Performance Enhancement of Ionic Polymer-Metal Composite Actuators with Polyethylene Oxide
Abstract
:1. Introduction
2. Experiments
2.1. Experimental Materials
2.2. Preparation of PEO/Nafion Membrane
2.3. Fabrication of IPMCs
2.4. Characterizations
2.4.1. Characterizations of PEO/Nafion Membranes
2.4.2. Electromechanical Test of IPMCs
3. Results and Discussion
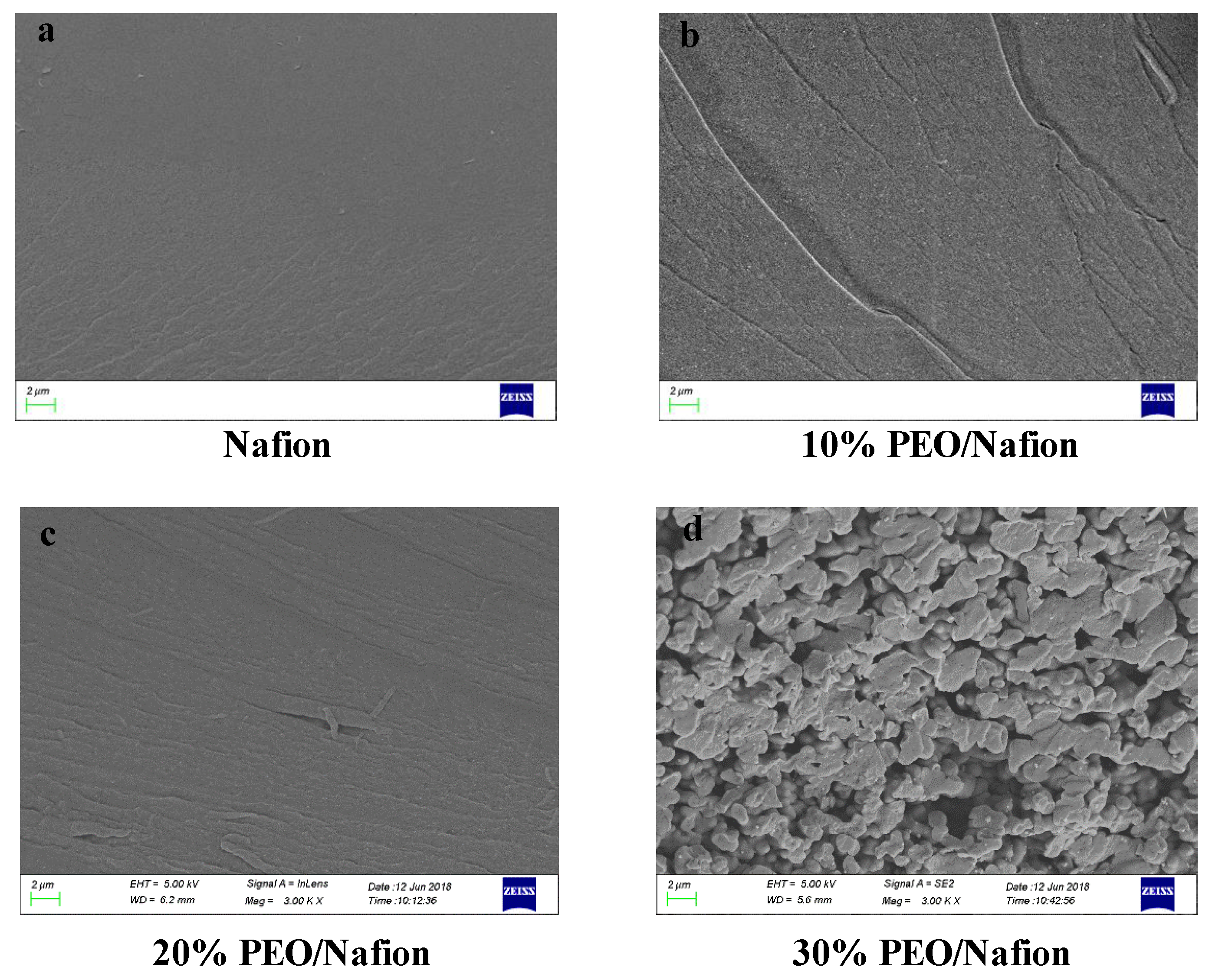
3.1. Influences of PEO Content on the Properties of the PEO/Nafion Membranes
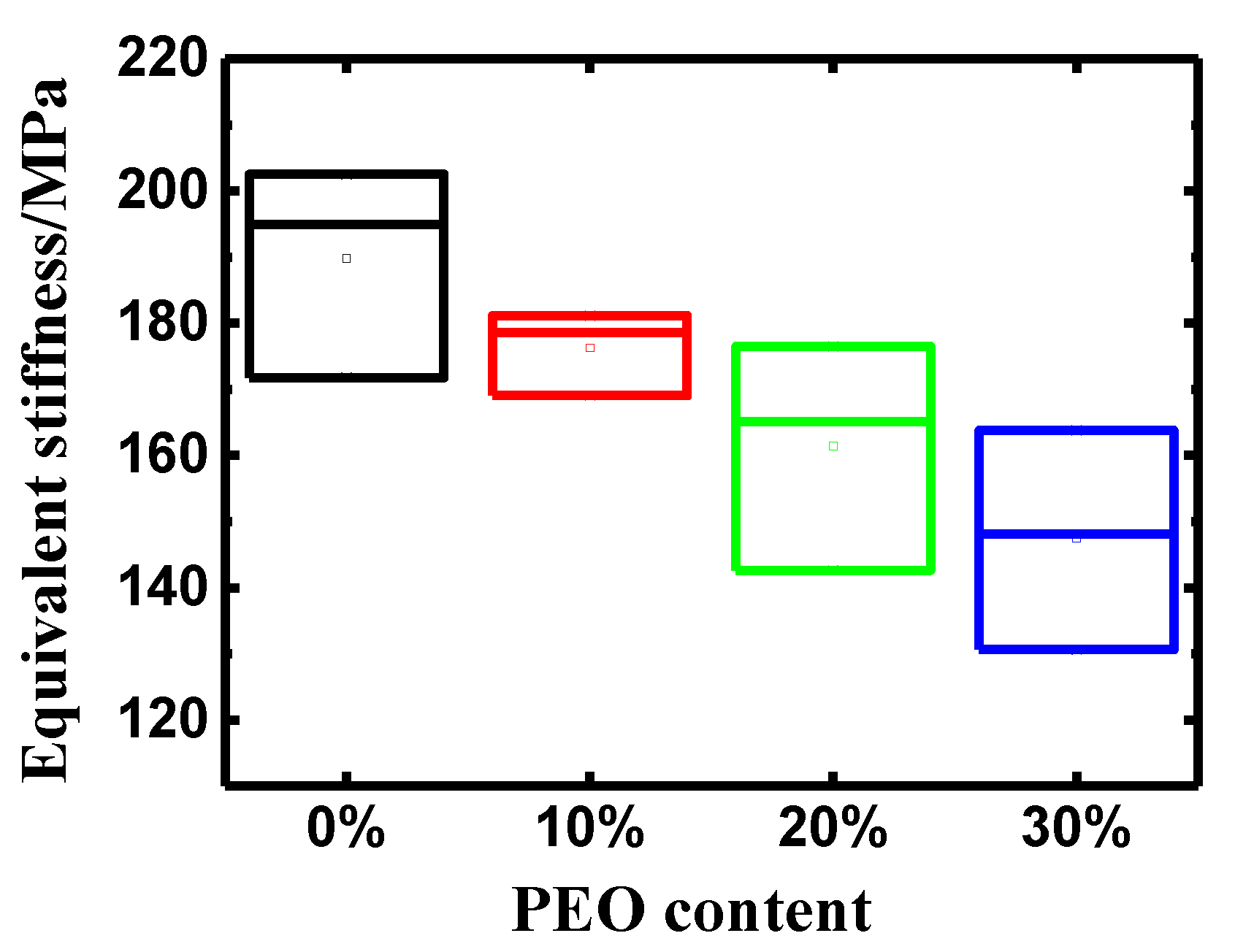
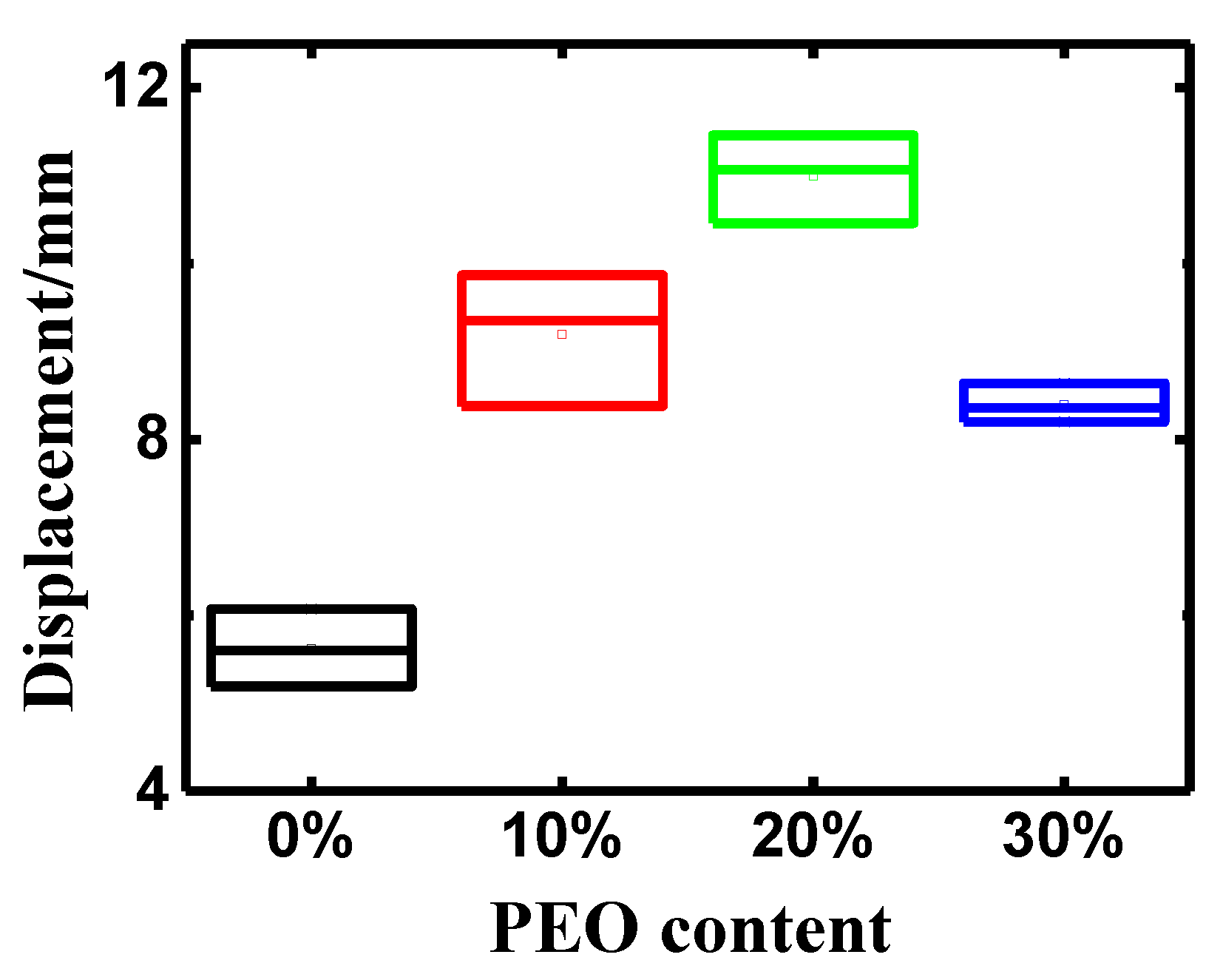
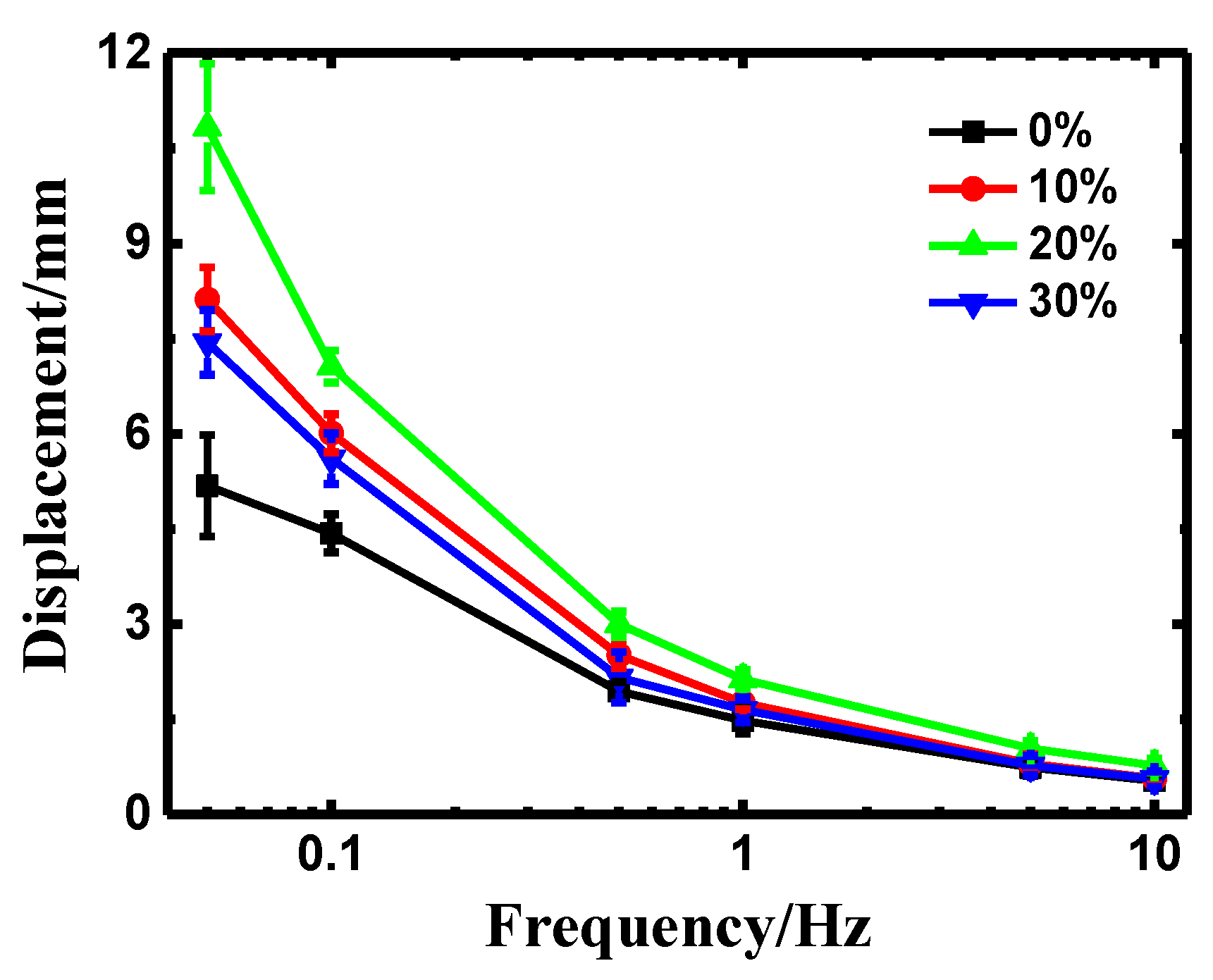
3.2. Influences of PEO Content on the Properties of the PEO/Nafion-IPMCs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, L.; Fu, J.; He, Y. A Review of 3D Printing Technologies for Soft Polymer Materials. Adv. Funct. Mater. 2020, 30, 2000187. [Google Scholar] [CrossRef]
- Ma, S.Q.; Zhang, Y.P.; Liang, Y.H.; Ren, L.; Tian, W.J.; Ren, L.Q. High-Performance Ionic-Polymer-Metal Composite: Toward Large-Deformation Fast-Response Artificial Muscles. Adv. Funct. Mater. 2019, 20, 1908508. [Google Scholar] [CrossRef]
- White, B.T.; Long, T.E. Advances in Polymeric Materials for Electromechanical Devices. Macromol. Rapid Commun. 2018, 40, e1800521. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Stalbaum, T.; Minaian, N.; Oh, I.; Kim, K.J. A robotic multiple-shape-memory ionic polymer-metal composite (IPMC) actuator: Modeling approach. Smart Mater. Struct. 2018, 28, 015009. [Google Scholar] [CrossRef]
- Ru, J.; Zhu, Z.; Wang, Y.; Chen, H.; Li, D. Tunable actuation behavior of ionic polymer metal composite utilizing carboxylated carbon nanotube-doped Nafion matrix. RSC Adv. 2018, 8, 3090–3094. [Google Scholar] [CrossRef] [Green Version]
- Zhao, D.; Li, D.; Wang, Y.; Chen, H. Improved manufacturing technology for producing porous Nafion for high-performance ionic polymer–metal composite actuators. Smart Mater. Struct. 2016, 25, 075043. [Google Scholar] [CrossRef]
- Chen, Z.; Shatara, S.; Tan, X. Modeling of Biomimetic Robotic Fish Propelled by an Ionic Polymer-Metal Composite Caudal Fin. IEEE/ASME Trans. Mechatron. 2009, 15, 448–459. [Google Scholar] [CrossRef]
- Aureli, M.; Kopman, V.; Porfiri, M. Free-Locomotion of Underwater Vehicles Actuated by Ionic Polymer Metal Composites. IEEE/ASME Trans. Mechatron. 2009, 15, 603–614. [Google Scholar] [CrossRef]
- Zhu, Z.; Chang, L.; Takagi, K.; Wang, Y.; Chen, H.; Li, D. Water content criterion for relaxation deformation of Nafion based ionic polymer metal composites doped with alkali cations. Appl. Phys. Lett. 2014, 105, 054103. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, H.; Wang, Y.; Luo, B.; Chang, L.; Zhu, Z.; Li, B. Influence of additives on the properties of casting nafion membranes and SO-based ionic polymer-Metal composite actuators. Polym. Eng. Sci. 2013, 54, 818–830. [Google Scholar] [CrossRef]
- Shoji, E.; Hirayama, D. Effects of Humidity on the Performance of Ionic Polymer-Metal Composite Actuators: Experimental Study of the Back-Relaxation of Actuators. J. Phys. Chem. B 2007, 111, 11915–11920. [Google Scholar] [CrossRef] [PubMed]
- Nemat-Nasser, S.; Wu, Y. Comparative experimental study of ionic polymer-metal composites with different backbone ionomers and in various cation forms. J. Appl. Phys. 2003, 93, 5255–5267. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Z.; Asaka, K.; Chang, L.; Takagi, K.; Chen, H. Physical interpretation of deformation evolvement with water content of ionic polymer-metal composite actuator. J. Appl. Phys. 2013, 114, 184902. [Google Scholar] [CrossRef]
- Guo, D.; Wang, L.; Wang, X.; Xiao, Y.; Wang, C.; Chen, L.; Ding, Y. PEDOT coating enhanced electromechanical performances and prolonged stable working time of IPMC actuator. Sens. Actuators B Chem. 2019, 305, 127488. [Google Scholar] [CrossRef]
- Franklin, J.W. Electromechanical Modeling of Encapsulated Ionic Polymer Transducers. Master’s Thesis, Virginia Tech, Blacksburg, VA, USA, 2003. [Google Scholar]
- Yu, C.-Y.; Zhang, Y.-W.; Su, G.-D. Reliability tests of ionic polymer metallic composites in dry air for actuator applications. Sens. Actuators A Phys. 2015, 232, 183–189. [Google Scholar] [CrossRef]
- Kim, S.J.; Lee, I.T.; Lee, H.-Y.; Kim, Y.H. Performance improvement of an ionic polymer-metal composite actuator by parylene thin film coating. Smart Mater. Struct. 2006, 15, 1540–1546. [Google Scholar] [CrossRef]
- Chung, C.-K.; Fung, P.; Hong, Y.; Ju, M.; Lin, C.-C.; Wu, T. A novel fabrication of ionic polymer-metal composites (IPMC) actuator with silver nano-powders. Sens. Actuators B Chem. 2006, 117, 367–375. [Google Scholar] [CrossRef]
- Bennett, M.D.; Leo, D.J. Ionic Liquids as Hyper-Stable Solvents for Ionic Polymer Transducers. In Proceedings of the ASME International Mechanical Engineering Congress & Exposition, Washington, DC, USA, 15–21 November 2003; pp. 415–423. [Google Scholar]
- Nemat-Nasser, S.; Zamani, S.; Tor, Y. Effect of solvents on the chemical and physical properties of ionic polymer-metal com-posites. J. Appl. Phys. 2006, 99, 51–191. [Google Scholar]
- Tant, M.R.; Mauritz, K.A.; Wilkes, G.L. Ionomers: Synthesis, Structure, Properties and Applications, 1st ed.; Van Nostrand Reinhold Co.: New York, NY, USA, 1997. [Google Scholar]
- Thangamuthu, R.; Lin, C. DBSA-doped PEG/SiO2 proton-conducting hybrid membranes for low-temperature fuel cell applications. Solid State Ionics 2005, 176, 531–538. [Google Scholar] [CrossRef]
- Bijay, P.T.; Vinod, K.S. Organic-inorganic nanocomposite polymer electrolyte membranes for fuel cell applications. Prog. Polym. Sci. 2011, 36, 945–979. [Google Scholar]
- Kang, M.-S.; Lee, M.-J. Anhydrous solid proton conductors based on perfluorosulfonic ionomer with polymeric solvent for polymer electrolyte fuel cell. Electrochem. Commun. 2009, 11, 457–460. [Google Scholar] [CrossRef]
- Smith, G.D.; Bedrov, D.; Borodin, O. Conformations and Chain Dimensions of Poly(ethylene oxide) in Aqueous Solution: A Molecular Dynamics Simulation Study. J. Am. Chem. Soc. 2000, 122, 9548–9549. [Google Scholar] [CrossRef]
- Thangamuthu, R.; Lin, C. Preparation of gas diffusion electrodes using PEG/SiO2 hybrid materials and the effect of their composition on microstructure of the catalyst layer and on fuel cell performance. J. Power Sources 2006, 161, 160–167. [Google Scholar] [CrossRef]
- Chang, H.; Lin, C.W. Proton conducting membranes based on PEG/SiO2 nanocomposites for direct methanol fuel cells. J. Membr. Sci. 2003, 218, 295–306. [Google Scholar] [CrossRef]
- Chang, H.; Thangamuthu, R.; Lin, C. Structure–property relationships in PEG/SiO2 based proton conducting hybrid membranes—A 29Si CP/MAS solid-state NMR study. J. Membr. Sci. 2004, 228, 217–226. [Google Scholar] [CrossRef]
- Wang, Y.J.; Chen, H.L.; Wang, Y.Q.; Zhu, Z.C.; Li, D.C. Effect of dehydration on the mechanical and physicochemical prop-erties of gold-and palladium-ionomeric polymer-metal composite (IPMC) actuators. Electrochim. Acta 2014, 129, 450–458. [Google Scholar] [CrossRef]
- Park, J.; Palmre, V.; Hwang, T.; Kim, K.; Yim, W.; Bae, C. Electromechanical performance and other characteristics of IPMCs fabricated with various commercially available ion exchange membranes. Smart Mater. Struct. 2014, 23, 74001. [Google Scholar] [CrossRef]
- Terasawa, N.; Ono, N.; Mukai, K.; Koga, T.; Higashi, N.; Asaka, K. A multi-walled carbon nanotube/polymer actuator that surpasses the performance of a single-walled carbon nanotube/polymer actuator. Carbon 2012, 50, 311–320. [Google Scholar] [CrossRef]
- An, Y. Mechanical and Electromechanical Performance of Ionic Polymer Metal Composite (IPMC). Ph.D. Thesis, Nanjing Uni-versity of Aeronautics and Astronautis, Nanjing, China, 2009. [Google Scholar]
- Lee, J.-W.; Hong, S.M.; Koo, C.M. High-performance polymer ionomer–ionic liquid membrane IPMC actuator. Res. Chem. Intermed. 2013, 40, 41–48. [Google Scholar] [CrossRef]
- Mousavi, M.S.S.; Alaei, A.; Hasani, M.; Kolahdouz, M.; Manteghi, F.; Ataei, F. Fabrication of ionic polymer metal composite for bio-actuation application: Sputtering and electroless plating methods. Mater. Res. Express 2018, 6, 035312. [Google Scholar] [CrossRef]
- Ishiki, A.; Nabae, H.; Kodaira, A.; Suzumori, K. PF-IPMC: Paper/Fabric Assisted IPMC Actuators for 3D Crafts. IEEE Robot. Autom. Lett. 2020, 5, 4035–4041. [Google Scholar] [CrossRef]
- Luo, B.; Chen, Z. Comparative Experimental Study on Ionic Polymer Mental Composite based on Nafion and Aquivion Membrane as Actuators. IOP Conf. Ser. Mater. Sci. Eng. 2017, 269, 012014. [Google Scholar] [CrossRef]
- Asaka, K.; Mukai, K.; Sugino, T.; Kiyohara, K. Ionic electroactive polymer actuators based on nano-carbon electrodes. Polym. Int. 2013, 62, 1263–1270. [Google Scholar] [CrossRef]
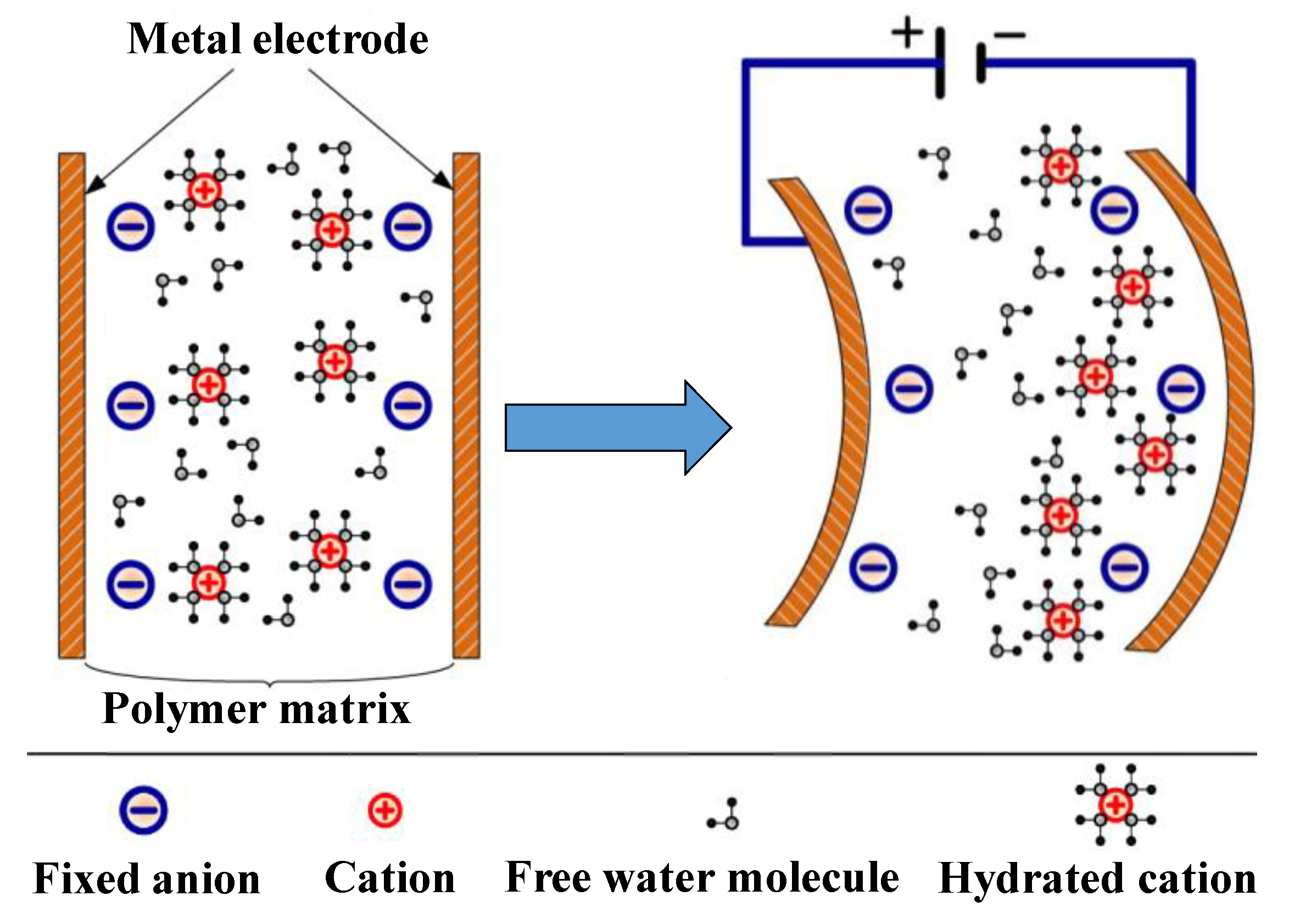



| PEO Content | Nafion (aq) | PEO (s) | DMF (l) | H2O (l) |
|---|---|---|---|---|
| 0 wt% | 26.250 g | 0 g | 6.3 g | 0 g |
| 10 wt% | 23.625 g | 0.1313 g | 6.3 g | 2.494 g |
| 20 wt% | 21.000 g | 0.2625 g | 6.3 g | 4.988 g |
| 30 wt% | 18.375 g | 0.3940 g | 6.3 g | 7.480 g |
| Sample | 0% | 10% | 20% | 30% |
|---|---|---|---|---|
| WD (KJ/m3) | 0.60 | 0.87 | 0.88 | 0.69 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, D.; Ru, J.; Wang, T.; Wang, Y.; Chang, L. Performance Enhancement of Ionic Polymer-Metal Composite Actuators with Polyethylene Oxide. Polymers 2022, 14, 80. https://doi.org/10.3390/polym14010080
Zhao D, Ru J, Wang T, Wang Y, Chang L. Performance Enhancement of Ionic Polymer-Metal Composite Actuators with Polyethylene Oxide. Polymers. 2022; 14(1):80. https://doi.org/10.3390/polym14010080
Chicago/Turabian StyleZhao, Dongxu, Jie Ru, Tong Wang, Yanjie Wang, and Longfei Chang. 2022. "Performance Enhancement of Ionic Polymer-Metal Composite Actuators with Polyethylene Oxide" Polymers 14, no. 1: 80. https://doi.org/10.3390/polym14010080






