.; Weinhart, M. A perfect match: Fast and truly random copolymerization of glycidyl ether monomers to thermoresponsive copolymers. Macromolecules 2017, 50, 44–53. [Google Scholar] [CrossRef]
- Steffen, E.; Tilman, E.; Volker, A. Double thermoresponsive block–random copolymers with adjustable phase transition temperatures: From block-like to gradient-like behavior. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 399–411. [Google Scholar]
- Dan, M.; Su, Y.; Xiao, X.; Li, S.; Zhang, W. A new family of thermo-responsive polymers based on poly [N-(4-vinylbenzyl)-N, N-dialkylamine]. Macromolecules 2013, 46, 3137–3146. [Google Scholar] [CrossRef]
- Pietsch, C.; Mansfeld, U.; Guerrero-Sanchez, C.; Hoeppener, S.; Vollrath, A.; Wagner, M.; Hoogenboom, R.; Saubern, S.; Thang, S.H.; Becer, C.R. Thermo-induced self-assembly of responsive poly (DMAEMA-b-DEGMA) block copolymers into multi-and unilamellar vesicles. Macromolecules 2012, 45, 9292–9302. [Google Scholar] [CrossRef]
- Eggers, S.; Abetz, V. Surfactant-Free RAFT Emulsion Polymerization of Styrene Using Thermoresponsive macroRAFT Agents: Towards Smart Well-Defined Block Copolymers with High Molecular Weights. Polymers 2017, 9, 668. [Google Scholar] [CrossRef] [Green Version]
- Cammas, S.; Suzuki, K.; Sone, C.; Sakurai, Y.; Kataoka, K.; Okano, T. Thermo-responsive polymer nanoparticles with a core-shell micelle structure as site-specific drug carriers. J. Control. Release 1997, 48, 157–164. [Google Scholar] [CrossRef]
- Wu, Y.; Deng, Y.; Yuan, Q.; Ling, Y.; Tang, H. Thermoresponsive poly (γ-propyl-l-glutamate)-graft-(oligo ethylene glycol) s: Synthesis, characterization, and properties. J. Appl. Polym. Sci. 2014, 131, 41022. [Google Scholar] [CrossRef]
- Cetintas, M.; De Grooth, J.; Hofman, A.H.; Van der Kooij, H.M.; Loos, K.; De Vos, W.M.; Kamperman, M. Free-standing thermo-responsive nanoporous membranes from high molecular weight PS-PNIPAM block copolymers synthesized via RAFT polymerization. Polym. Chem. 2017, 8, 2235–2243. [Google Scholar] [CrossRef]
- Lauterbach, F.; Abetz, V. An eco-friendly pathway to thermosensitive micellar nanoobjects via photoRAFT PISA: The full guide to poly (N-acryloylpyrrolidin)-block-polystyrene diblock copolymers. Soft Matter 2020, 16, 2321–2331. [Google Scholar] [CrossRef] [Green Version]
- Harkins, W.D. A general theory of the mechanism of emulsion polymerization1. J. Am. Chem. Soc. 1947, 69, 1428–1444. [Google Scholar] [CrossRef]
- Hill, M.R.; Carmean, R.N.; Sumerlin, B.S. Expanding the Scope of RAFT Polymerization: Recent Advances and New Horizons. Macromolecules 2015, 48, 5459–5469. [Google Scholar] [CrossRef]
- Harkins, W.D. General theory of mechanism of emulsion polymerization. II. J. Polym. Sci. 1950, 5, 217–251. [Google Scholar] [CrossRef]
- Gilbert, R.G. Emulsion Polymerization: A Mechanistic Approach; Academic Press: London, UK, 1995. [Google Scholar]
- Semsarilar, M.; Perrier, S. ‘Green’ reversible addition-fragmentation chain-transfer (RAFT) polymerization. Nat. Chem. 2010, 2, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, M.F.; Campbell, J.D.; Fu, Z.; Bohling, J.; Leroux, J.G.; Mabee, W.; Robert, T. Future green chemistry and sustainability needs in polymeric coatings. Green Chem. 2019, 21, 4919–4926. [Google Scholar] [CrossRef]
- Nieswandt, K.; Georgopanos, P.; Abetz, C.; Filiz, V.; Abetz, V. Synthesis of poly (3-vinylpyridine)-block-polystyrene diblock copolymers via surfactant-free RAFT emulsion polymerization. Materials 2019, 12, 3145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nieswandt, K.; Georgopanos, P.; Abetz, V. Well-defined polyvinylpyridine-block-polystyrene diblock copolymers via RAFT aqueous-alcoholic dispersion polymerization: Synthesis and isoporous thin film morphology. Polym. Chem. 2021, 12, 2210–2221. [Google Scholar] [CrossRef]
- Canning, S.L.; Smith, G.N.; Armes, S.P. A critical appraisal of RAFT-mediated polymerization-induced self-assembly. Macromolecules 2016, 49, 1985–2001. [Google Scholar] [CrossRef]
- Truong, N.P.; Quinn, J.F.; Whittaker, M.R.; Davis, T.P. Polymeric filomicelles and nanoworms: Two decades of synthesis and application. Polym. Chem. 2016, 7, 4295–4312. [Google Scholar] [CrossRef]
- Yeow, J.; Boyer, C. Photoinitiated Polymerization-Induced Self-Assembly (Photo-PISA): New Insights and Opportunities. Adv. Sci. 2017, 4, 1700137. [Google Scholar] [CrossRef]
- Pham, B.T.T.; Nguyen, D.; Ferguson, C.J.; Hawkett, B.S.; Serelis, A.K.; Such, C.H. Miniemulsion Polymerization Stabilized by Amphipathic Macro RAFT Agents. Macromolecules 2003, 36, 8907–8909. [Google Scholar] [CrossRef]
- Charleux, B.; Delaittre, G.; Rieger, J.; D’Agosto, F. Polymerization-Induced Self-Assembly: From Soluble Macromolecules to Block Copolymer Nano-Objects in One Step. Macromolecules 2012, 45, 6753–6765. [Google Scholar] [CrossRef]
- Ganeva, D.E.; Sprong, E.; De Bruyn, H.; Warr, G.G.; Such, C.H.; Hawkett, B.S. Particle formation in ab initio RAFT mediated emulsion polymerization systems. Macromolecules 2007, 40, 6181–6189. [Google Scholar] [CrossRef] [Green Version]
- Chaduc, I.; Crepet, A.s.; Boyron, O.; Charleux, B.; D’Agosto, F.; Lansalot, M. Effect of the pH on the RAFT polymerization of acrylic acid in water. Application to the synthesis of poly (acrylic acid)-stabilized polystyrene particles by RAFT emulsion polymerization. Macromolecules 2013, 46, 6013–6023. [Google Scholar] [CrossRef]
- Liu, S.; Tong, Y.; Yang, Y. Thermally sensitive micelles self-assembled from poly (N-isopropylacrylamide-co-N, N-dimethylacrylamide)-b-poly (D, L-lactide-co-glycolide) for controlled delivery of paclitaxel. Mol. BioSystems 2005, 1, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Tong, Y.; Yang, Y.-Y. Incorporation and in vitro release of doxorubicin in thermally sensitive micelles made from poly (N-isopropylacrylamide-co-N, N-dimethylacrylamide)-b-poly (D, L-lactide-co-glycolide) with varying compositions. Biomaterials 2005, 26, 5064–5074. [Google Scholar] [CrossRef]
- Barker, I.; Cowie, J.; Huckerby, T.; Shaw, D.; Soutar, I.; Swanson, L. Studies of the “smart” thermoresponsive behavior of copolymers of N-isopropylacrylamide and N, N-dimethylacrylamide in dilute aqueous solution. Macromolecules 2003, 36, 7765–7770. [Google Scholar] [CrossRef]
- Shen, Z.; Terao, K.; Maki, Y.; Dobashi, T.; Ma, G.; Yamamoto, T. Synthesis and phase behavior of aqueous poly (N-isopropylacrylamide-co-acrylamide), poly (N-isopropylacrylamide-co-N, N-dimethylacrylamide) and poly (N-isopropylacrylamide-co-2-hydroxyethyl methacrylate). Colloid Polym. Sci. 2006, 284, 1001–1007. [Google Scholar] [CrossRef] [Green Version]
- Kohori, F.; Sakai, K.; Aoyagi, T.; Yokoyama, M.; Yamato, M.; Sakurai, Y.; Okano, T. Control of adriamycin cytotoxic activity using thermally responsive polymeric micelles composed of poly (N-isopropylacrylamide-co-N, N-dimethylacrylamide)-b-poly (D, L-lactide). Colloids Surf. B Biointerfaces 1999, 16, 195–205. [Google Scholar] [CrossRef]
- Yeh, J.-C.; Yang, H.-H.; Hsu, Y.-T.; Su, C.-M.; Lee, T.-H.; Lou, S.-L. Synthesis and characteristics of biodegradable and temperature responsive polymeric micelles based on poly (aspartic acid)-g-poly (N-isopropylacrylamide-co-N, N-dimethylacrylamide). Colloids Surf. A Physicochem. Eng. Asp. 2013, 421, 1–8. [Google Scholar] [CrossRef]
- Qiu, L.Y.; Wu, X.L.; Jin, Y. Doxorubicin-loaded polymeric micelles based on amphiphilic polyphosphazenes with poly (N-isopropylacrylamide-co-N, N-dimethylacrylamide) and ethyl glycinate as side groups: Synthesis, preparation and in vitro evaluation. Pharm. Res. 2009, 26, 946–957. [Google Scholar] [CrossRef]
- Zou, X.-Y.; Xie, R.; Ju, X.-J.; Wang, W.; Liu, Z.; Li, X.-Y.; Chu, L.-Y. Visual detection of methanol in alcoholic beverages using alcohol-responsive poly (N-isopropylacrylamide-co-N, N-dimethylacrylamide) copolymers as indicators. RSC Adv. 2014, 4, 61711–61721. [Google Scholar] [CrossRef]
- Bauri, K.; Roy, S.G.; Arora, S.; Dey, R.K.; Goswami, A.; Madras, G.; De, P. Thermal degradation kinetics of thermoresponsive poly (N-isopropylacrylamide-co-N, N-dimethylacrylamide) copolymers prepared via RAFT polymerization. J. Therm. Anal. Calorim. 2013, 111, 753–761. [Google Scholar] [CrossRef]
- Lauterbach, F. Advances in RAFT Polymerization Process Design and Analysis. Ph.D. Thesis, University of Hamburg, Hamburg, Germany, 2020. [Google Scholar]
- Gody, G.; Maschmeyer, T.; Zetterlund, P.B.; Perrier, S. Exploitation of the degenerative transfer mechanism in RAFT polymerization for synthesis of polymer of high livingness at full monomer conversion. Macromolecules 2014, 47, 639–649. [Google Scholar] [CrossRef]
- Gody, G.; Maschmeyer, T.; Zetterlund, P.B.; Perrier, S. Pushing the limit of the RAFT process: Multiblock copolymers by one-pot rapid multiple chain extensions at full monomer conversion. Macromolecules 2014, 47, 3451–3460. [Google Scholar] [CrossRef]
- Guillaneuf, Y.; Castignolles, P. Using apparent molecular weight from SEC in controlled/living polymerization and kinetics of polymerization. Part A Polym. Chem. 2008, 46, 897–911. [Google Scholar] [CrossRef]
- Tüdös, F.; Kelen, T. Analysis of the linear methods for determining copolymerization reactivity ratios. V. Planning of experiments. J. Macromol. Sci.-Chem. 1981, 16, 1283–1297. [Google Scholar] [CrossRef]
- d’Agosto, F.; Rieger, J.; Lansalot, M. RAFT-Mediated Polymerization-Induced Self-Assembly. Angew. Chem. Int. Ed. 2020, 59, 8368–8392. [Google Scholar] [CrossRef]
- Rieger, J.; Zhang, W.; Stoffelbach, F.; Charleux, B. Surfactant-Free RAFT Emulsion Polymerization Using Poly(N,N-dimethylacrylamide) Trithiocarbonate Macromolecular Chain Transfer Agents. Macromolecules 2010, 43, 6302–6310. [Google Scholar] [CrossRef]
- Taylor, L.D.; Cerankowski, L.D. Preparation of films exhibiting a balanced temperature dependence to permeation by aqueous solutions—A study of lower consolute behavior. J. Polym. Sci. Polym. Chem. Ed. 1975, 13, 2551–2570. [Google Scholar] [CrossRef]
- Feil, H.; Bae, Y.H.; Feijen, J.; Kim, S.W. Effect of comonomer hydrophilicity and ionization on the lower critical solution temperature of N-isopropylacrylamide copolymers. Macromolecules 1993, 26, 2496–2500. [Google Scholar] [CrossRef]
- Zheng, J.Y.; Tan, M.J.; Thoniyot, P.; Loh, X.J. Unusual thermogelling behaviour of poly [2-(dimethylamino) ethyl methacrylate](PDMAEMA)-based polymers polymerized in bulk. RSC Adv. 2015, 5, 62314–62318. [Google Scholar] [CrossRef]
- Cho, S.H.; Jhon, M.S.; Yuk, S.H. Temperature-sensitive swelling behavior of polymer gel composed of poly (N, N-dimethylaminoethyl methacrylate) and its copolymers. Eur. Polym. J. 1999, 35, 1841–1845. [Google Scholar] [CrossRef]
- Wang, X.; Qiu, X.; Wu, C. Comparison of the coil-to-globule and the globule-to-coil transitions of a single poly (N-isopropylacrylamide) homopolymer chain in water. Macromolecules 1998, 31, 2972–2976. [Google Scholar] [CrossRef]
- Algaer, E.A.; van der Vegt, N.F. Hofmeister ion interactions with model amide compounds. J. Phys. Chem. B 2011, 115, 13781–13787. [Google Scholar] [CrossRef] [PubMed]
- Heyda, J.; Dzubiella, J. Thermodynamic description of Hofmeister effects on the LCST of thermosensitive polymers. J. Phys. Chem. B 2014, 118, 10979–10988. [Google Scholar] [CrossRef] [PubMed]
- Von Hippel, P.H.; Schleich, T. Ion effects on the solution structure of biological macromolecules. Acc. Chem. Res. 1969, 2, 257–265. [Google Scholar] [CrossRef]
- Bozorg, M.; Hankiewicz, B.; Abetz, V. Solubility behaviour of random and gradient copolymers of di-and oligo (ethylene oxide) methacrylate in water: Effect of various additives. Soft Matter 2020, 16, 1066–1081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Furyk, S.; Bergbreiter, D.E.; Cremer, P.S. Specific ion effects on the water solubility of macromolecules: PNIPAM and the Hofmeister series. J. Am. Chem. Soc. 2005, 127, 14505–14510. [Google Scholar] [CrossRef]
- Marcus, Y. Ion Properties; CRC Press: Boca Raton, FL, USA, 1997. [Google Scholar]
- Zhang, Y.; Furyk, S.; Sagle, L.B.; Cho, Y.; Bergbreiter, D.E.; Cremer, P.S. Effects of Hofmeister anions on the LCST of PNIPAM as a function of molecular weight. J. Phys. Chem. C 2007, 111, 8916–8924. [Google Scholar] [CrossRef] [Green Version]
- Taylor, M.J.; Tomlins, P.; Sahota, T.S. Thermoresponsive gels. Gels 2017, 3, 4. [Google Scholar] [CrossRef]
- Eggers, S.; Lauterbach, F.; Abetz, V. Synthesis and self-assembly of high molecular weight polystyrene-block-poly [2-(N-morpholino) ethyl methacrylate]: A story about microphase separation, amphiphilicity, and stimuli-responsivity. Polymer 2016, 107, 357–367. [Google Scholar] [CrossRef]
- Zeng, F.; Tong, Z.; Sato, T. Molecular chain properties of poly (N-isopropyl acrylamide). Sci. China Ser. B Chem. 1999, 42, 290–297. [Google Scholar] [CrossRef]
- Radjabian, M.; Abetz, C.; Fischer, B.; Meyer, A.; Abetz, V. Influence of solvent on the structure of an amphiphilic block copolymer in solution and in formation of an integral asymmetric membrane. ACS Appl. Mater. Interfaces 2017, 9, 31224–31234. [Google Scholar] [CrossRef]
- Truong, N.P.; Dussert, M.V.; Whittaker, M.R.; Quinn, J.F.; Davis, T.P. Rapid synthesis of ultrahigh molecular weight and low polydispersity polystyrene diblock copolymers by RAFT-mediated emulsion polymerization. Polym. Chem. 2015, 6, 3865–3874. [Google Scholar] [CrossRef]
- Luo, Y.; Cui, X. Reversible addition–fragmentation chain transfer polymerization of methyl methacrylate in emulsion. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 2837–2847. [Google Scholar] [CrossRef]
- Sugihara, S.; Blanazs, A.; Armes, S.P.; Ryan, A.J.; Lewis, A.L. Aqueous dispersion polymerization: A new paradigm for in situ block copolymer self-assembly in concentrated solution. J. Am. Chem. Soc. 2011, 133, 15707–15713. [Google Scholar] [CrossRef]
- Blanazs, A.; Ryan, A.; Armes, S. Predictive phase diagrams for RAFT aqueous dispersion polymerization: Effect of block copolymer composition, molecular weight, and copolymer concentration. Macromolecules 2012, 45, 5099–5107. [Google Scholar] [CrossRef]











| Copolymer a | Conv.DMA [%] | Conv.NIPAM [%] | Number of DMA Units d | Number of NIPAM Units d | Đ | fDMA feed [%] | fDMA copolymer [%] | ||
|---|---|---|---|---|---|---|---|---|---|
| P(DMA148-NIPAM127)29 | 29 | 38 | 99.0 | 97.0 | 148 | 127 | 1.20 | 50 | 50.5 |
| P(DMA199-NIPAM171)39 | 39 | 52 | 99.6 | 97.0 | 199 | 171 | 1.20 | 50 | 50.5 |
| P(DMA43-NIPAM69)13 | 13 | 18 | 90.0 | 78.0 | 43 | 69 | 1.08 | 30 | 33.2 |
| P(DMA29-NIPAM180)24 | 24 | 39 | 98.5 | 94.0 | 29 | 180 | 1.09 | 12 | 12.5 |
| P(DMA35-NIPAM212)28 | 28 | 48 | 98.0 | 92.0 | 35 | 212 | 1.09 | 12 | 12.7 |
| P(DMA10-NIPAM153)19 | 19 | 33 | 99.9 | 93.0 | 10 | 153 | 1.08 | 5 | 5.4 |
| P(DMA6-NIPAM160)19 | 19 | 34 | 99.9 | 95.5 | 6 | 160 | 1.08 | 3 | 3.1 |
| P(DMA4-NIPAM138)16 | 16 | 30 | 95.0 | 82.0 | 4 | 138 | 1.09 | 2 | 2.4 |
| P(DMA2-NIPAM165)19 | 19 | 38 | 99.9 | 96.0 | 2 | 165 | 1.08 | 1 | 1.1 |
| Diblock Copolymer a | Conv. [%] | Đ | DPPS d | fP(DMA-co-NIPAM) [wt.%] | ||
|---|---|---|---|---|---|---|
| P(DMA148-NIPAM127)-PS887122 | 122 | 127 | 98 | 1.6 | 887 | 23 |
| P(DMA148-NIPAM127)-PS1024136 | 136 | 125 | 96 | 1.6 | 1024 | 26 |
| P(DMA148-NIPAM127)-PS66398 | 98 | 131 | 66 | 1.5 | 663 | 30 |
| P(DMA148-NIPAM127)-PS8381116 | 116 | 127 | 98 | 1.5 | 838 | 25 |
| P(DMA148-NIPAM127)-PS794112 | 112 | 126 | 98 | 1.5 | 794 | 26 |
| P(DMA29-NIPAM180)-PS937121 | 121 | 84 | 71 | 1.4 | 937 | 20 |
| P(DMA29-NIPAM180)-PS944122 | 122 | 87 | 76 | 1.5 | 944 | 19 |
| P(DMA35-NIPAM212)-PS1125141 | 141 | 85 | 79 | 1.5 | 1125 | 17 |
| P(DMA35-NIPAM212)-PS982130 | 130 | 105 | 82 | 1.5 | 982 | 21 |
| P(DMA35-NIPAM212)-PS1089141 | 141 | 129 | 84 | 1.4 | 1089 | 20 |
| P(DMA29-NIPAM180)-PS1120140 | 140 | 111 | 91 | 1.4 | 1120 | 17 |
| Copolymer | CP [°C] a | f PDMA Copolymer [%] |
|---|---|---|
| P(DMA2-NIPAM165)19 | 34.5 | 1.1 |
| P(DMA4-NIPAM138)16 | 36.6 | 2.4 |
| P(DMA10-NIPAM153)19 | 38.8 | 5.4 |
| P(DMA35-NIPAM212)28 | 42.5 | 12.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nieswandt, K.; Georgopanos, P.; Held, M.; Sperling, E.; Abetz, V. RAFT Emulsion Polymerization of Styrene Using a Poly((N,N-dimethyl acrylamide)-co-(N-isopropyl acrylamide)) mCTA: Synthesis and Thermosensitivity. Polymers 2022, 14, 62. https://doi.org/10.3390/polym14010062
Nieswandt K, Georgopanos P, Held M, Sperling E, Abetz V. RAFT Emulsion Polymerization of Styrene Using a Poly((N,N-dimethyl acrylamide)-co-(N-isopropyl acrylamide)) mCTA: Synthesis and Thermosensitivity. Polymers. 2022; 14(1):62. https://doi.org/10.3390/polym14010062
Chicago/Turabian StyleNieswandt, Katharina, Prokopios Georgopanos, Martin Held, Evgeni Sperling, and Volker Abetz. 2022. "RAFT Emulsion Polymerization of Styrene Using a Poly((N,N-dimethyl acrylamide)-co-(N-isopropyl acrylamide)) mCTA: Synthesis and Thermosensitivity" Polymers 14, no. 1: 62. https://doi.org/10.3390/polym14010062
APA StyleNieswandt, K., Georgopanos, P., Held, M., Sperling, E., & Abetz, V. (2022). RAFT Emulsion Polymerization of Styrene Using a Poly((N,N-dimethyl acrylamide)-co-(N-isopropyl acrylamide)) mCTA: Synthesis and Thermosensitivity. Polymers, 14(1), 62. https://doi.org/10.3390/polym14010062