Formulation and Characterization of Gum Arabic Stabilized Red Rice Extract Nanoemulsion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
Chemicals
2.2. Methods
2.2.1. Extraction of Ethanolic Extract from Red Rice
2.2.2. Preparation of Oil in Water Nanoemulsion
2.2.3. Creaming Index for the Selection of Suitable Nanoemulsion
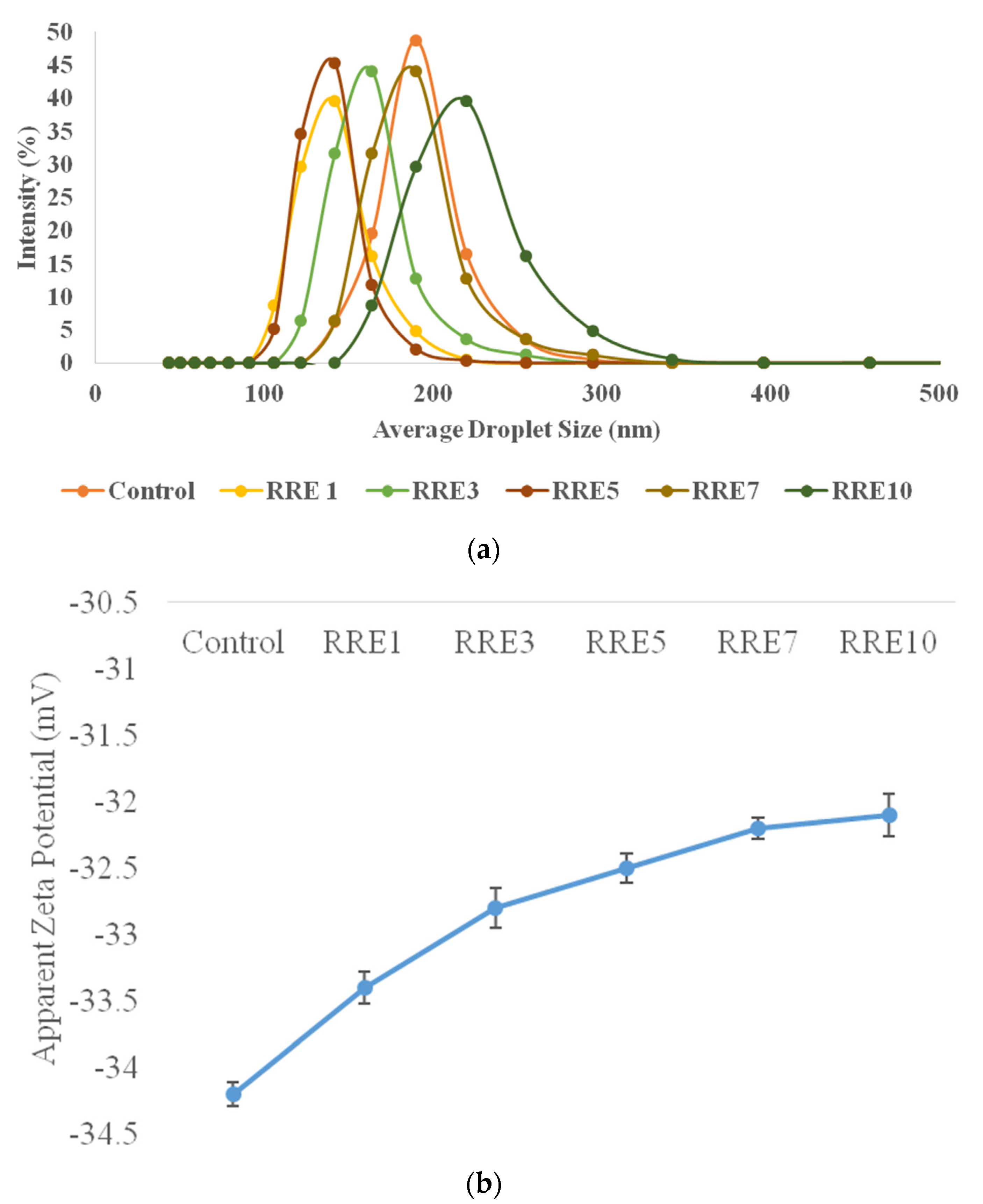
Droplet Size Distribution and Zeta Potential of the Nanoemulsion
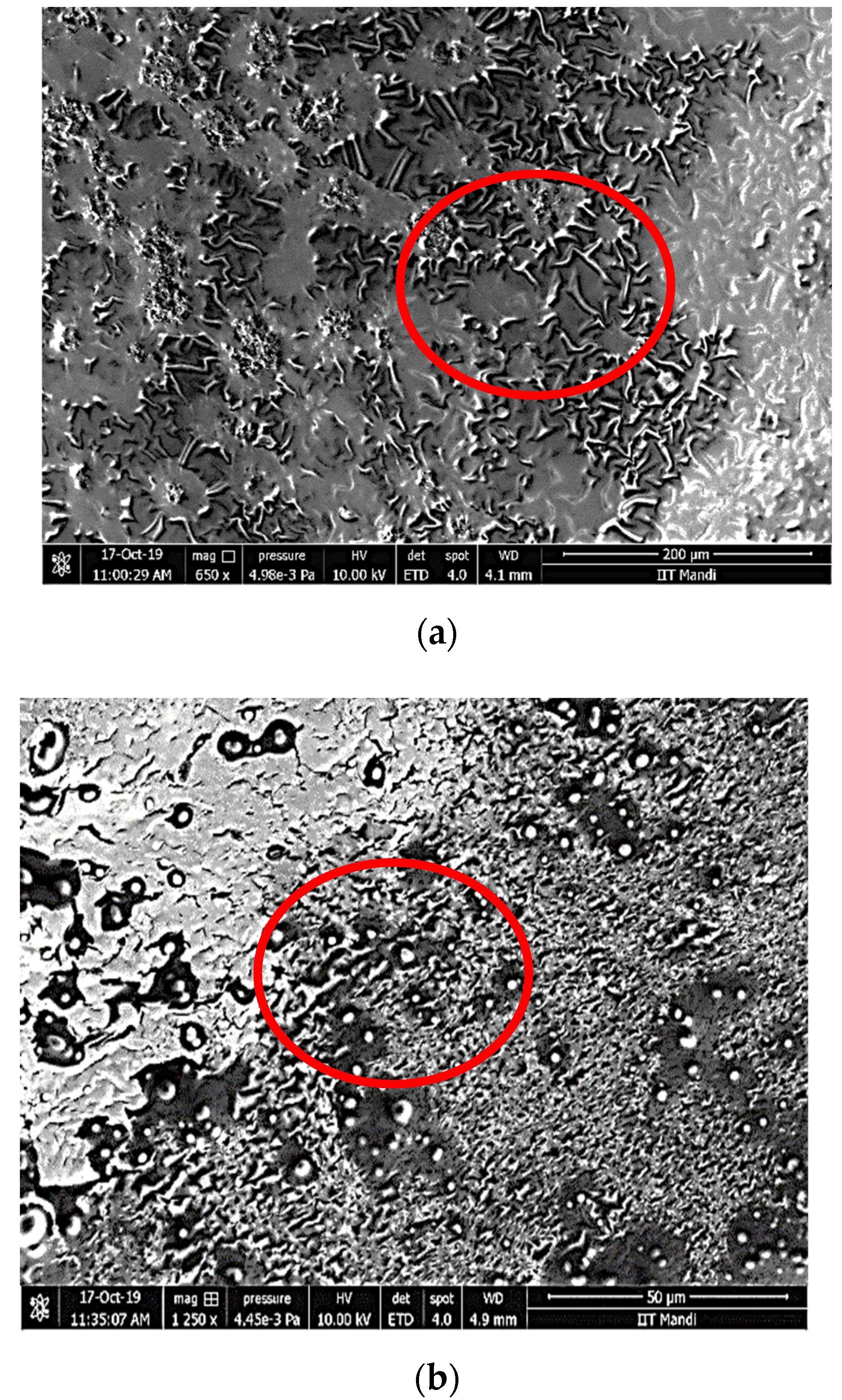
Scanning Electron Microscopy of the Nanoemulsion
Emulsion Stability Testing
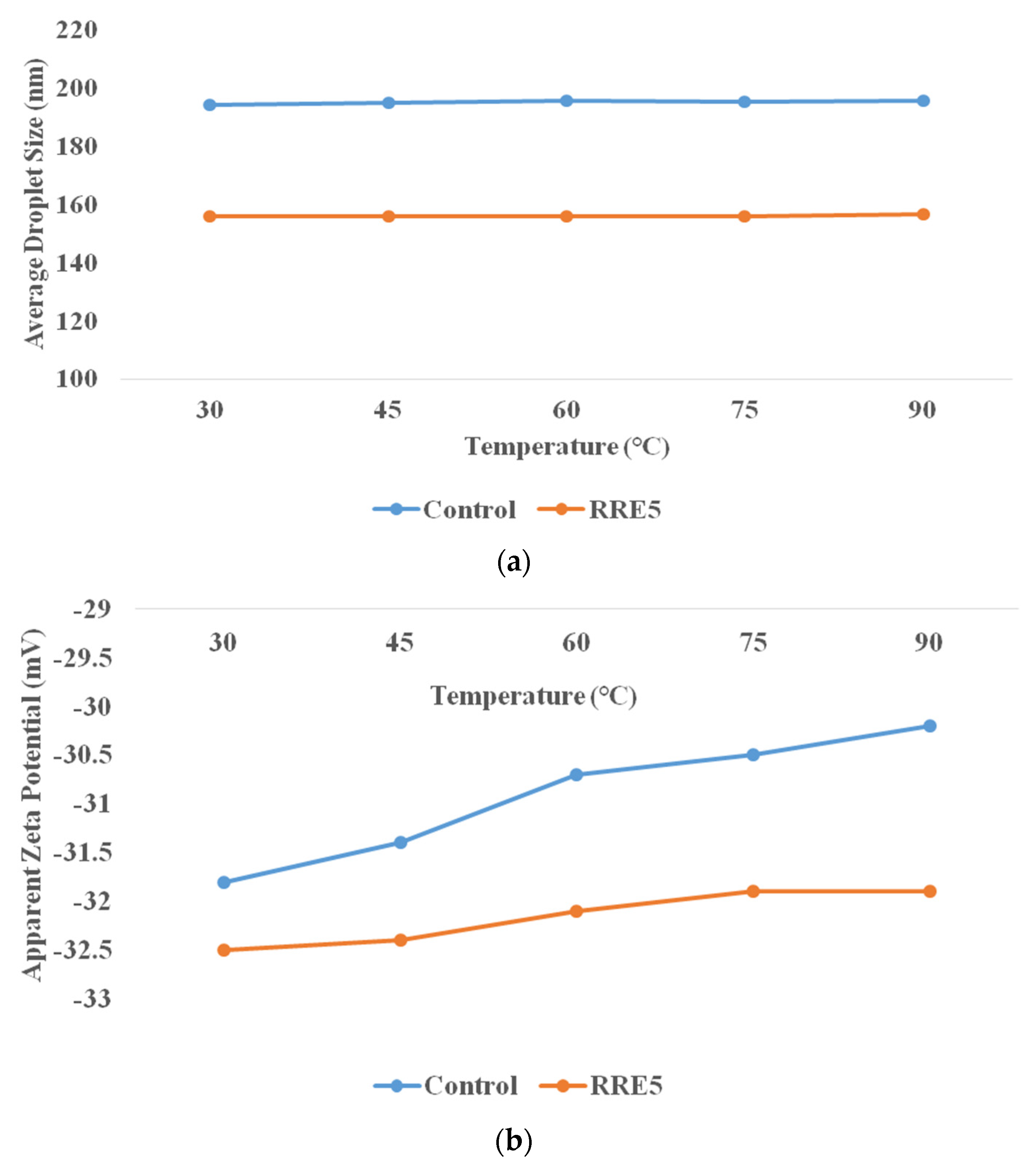
Effect of Thermal Processing
Effect of pH
Ionic Strength
2.3. Thiobarbituric Acid (TBA) Value of Nanoemulsion
2.4. Antioxidant Assay
DPPH (2,2-Diphenyl-1-picrylhydrazyl) Radical Scavenging of Nanoemulsion
2.5. Anti-Inflammatory Assay
2.5.1. Protein (BSA) Denaturation Assay
2.5.2. HRBC Membrane Stabilization Assay
2.6. Antimicrobial Efficiency of Nanoemulsion
Time-Kill Study
2.7. Statistical Analysis
3. Results and Discussion
3.1. Formation of Gum Arabic Stabilized Nanoemulsion
3.2. Creaming Index of Nanoemulsion
3.3. Morphology of Nanoemulsion
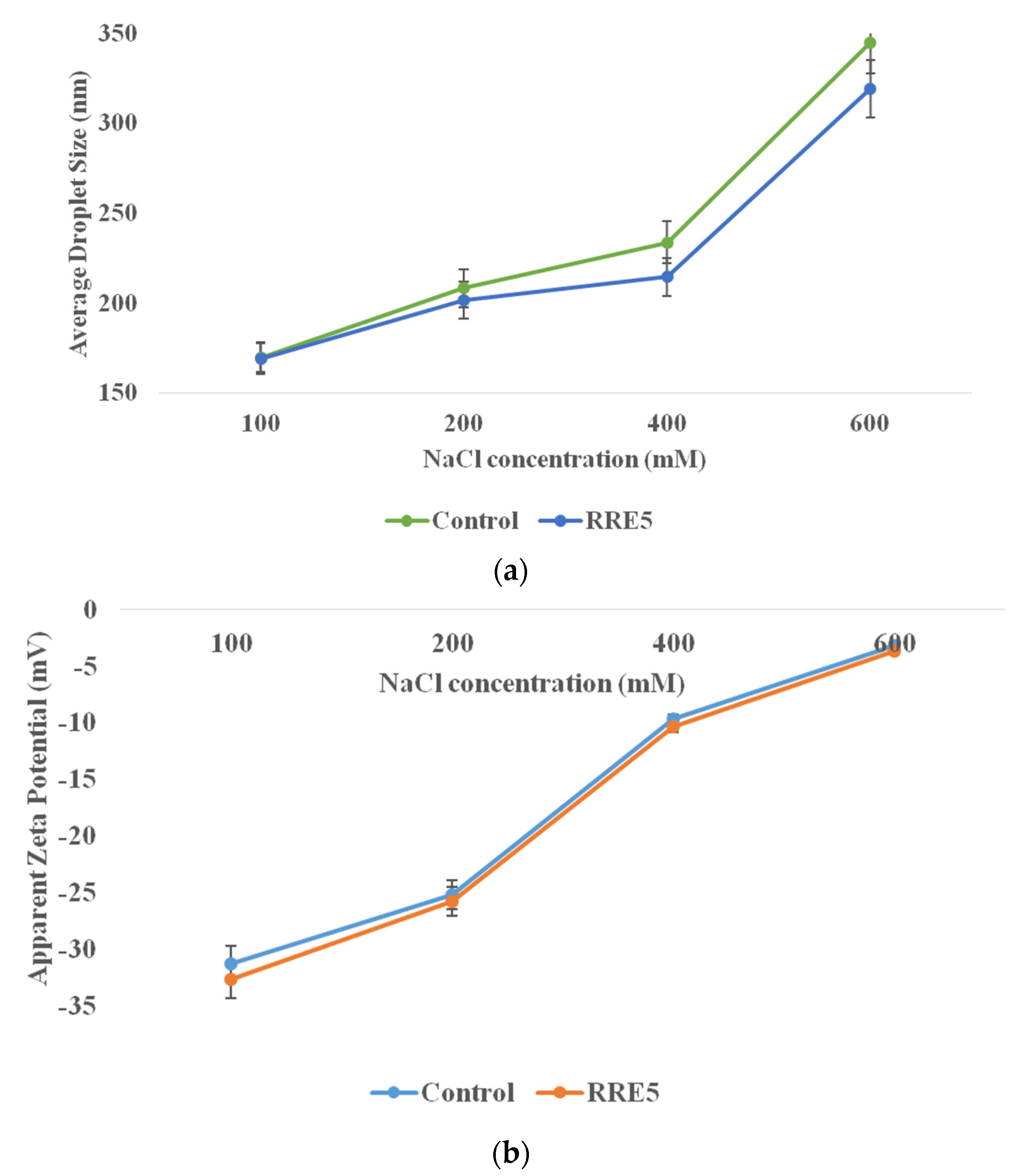
3.4. Thermal Stability of Nanoemulsion
3.5. The Influence of pH on Nanoemulsion Formation
3.6. The influence of Ionic Strength on Nanoemulsion Formation
3.7. TBA Value
3.8. Antioxidant Activity
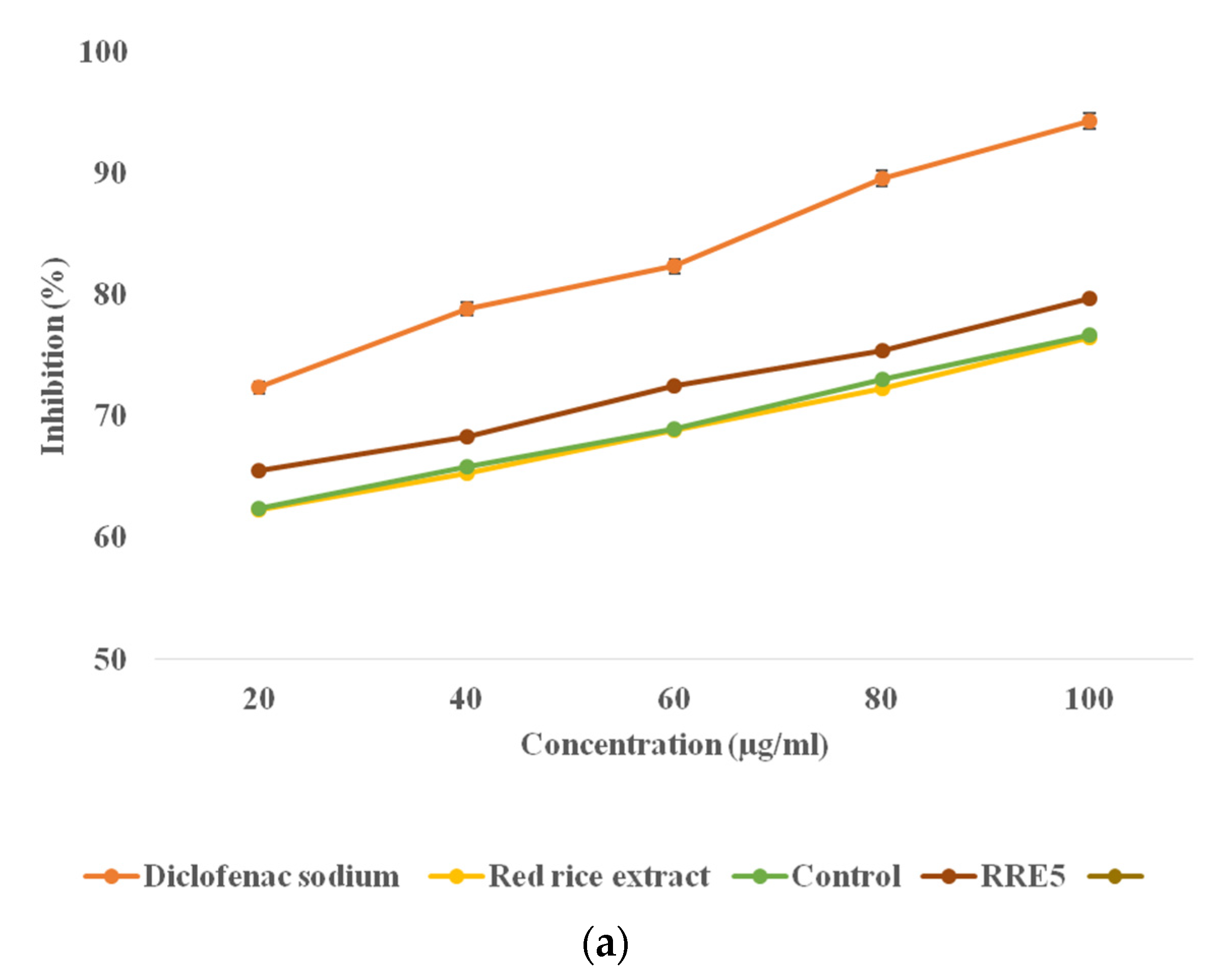
3.9. Anti-Inflammatory Activity
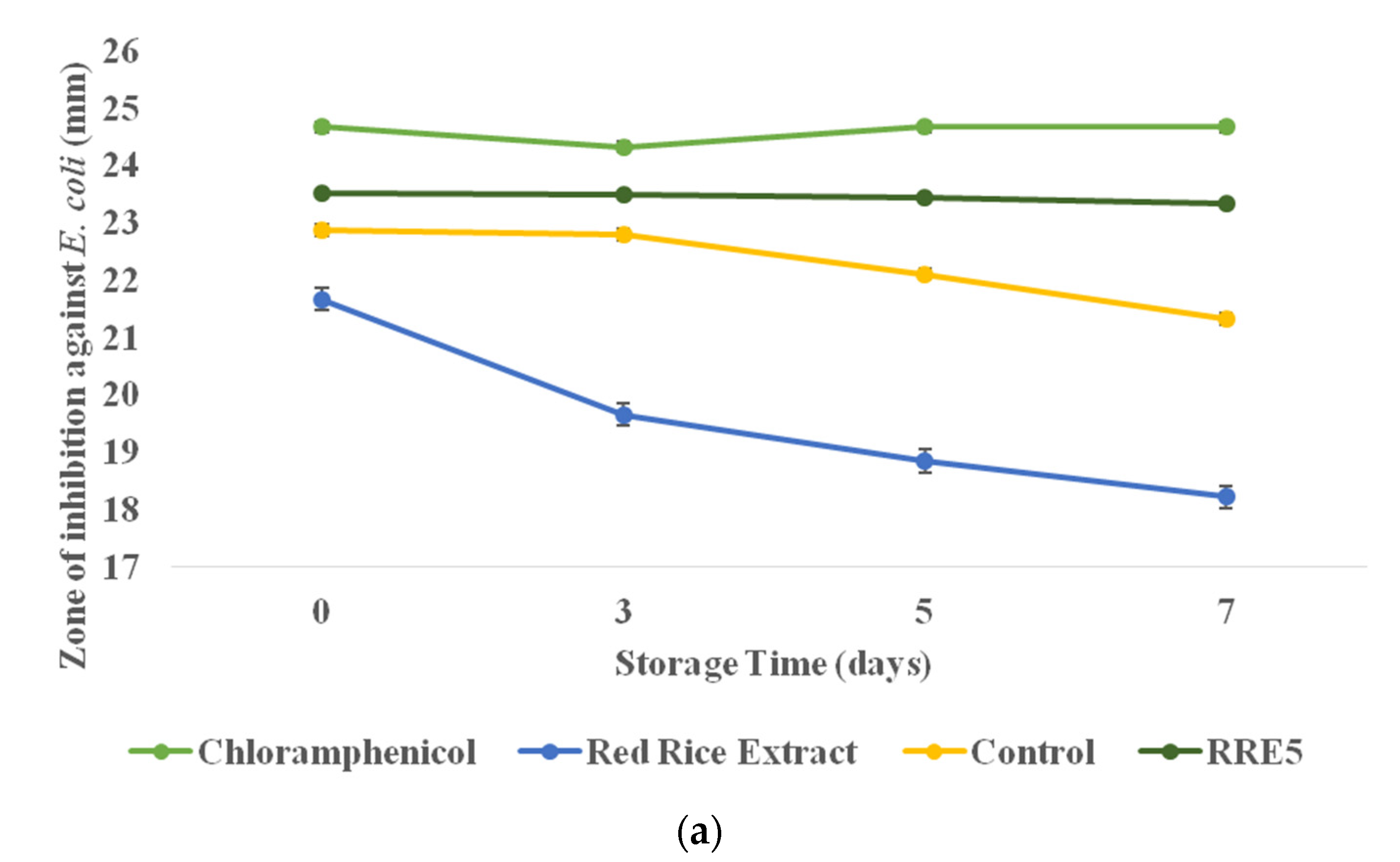
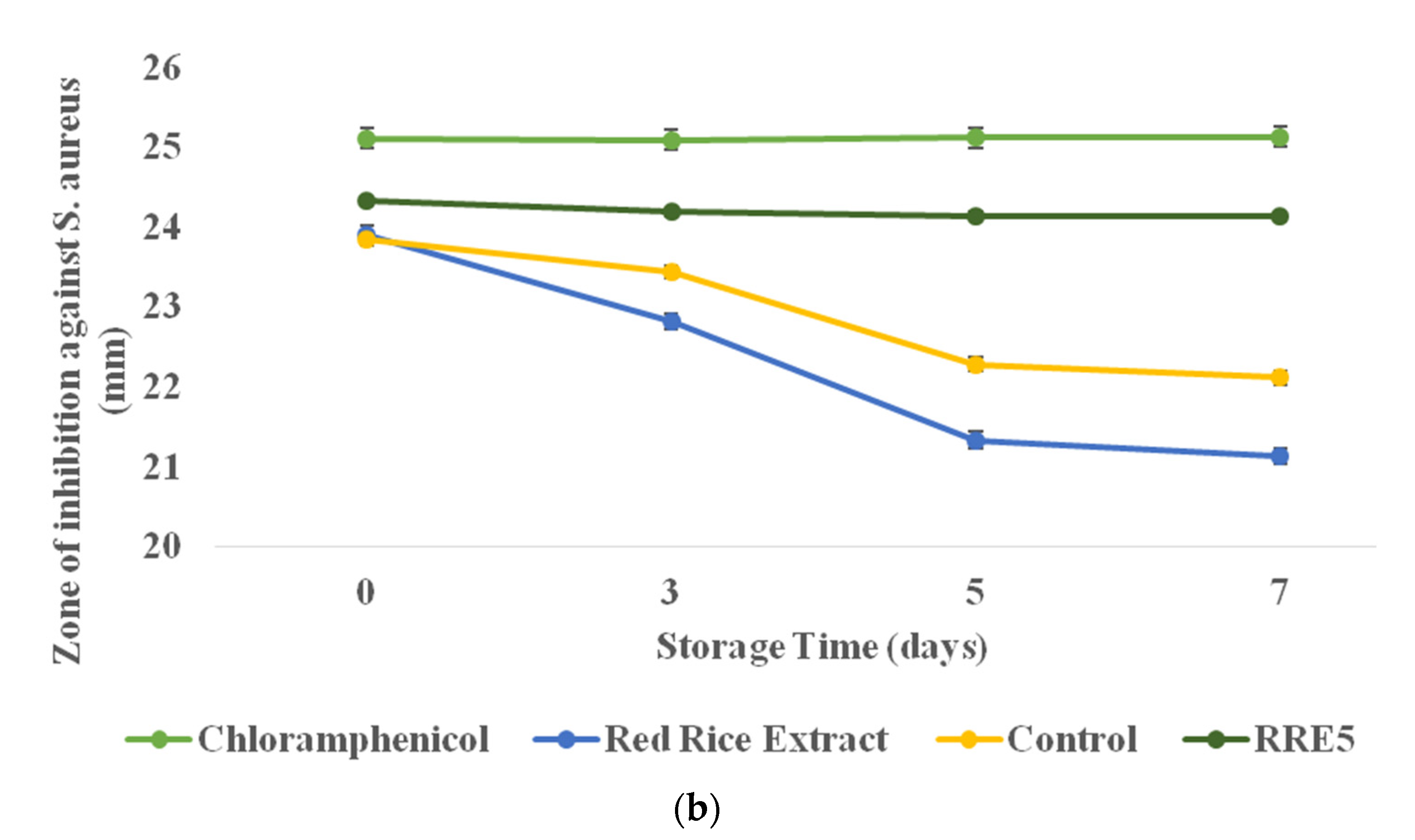
3.10. Antimicrobial Efficiency
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DLS | Dynamic light scattering |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| RRE | Red rice extract |
| GA | Gum arabic |
| TBA | 2-thiobarbituric acid |
| BSA | Bovine serum albumin |
References
- Kotamreddy, J.N.R.; Hansda, C.; Mitra, A. Semi-targeted metabolomic analysis provides the basis for enhanced antioxidant capacities in pigmented rice grains. J. Food Meas. Charact. 2020, 14, 1183–1191. [Google Scholar] [CrossRef]
- Mbanjo, E.G.N.; Kretzschmar, T.; Jones, H.; Ereful, N.; Blanchard, C.; Boyd, L.A.; Sreenivasulu, N. The Genetic Basis and Nutritional Benefits of Pigmented Rice Grain. Front. Genet. 2020, 11, 229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitisin, T.; Saewan, N.; Luplertlop, N. Potential anti-inflammatory and anti-oxidative properties of Thai colored-rice extracts. Plant Omics 2015, 8, 69–77. [Google Scholar]
- Limtrakul, P.; Yodkeeree, S.; Pitchakarn, P.; Punfa, W. Anti-inflammatory effects of proanthocyanidin-rich red rice extract via suppression of MAPK, AP-1 and NF-kappaB pathways in Raw 264.7 macrophages. Nutr. Res. Pract. 2016, 10, 251–258. [Google Scholar] [CrossRef] [Green Version]
- Samyor, D.; Das, A.B.; Deka, S.C. Pigmented rice a potential source of bioactive compounds: A review. Int. J. Food Sci. Technol. 2017, 52, 1073–1081. [Google Scholar] [CrossRef]
- Ginwala, R.; Bhavsar, R.; Chigbu, D.I.; Jain, P.; Khan, Z.K. Potential Role of Flavonoids in Treating Chronic Inflammatory Diseases with a Special Focus on the Anti-Inflammatory Activity of Apigenin. Antioxidants 2019, 8, 35. [Google Scholar] [CrossRef] [Green Version]
- Marin-Ocampo, L.; Veloza, L.A.; Abonia, R.; Sepulveda-Arias, J.C. Anti-inflammatory activity of triazine derivatives: A systematic review. Eur. J. Med. Chem. 2019, 162, 435–447. [Google Scholar] [CrossRef]
- Adnan, M.; Nazim Uddin Chy, M.; Mostafa Kamal, A.T.M.; Barlow, J.W.; Faruque, M.O.; Yang, X.; Uddin, S.B. Evaluation of anti-nociceptive and anti-inflammatory activities of the methanol extract of Holigarna caustica (Dennst.) Oken leaves. J. Ethnopharmacol. 2019, 236, 401–411. [Google Scholar] [CrossRef]
- Chung, J.H.; Kong, J.N.; Choi, H.E.; Kong, K.H. Antioxidant, anti-inflammatory, and anti-allergic activities of the sweet-tasting protein brazzein. Food Chem. 2018, 267, 163–169. [Google Scholar] [CrossRef]
- Gunathilake, K.; Ranaweera, K.; Rupasinghe, H.P.V. In Vitro Anti-Inflammatory Properties of Selected Green Leafy Vegetables. Biomedicines 2018, 6, 107. [Google Scholar] [CrossRef] [Green Version]
- Rashed, M.M.; Tong, Q.; Abdelhai, M.H.; Gasmalla, M.A.; Ndayishimiye, J.B.; Chen, L.; Ren, F. Effect of ultrasonic treatment on total phenolic extraction from Lavandula pubescens and its application in palm olein oil industry. Ultrason. Sonochem. 2016, 29, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Bains, A.; Tripathi, A. Evaluation of Antioxidant and Anti-Inflammatory Properties of Aqueous Extract of Wild Mushrooms Collected from Himachal Pradesh. Asian J. Pharm. Clin. Res. 2017, 10, 467. [Google Scholar] [CrossRef] [Green Version]
- Gonelimali, F.D.; Lin, J.; Miao, W.; Xuan, J.; Charles, F.; Chen, M.; Hatab, S.R. Antimicrobial Properties and Mechanism of Action of Some Plant Extracts Against Food Pathogens and Spoilage Microorganisms. Front. Microbiol. 2018, 9, 1639. [Google Scholar] [CrossRef] [PubMed]
- Manandhar, S.; Luitel, S.; Dahal, R.K. In Vitro Antimicrobial Activity of Some Medicinal Plants against Human Pathogenic Bacteria. J. Trop. Med. 2019, 2019, 1895340. [Google Scholar] [CrossRef] [Green Version]
- Pengon, S.; Chinatangkul, N.; Limmatvapirat, C.; Limmatvapirat, S. The effect of surfactant on the physical properties of coconut oil nanoemulsions. Asian J. Pharm. Sci. 2018, 13, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Taarji, N.; Rabelo da Silva, C.A.; Khalid, N.; Gadhi, C.; Hafidi, A.; Kobayashi, I.; Neves, M.A.; Isoda, H.; Nakajima, M. Formulation and stabilization of oil-in-water nanoemulsions using a saponins-rich extract from argan oil press-cake. Food Chem. 2018, 246, 457–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riquelme, N.; Zúñiga, R.N.; Arancibia, C. Physical stability of nanoemulsions with emulsifier mixtures: Replacement of tween 80 with quillaja saponin. LWT 2019, 111, 760–766. [Google Scholar] [CrossRef]
- Singh, P.; Dasgupta, N.; Singh, V.; Chandra Mishra, N.; Singh, H.; Purohit, S.D.; Srivastava, N.; Ranjan, S.; Yadav, N.P.; Mishra, B.N. Inhibitory effect of clove oil nanoemulsion on fumonisin isolated from maize kernels. LWT 2020, 134, 110237. [Google Scholar] [CrossRef]
- Gupta, A.; Eral, H.B.; Hattona, T.A.; Doyle, P.S. Nanoemulsions: Formation, properties and applications. Soft Matter 2016, 12, 2826–2841. [Google Scholar] [CrossRef] [Green Version]
- Xue, F.; Li, X.; Qin, L.; Liu, X.; Li, C.; Adhikari, B. Anti-aging properties of phytoconstituents and phyto-nanoemulsions and their application in managing aging-related diseases. Adv. Drug Deliv. Rev. 2021, 176, 113886. [Google Scholar] [CrossRef]
- Kumari, S.; Kumaraswamy, R.V.; Choudhary, R.C.; Sharma, S.S.; Pal, A.; Raliya, R.; Biswas, P.; Saharan, V. Thymol nanoemulsion exhibits potential antibacterial activity against bacterial pustule disease and growth promotory effect on soybean. Sci. Rep. 2018, 8, 6650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sethuraman, S.; Rajendran, K. Is Gum Arabic a Good Emulsifier Due to CH...π Interactions? How Urea Effectively Destabilizes the Hydrophobic CH...π Interactions in the Proteins of Gum Arabic than Amides and GuHCl? ACS Omega 2019, 4, 16418–16428. [Google Scholar] [CrossRef] [PubMed]
- Nishio, M. The CH/pi hydrogen bond in chemistry. Conformation, supramolecules, optical resolution and interactions involving carbohydrates. Phys. Chem. Chem. Phys. 2011, 13, 13873–13900. [Google Scholar] [CrossRef] [PubMed]
- Gupta, C.; Chawla, P.; Arora, S.; Tomar, S.K.; Singh, A.K. Iron microencapsulation with blend of gum arabic, maltodextrin and modified starch using modified solvent evaporation method–Milk fortification. Food Hydrocoll. 2015, 43, 622–628. [Google Scholar] [CrossRef]
- Sari, T.P.; Mann, B.; Kumar, R.; Singh, R.R.B.; Sharma, R.; Bhardwaj, M.; Athira, S. Preparation and characterization of nanoemulsion encapsulating curcumin. Food Hydrocoll. 2015, 43, 540–546. [Google Scholar] [CrossRef]
- Espitia, P.J.P.; Fuenmayor, C.A.; Otoni, C.G. Nanoemulsions: Synthesis, Characterization, and Application in Bio-Based Active Food Packaging. Compr. Rev. Food Sci. Food Saf. 2019, 18, 264–285. [Google Scholar] [CrossRef] [Green Version]
- Sadh, P.K.; Chawla, P.; Duhan, J.S. Fermentation approach on phenolic, antioxidants and functional properties of peanut press cake. Food Biosci. 2018, 22, 113–120. [Google Scholar] [CrossRef]
- Li, J.; Hwang, I.-C.; Chen, X.; Park, H.J. Effects of chitosan coating on curcumin loaded nano-emulsion: Study on stability and in vitro digestibility. Food Hydrocoll. 2016, 60, 138–147. [Google Scholar] [CrossRef]
- Sharma, A.; Shilpa Shree, B.G.; Arora, S.; Kapila, S. Preparation of lactose-iron complex and its cyto-toxicity, in-vitro digestion and bioaccessibility in Caco-2 cell model system. Food Biosci. 2017, 20, 125–130. [Google Scholar] [CrossRef]
- Bains, A.; Chawla, P. In vitro bioactivity, antimicrobial and anti-inflammatory efficacy of modified solvent evaporation assisted Trametes versicolor extract. 3 Biotech 2020, 10, 404. [Google Scholar] [CrossRef]
- Chawla, P.; Kumar, N.; Bains, A.; Dhull, S.B.; Kumar, M.; Kaushik, R.; Punia, S. Gum arabic capped copper nanoparticles: Synthesis, characterization, and applications. Int. J. Biol. Macromol. 2020, 146, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, R.; Chawla, P.; Kumar, N.; Janghu, S.; Lohan, A. Effect of premilling treatments on wheat gluten extraction and noodle quality. Food Sci. Technol. Int. 2018, 24, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Khan, B.A.; Akhtar, N.; Khan, H.; Braga, V.D.A. Development, characterization and antioxidant activity of polysorbate based O/W emulsion containing polyphenols derived from Hippophae rhamnoides and Cassia fistula. Braz. J. Pharm. Sci. 2013, 49, 763–773. [Google Scholar] [CrossRef] [Green Version]
- Yao, X.; Nie, K.; Chen, Y.; Jiang, F.; Kuang, Y.; Yan, H.; Fang, Y.; Yang, H.; Nishinari, K.; Phillips, G.O. The influence of non-ionic surfactant on lipid digestion of gum Arabic stabilized oil-in-water emulsion. Food Hydrocoll. 2018, 74, 78–86. [Google Scholar] [CrossRef]
- Prashar, S.; Sharma, S.; Kumar, N.; Kaushik, R.; Chawla, P. Formulation, Characterization, and In Vitro Mineral Absorption of Ficus Palmata Fruit Extract Nanoemulsion. J. Am. Coll. Nutr. 2022, 41, 291–300. [Google Scholar] [CrossRef]
- Galvão, K.C.S.; Vicente, A.A.; Sobral, P.J.A. Development, Characterization, and Stability of O/W Pepper Nanoemulsions Produced by High-Pressure Homogenization. Food Bioprocess Technol. 2017, 11, 355–367. [Google Scholar] [CrossRef] [Green Version]
- Hatziantoniou, S.; Deli, G.; Nikas, Y.; Demetzos, C.; Papaioannou, G.T. Scanning electron microscopy study on nanoemulsions and solid lipid nanoparticles containing high amounts of ceramides. Micron 2007, 38, 819–823. [Google Scholar] [CrossRef]
- Chawla, P.; Kumar, N.; Kaushik, R.; Dhull, S.B. Synthesis, characterization and cellular mineral absorption of nanoemulsions of Rhododendron arboreum flower extracts stabilized with gum arabic. J. Food Sci. Technol. 2019, 56, 5194–5203. [Google Scholar] [CrossRef]
- Nazarzadeh, E.; Anthonypillai, T.; Sajjadi, S. On the growth mechanisms of nanoemulsions. J. Colloid Interface Sci. 2013, 397, 154–162. [Google Scholar] [CrossRef]
- Yesmin, S.; Paul, A.; Naz, T.; Rahman, A.B.M.A.; Akhter, S.F.; Wahed, M.I.I.; Emran, T.B.; Siddiqui, S.A. Membrane stabilization as a mechanism of the anti-inflammatory activity of ethanolic root extract of Choi (Piper chaba). Clin. Phytoscience 2020, 6, 59. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, Y.; Wang, Y.W.; Huang, M.T.; Ho, C.T.; Huang, Q. Enhancing anti-inflammation activity of curcumin through O/W nanoemulsions. Food Chem. 2008, 108, 419–424. [Google Scholar] [CrossRef]
- Baba, S.A.; Malik, S.A. Evaluation of antioxidant and antibacterial activity of methanolic extracts of Gentiana kurroo royle. Saudi J. Biol. Sci. 2014, 21, 493–498. [Google Scholar] [CrossRef] [Green Version]
- Bains, A.; Chawla, P.; Tripathi, A.; Sadh, P.K. A comparative study of antimicrobial and anti-inflammatory efficiency of modified solvent evaporated and vacuum oven dried bioactive components of Pleurotus floridanus. J. Food Sci. Technol. 2021, 58, 3328–3337. [Google Scholar] [CrossRef]
- Malik, A.; Najda, A.; Bains, A.; Nurzynska-Wierdak, R.; Chawla, P. Characterization of Citrusnobilis Peel Methanolic Extract for Antioxidant, Antimicrobial, and Anti-Inflammatory Activity. Molecules 2021, 26, 4310. [Google Scholar] [CrossRef]





| Time (h) | Red Rice Extract (Log CFU/mL) | Control Nanoemulsion (Log CFU/mL) | RRE5 Nanoemulsion (Log CFU/mL) |
|---|---|---|---|
| 0 | 8.40 ± 0.29 a | 8.39 ± 0.42 a | 8.38 ± 0.37 a |
| 18 | 8.29 ± 0.34 a | 8.28 ± 0.22 a | 8.26 ± 0.59 a |
| 24 | 8.05 ± 0.27 c | 7.95 ± 0.55 b | 7.92 ± 0.24 a |
| 48 | 7.99 ± 0.31 c | 7.91 ± 0.59 b | 7.85 ± 0.33 a |
| Time (h) | Red rice Extract (Log CFU/mL) | Control Nanoemulsion (Log CFU/mL) | RRE5 Nanoemulsion (Log CFU/mL) |
|---|---|---|---|
| 0 | 8.26 ± 0.36 a | 8.27 ± 0.19 a | 8.25 ± 0.39 a |
| 18 | 8.18 ± 0.43 a | 8.17 ± 0.38 a | 8.15 ± 0.32 a |
| 24 | 7.97 ± 0.23 c | 7.93 ± 0.51 b | 7.87 ± 0.26 a |
| 48 | 7.89 ± 0.44 c | 7.84 ± 0.47 b | 7.79 ± 0.41 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bains, A.; Najda, A.; Chawla, P.; Klepacka, J.; Dhull, S.B.; Sadh, P.K.; Khan, M.A.; Kaushik, R. Formulation and Characterization of Gum Arabic Stabilized Red Rice Extract Nanoemulsion. Polymers 2022, 14, 1938. https://doi.org/10.3390/polym14101938
Bains A, Najda A, Chawla P, Klepacka J, Dhull SB, Sadh PK, Khan MA, Kaushik R. Formulation and Characterization of Gum Arabic Stabilized Red Rice Extract Nanoemulsion. Polymers. 2022; 14(10):1938. https://doi.org/10.3390/polym14101938
Chicago/Turabian StyleBains, Aarti, Agnieszka Najda, Prince Chawla, Joanna Klepacka, Sanju Bala Dhull, Pardeep Kumar Sadh, Mohammed Azhar Khan, and Ravinder Kaushik. 2022. "Formulation and Characterization of Gum Arabic Stabilized Red Rice Extract Nanoemulsion" Polymers 14, no. 10: 1938. https://doi.org/10.3390/polym14101938