New Potentiometric Screen-Printed Platforms Modified with Reduced Graphene Oxide and Based on Man-Made Imprinted Receptors for Caffeine Assessment
Abstract
1. Introduction
2. Materials and Methods
2.1. Apparatus
2.2. Reagents and Chemicals
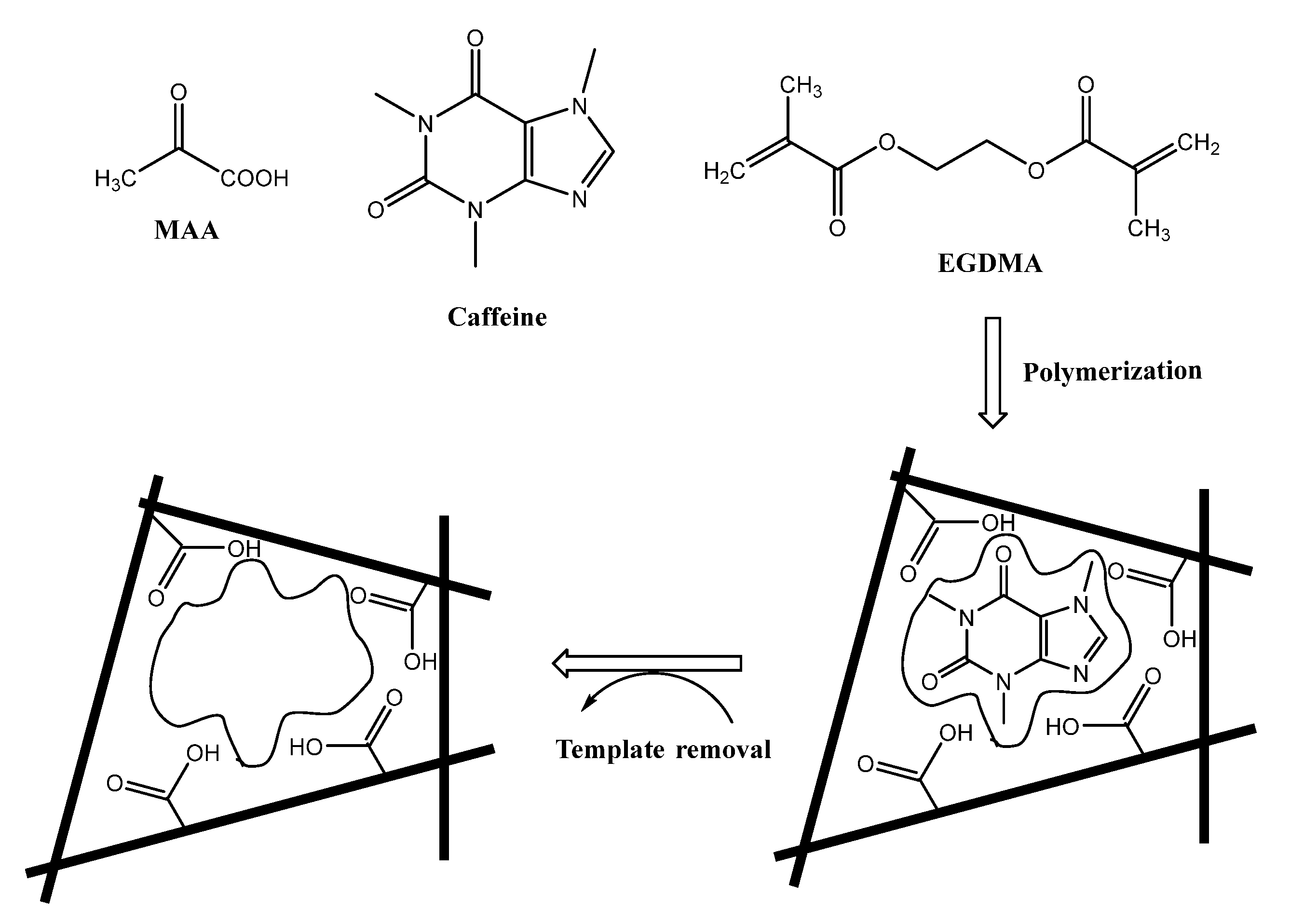
2.3. Preparation of the Imprinted Beads
2.4. Sensor Fabrication
3. Results and Discussion
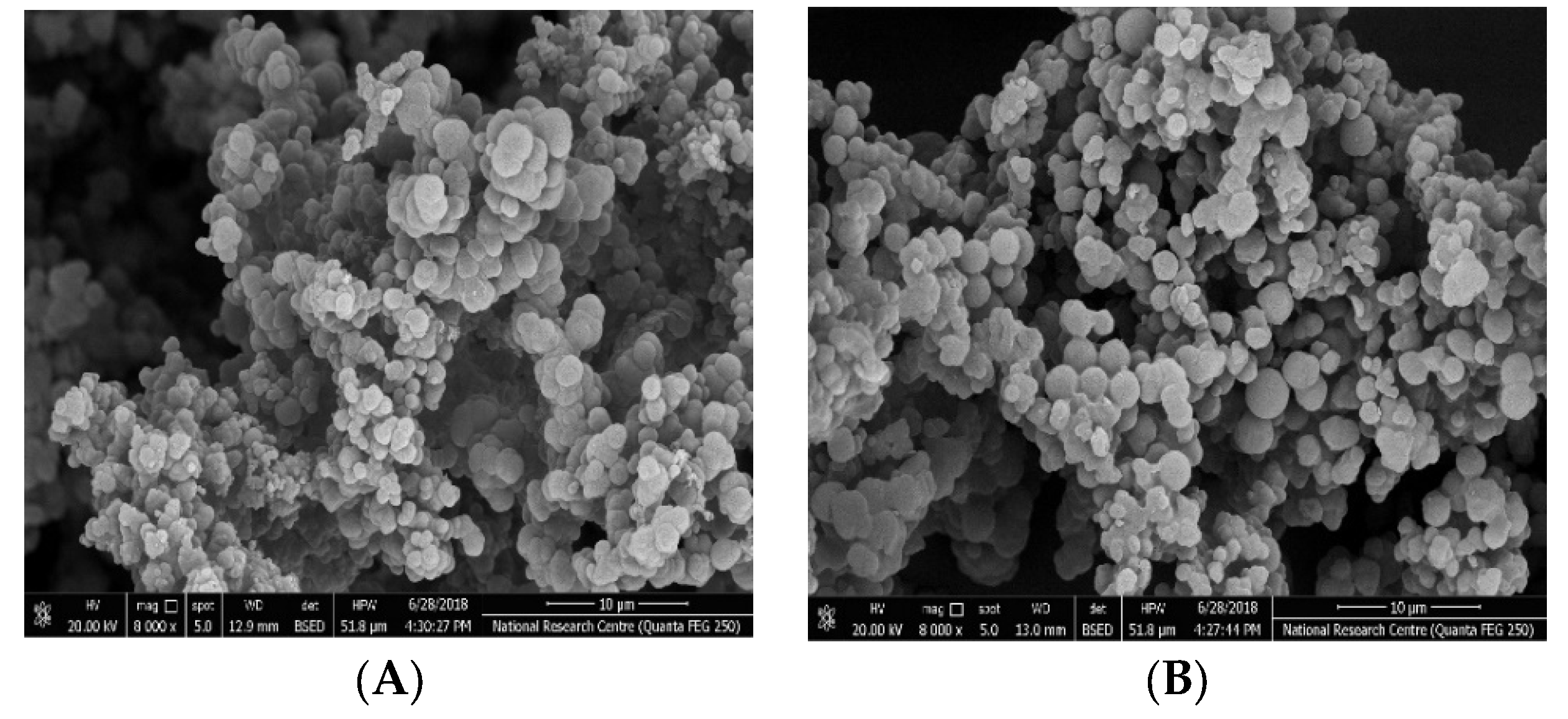
3.1. MIPs Characterization
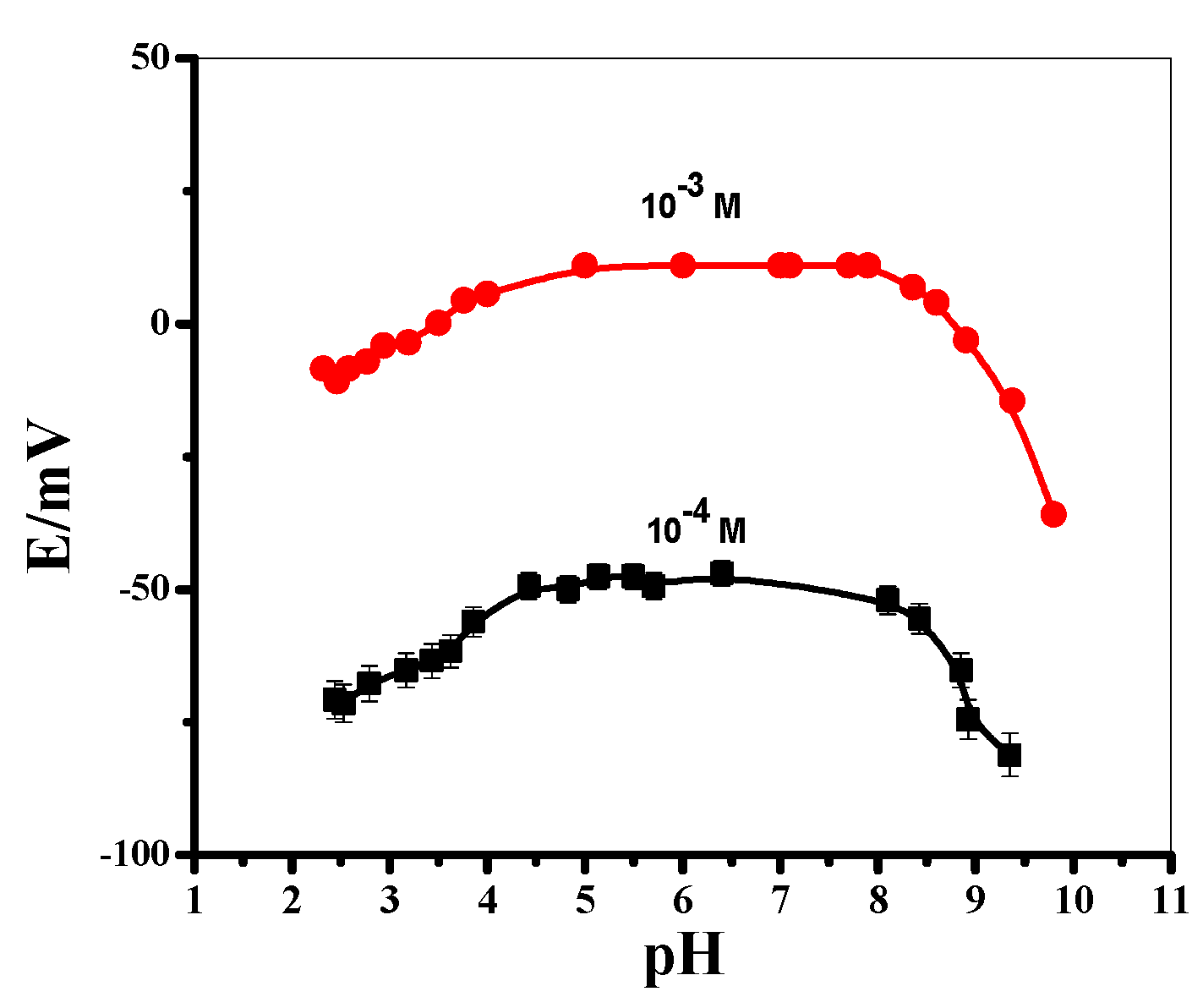
3.2. Sensors’ Characteristics
3.3. Transduction Mechanism
3.4. Method Validation
3.4.1. Limit of Detection (LOD) and Linearity
3.4.2. Reproducibility and Repeatability
3.4.3. Trueness, Bias and Recovery
3.4.4. Ruggedness and Robustness
3.5. Sensors’ Selectivity
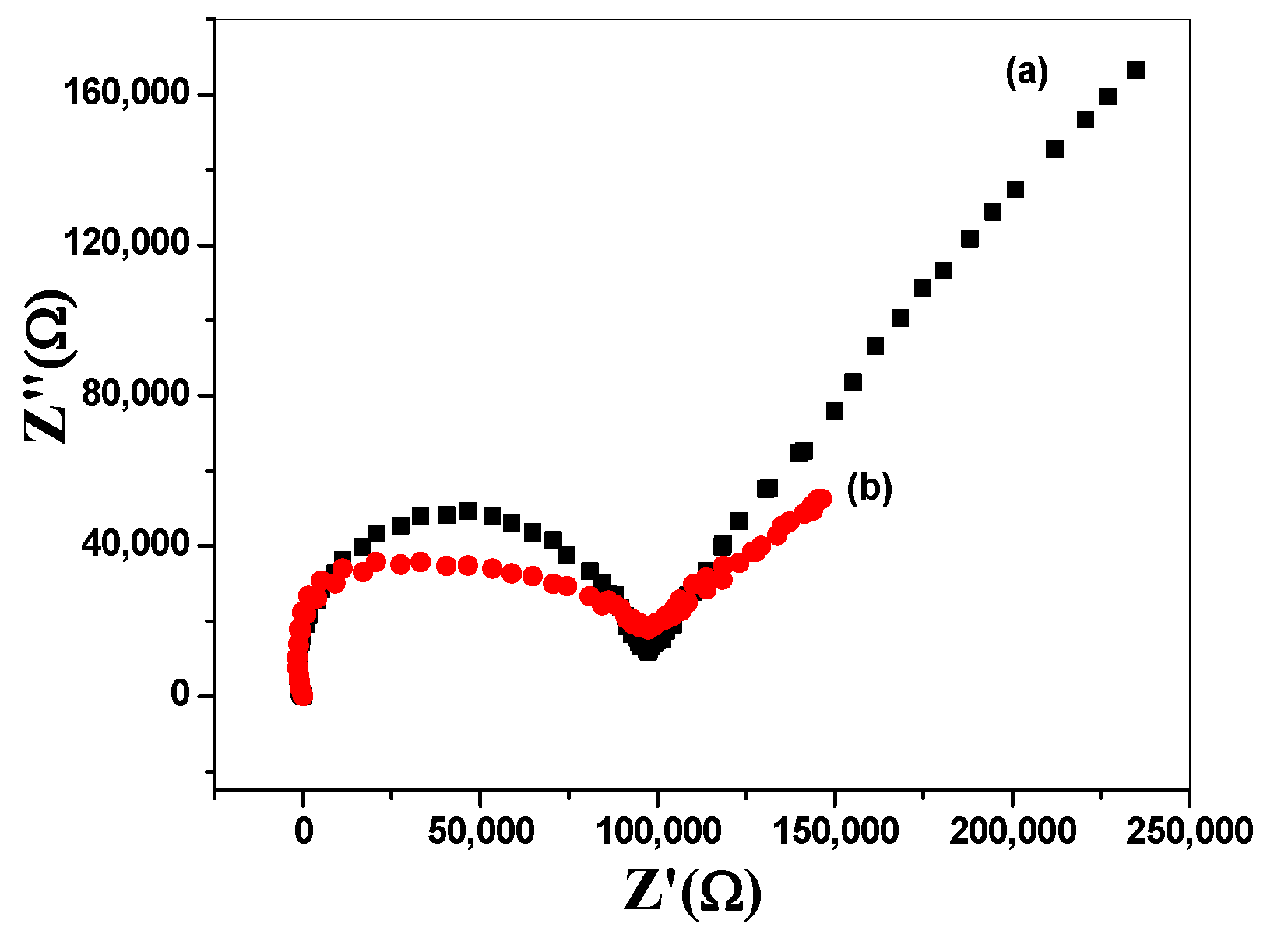
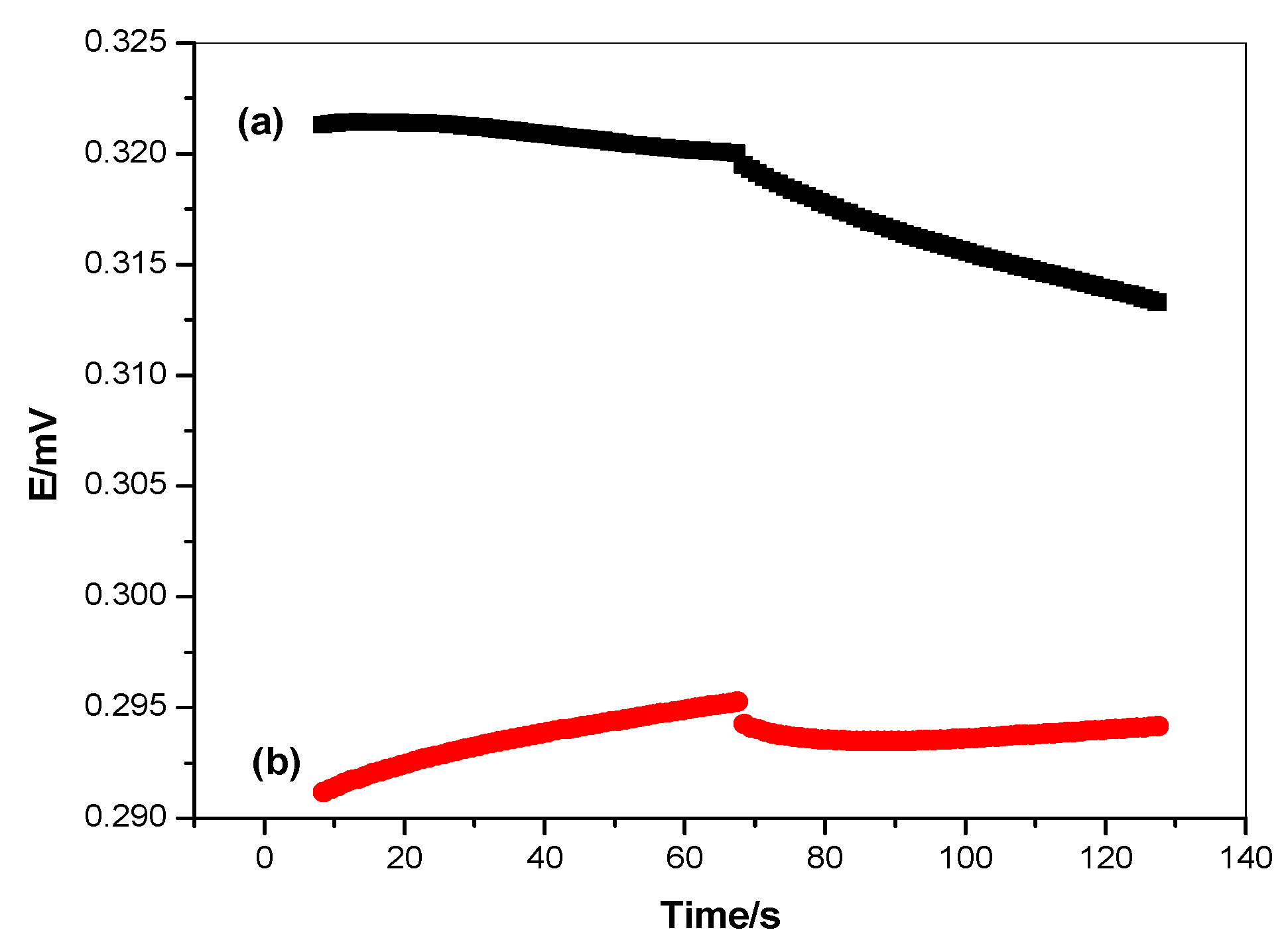
3.6. Chronopotentiometry and Electrochemical Impedance Spectroscopy (EIS) Measurements
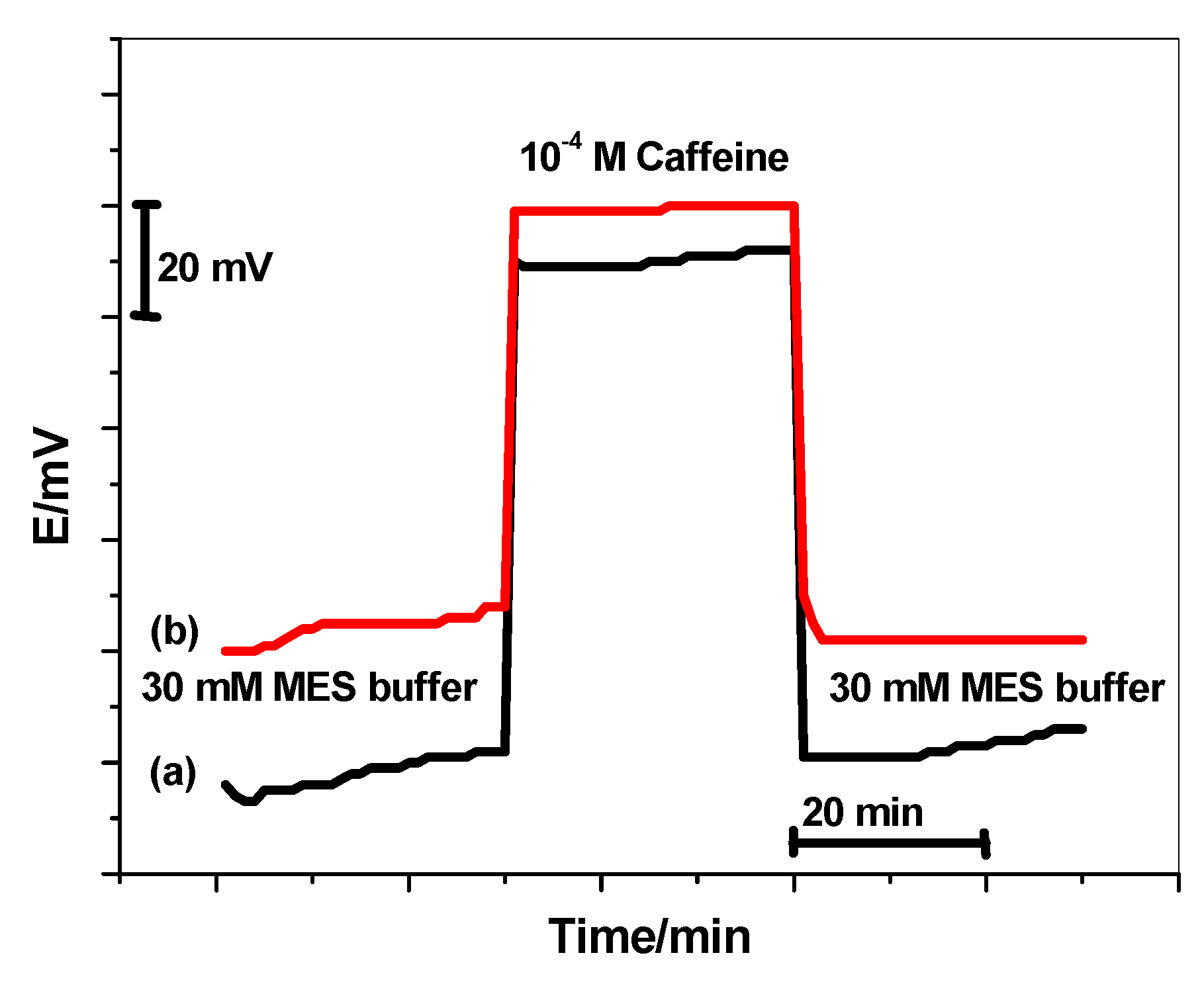
3.7. Water-Layer Test
3.8. Caffeine Assay in Different Pharmaceutical Formulations
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mitchell, D.C.; Knight, C.A.; Hockenberry, J.; Teplansky, R.; Hartman, T.J. Beverage caffeine intakes in the U.S. Food Chem. Toxicol. 2014, 63, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.C.; de Oliveira Ribeiro, N.P.; de Mello Schier, A.R.; Pereira, V.M.; Vilarim, M.M.; Pessoa, T.M.; Arias-Carrión, O.; Machado, S.; Nardi, A.E. Caffeine and suicide: A systematic review. CNS Neurol. Disord. Drug Targets 2014, 13, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Szczepanik, J.C.; de Oliveira, P.A.; de Oliveira, J.; Mack, J.M.; Engel, D.F.; Rial, D.; Moreira, E.L.G.; de Bem, A.F.; Prediger, R.D. Caffeine Mitigates the Locomotor Hyperactivity in Middle-aged Low-density Lipoprotein Receptor (LDL r)-Knockout Mice. CNS Neurosci. Ther. 2016, 22, 420–422. [Google Scholar] [CrossRef]
- Liszt, K.I.; Ley, J.P.; Lieder, B.; Behrens, M.; Stoger, V.; Reiner, A.; Hochkogler, C.M.; Kock, E.; Marchiori, A.; Hans, J.; et al. Caffeine induces gastric acid secretion via bitter taste signaling in gastric parietal cells. Proc. Natl. Acad. Sci. USA 2017, 114, E6260–E6269. [Google Scholar] [CrossRef] [PubMed]
- Marx, B.; Scuvee, E.; Scuvee-Moreau, J.; Seutin, V.; Jouret, F. Mechanisms of caffeine-induced diuresis. Med. Sci. 2016, 32, 485–490. [Google Scholar]
- Teng, C.L.; Lim, W.Y.; Chua, C.Z.; Teo, R.S.K.; Lin, K.T.H.; Yeoh, J.C. Does a single cup of caffeinated drink significantly increase blood pressure in young adults? A randomised controlled trial. Aust. Fam. Physician 2016, 45, 65. [Google Scholar] [PubMed]
- Sheak, K.; Richards, A.; Greene, A. 434 myocardial infarct (mi) caused by low dose of caffeine in patient with unsuspected coronary artery anomaly. J. Investig. Med. 2018, 66, A246. [Google Scholar]
- Abusnina, A.; Lugnier, C. Therapeutic potentials of natural compounds acting on cyclic nucleotide phosphodiesterase families. Cell. Signal. 2017, 39, 55–65. [Google Scholar] [CrossRef]
- Ceja-Navarro, J.A.; Vega, F.E.; Karaoz, U.; Hao, Z.; Jenkins, S.; Lim, H.C.; Kosina, P.; Infante, F.; Northen, T.R.; Brodie, E.L. Gut microbiota mediate caffeine detoxification in the primary insect pest of coffee. Nat. Commun. 2015, 6, 7618. [Google Scholar] [CrossRef]
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on iron and its importance for human health. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2014, 19, 164. [Google Scholar]
- Hashibe, M.; Galeone, C.; Buys, S.S.; Gren, L.; Boffetta, P.; Zhang, Z.F.; La Vecchia, C. Coffee, tea, caffeine intake, and the risk of cancer in the PLCO cohort. Br. J. Cancer 2015, 113, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Mejia, E.G.D.; Ramirez-Mares, M.V. Impact of caffeine and coffee on our health. Trends Endocrinol. Metab. 2014, 25, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Fulay, A.P.; Rifas-Shiman, S.L.; Oken, E.; Perng, W. Associations of the dietary approaches to stop hypertension (DASH) diet with pregnancy complications in Project Viva. Eur. J. Clin. Nutr. 2018, 72, 1385–1395. [Google Scholar] [CrossRef] [PubMed]
- Korekar, G.; Kumar, A.; Ugale, C. Occurrence, fate, persistence and remediation of caffeine: A review. Environ. Sci. Pollut. Res. 2020, 27, 34715–34733. [Google Scholar] [CrossRef]
- Rudolph, E.; Farbinger, A.; Konig, J. Determination of the caffeine contents of various food items within the Austrian market and validation of a caffeine assessment tool (CAT). Food Addit. Contam. Part A 2012, 29, 1849–1860. [Google Scholar] [CrossRef] [PubMed]
- Frary, C.D.; Johnson, R.K.; Wang, M.Q. Food sources and intakes of caffeine in the diets of persons in the United States. J. Am. Diet Assoc. 2005, 105, 110–113. [Google Scholar] [CrossRef] [PubMed]
- Fulgoni, V.L.; Keast, D.R.; Lieberman, H.R. Trends in intake and sources of caffeine in the diets of US adults: 2001–2010. Am. J. Clin. Nutr. 2015, 101, 1081–1087. [Google Scholar] [CrossRef]
- Nehlig, A. Interindividual differences in caffeine metabolism and factors driving caffeine consumption. Pharmacol. Rev. 2018, 70, 384–411. [Google Scholar] [CrossRef]
- Ogawa, N.; Ueki, H. Clinical importance of caffeine dependence and abuse. Psychiatry Clin. Neurosci. 2007, 61, 263–268. [Google Scholar] [CrossRef]
- Jain, S.; Srivastava, A.S.; Verma, R.P.; Maggu, G. Caffeine addiction: Need for awareness and research and regulatory measures. Asian J. Psychiatry 2019, 41, 73–75. [Google Scholar] [CrossRef]
- Dos Santos, M.K.F.; Gavioli, E.C.; Rosa, L.S.; de Paula Soares-Rachetti, V.; Lobão-Soares, B. Craving espresso: The dialetics in classifying caffeine as an abuse drug. Naunyn Schmiedebergs Arch. Pharmcol. 2018, 391, 1301–1318. [Google Scholar] [CrossRef] [PubMed]
- Heinz, A.; Daedelow, L.S.; Wackerhagen, C.; Di Chiara, G. Addiction theory matters- why there is no dependence on caffeine or antidepressant medication. Addict. Biol. 2020, 25, e12735. [Google Scholar] [CrossRef] [PubMed]
- Ahmad Bhawani, S.; Fong, S.S.; Mohamad Ibrahim, M.N. Spectrophotometric analysis of caffeine. Int. J. Anal. Chem. 2015, 2015, 170239. [Google Scholar] [CrossRef] [PubMed]
- Katayama, M.; Taniguchi, H. Fluorometric reactions of purines and determination of caffeine. Talanta 1989, 36, 1171–1175. [Google Scholar] [CrossRef]
- Maeso, N.; del Castillo, C.; Cornejo, L.; García-Acicollar, M.; Alguacil, L.F.; Barbas, C. Capillary electrophoresis for caffeine and pyroglutamate determination in coffees study of the in vivo effect on learning and locomotor activity in mice. J. Pharm. Biomed. Anal. 2006, 41, 1095–1100. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Chen, J.; He, M.; Hu, B. Simultaneous quantification of amphetamines, caffeine and ketamine in urine by hollow fiber liquid phase microextraction combined with gas chromatography-flame ionization detector. Talanta 2010, 82, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Ponce-Rodríguez, H.D.; García-Robles, A.A.; Sáenz-González, P.; Verdú-Andrés, J.; Campíns-Falcó, P. On-line in-tube solid phase microextraction coupled to capillary liquid chromatography-diode array detection for the analysis of caffeine and its metabolites in small amounts of biological samples. J. Pharm. Biomed. Anal. 2020, 178, 112914. [Google Scholar] [CrossRef]
- Mizuno, A.; Uematsu, T.; Gotoh, S.; Katoh, E.; Nakashima, M. The measurement of caffeine concentration in scalp hair as an indicator of liver function. J. Pharm. Pharmcol. 1996, 48, 660–664. [Google Scholar] [CrossRef]
- Dussy, F.; Carson, N.; Hangartner, S.; Briellmann, T. Is one hair lock really representative? Drug Testing Anal. 2014, 6 (Suppl. 1), 5–8. [Google Scholar] [CrossRef]
- Meier, U.; Briellmann, T.; Scheurer, E.; Dussy, F. Distribution pattern of ethyl glucuronide and caffeine concentrations over the scalp of a single person in a forensic context. Drug Test. Anal. 2017, 9, 1594–1603. [Google Scholar] [CrossRef]
- De Kesel, P.M.; Lambert, W.E.; Stove, C.P. An optimized and validated SPE-LC-MS/ MS method for the determination of caffeine and paraxanthine in hair. Talanta 2015, 144, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Musshoff, F.; Fels, H.; Carli, A.; Piombino-Mascali, D. The anatomical mummies of Mombello: Detection of cocaine, nicotine, and caffeine in the hair of psychiatric patients of the early 20th century. Forensic Sci. Int. 2017, 270, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Bertol, E.; Vaiano, F.; Boscolo-Berto, R.; Fioravanti, A.; Palumbo, D.; Catalani, V.; Mari, F.; Patussi, V.; Serpelloni, G. Alcohol, caffeine, and nicotine consumption in adolescents: Hair analysis versus self-report. Am. J. Drug Alcohol Abus. 2017, 43, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Mondal, G.; Butawan, M.; Bloomer, R.J.; Yates, C.R. Development of a liquid chromatography-tandem mass spectrometry (LC–MS/MS) method for characterizing caffeine, methylliberine, and theacrine pharmacokinetics in humans. J. Chromatogr. B 2020, 1155, 122278. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Zhong, Y.; Qiao, H.; Dia, B.; Chen, J.; Su, M. UPLC-MS/MS method for the simultaneous quantification of caffeine and illicit psychoactive drugs in hair using a single-step high-speed grinding extraction—Insights into a cut-off value for caffeine abuse. J. Pharm. Biomed. Anal. 2021, 209, 114489. [Google Scholar] [CrossRef]
- Filik, H.; Aslıhan Avan, A. Conducting polymer modified screen-printed carbon electrode coupled with magnetic solid phase microextraction for determination of caffeine. Food Chem. 2018, 242, 301–307. [Google Scholar] [CrossRef]
- Ashmawy, N.H.; Almehizia, A.A.; Youssef, T.A.; El-Galil, E.A.A.; Al-Omar, M.A.; Kamel, A.H. Novel Carbon/PEDOT/PSS-Based Screen-Printed Biosensors for Acetylcholine Neurotransmitter and Acetylcholinesterase Detection in Human Serum. Molecules 2019, 24, 1539. [Google Scholar] [CrossRef]
- Molaakbari, E.; Mostafavi, A.; Beitollahi, H. Simultaneous electrochemical determination of dopamine, melatonin, methionine and caffeine. Sens. Actuators B 2015, 208, 195–203. [Google Scholar] [CrossRef]
- Fernandes, D.M.; Silva, N.; Pereira, C.; Moura, C.; Magalhaes, J.M.; Bachiller-Baeza, B.; Rodríguez-Ramos, I.; Guerrero-Ruiz, A.; Delerue-Matos, C.; Freire, C. MnFe2O4@ CNT-N as novel electrochemical nanosensor for determination of caffeine, acetaminophen and ascorbic acid. Sens. Actuators B 2015, 218, 128–136. [Google Scholar] [CrossRef]
- Alpar, N.; Yardım, Y.; Sentürk, Z. Selective and simultaneous determination of total chlorogenic acids, vanillin and caffeine in foods and beverages by adsorptive stripping voltammetry using a cathodically pretreated boron-doped diamond electrode. Sens. Actuators B 2018, 257, 398–408. [Google Scholar] [CrossRef]
- Hallaj, R.; Soltani, E.; Mafakheri, S.; Ghadermazi, M. A surface-modified silicon carbide nanoparticles based electrochemical sensor for free interferences determination of caffeine in tea and coffee. Mater. Sci. Eng. B 2021, 274, 115473. [Google Scholar] [CrossRef]
- Kamel, A.H.; Sayour, H.E.M. Flow-through assay of quinine using solid contact potentiometric sensors based on molecularly imprinted polymers. Electroanalysis 2009, 21, 2701–2708. [Google Scholar] [CrossRef]
- Guerreiro, J.R.L.; Kamel, A.H.; Sales, M.G.F. FIA potentiometric system based on periodate polymeric membrane sensors for the assessment of ascorbic acid in commercial drinks. Food Chem. 2010, 120, 934. [Google Scholar] [CrossRef][Green Version]
- Kamel, A.H.; Galal, H.R.; Hana, A.A. Novel planar chip biosensors for potentiometric immunoassay of acid phosphatase activity based on the use of ion association complexes as novel electroactive materials. Int. J. Electrochem. Sci. 2014, 9, 5776–5787. [Google Scholar]
- Kamel, A.H.; Amr, A.E.; Galal, H.R.; Al-Omar, M.A.; Almehizia, A.A. Screen-Printed Sensor Based on Potentiometric Transduction for Free Bilirubin Detection as a Biomarker for Hyperbilirubinemia Diagnosis. Chemosensors 2020, 8, 86. [Google Scholar] [CrossRef]
- Hassan, S.S.M.; Elnemma, E.M.; Mohamed, A.H. Novel biomedical sensors for flow injection potentiometric determination of creatinine in human serum. Electroanalysis 2005, 17, 2246–2253. [Google Scholar] [CrossRef]
- Abdalla, N.S.; Youssef, M.A.; Algarni, H.; Awwad, N.S.; Kamel, A.H. All Solid-State Poly (Vinyl Chloride) Membrane Potentiometric Sensor Integrated with Nano-Beads Imprinted Polymers for Sensitive and Rapid Detection of Bispyribac Herbicide as Organic Pollutant. Molecules 2019, 24, 712. [Google Scholar] [CrossRef]
- Kamel, A.H.; Kalifa, M.E.; Abd El-Maksoud, S.A.; Elgendy, F.A. Fabrication of novel sensors based on a synthesized acyclic pyridine derivative ionophore for potentiometric monitoring of copper. Anal. Methods 2014, 6, 7814–7822. [Google Scholar] [CrossRef]
- Hassan, S.S.M.; Amr, A.E.; Elbehery, N.H.A.; Al-Omar, M.A.; Kamel, A.H. Non-equilibrium potential responses towards neutral orcinol using all-solid-state potentiometric sensors integrated with molecularly imprinted polymers. Polymers 2019, 11, 1232. [Google Scholar] [CrossRef]
- Kamel, A.H.; Galal, H.R. MIP-Based Biomimetic Sensors for Static and Hydrodynamic Potentiometric Transduction of Sitagliptin in Biological fluids. Int. J. Electrochem. Sci. 2014, 9, 4361–4373. [Google Scholar]
- Yan, R.; Qiu, S.; Tong, L.; Qian, Y. Review of progresses on clinical applications of ion selective electrodes for electrolytic ion tests: From conventional ISEs to graphene-based ISEs. Chem. Speciat. Bioavailab. 2016, 28, 72–77. [Google Scholar] [CrossRef]
- Cuartero, M.; Crespo, G.A. All-solid-state potentiometric sensors: A new wave for in situ aquatic research. Curr. Opin. Electrochem. 2018, 10, 98–106. [Google Scholar] [CrossRef]
- Yin, T.; Qin, W. Applications of nanomaterials in potentiometric sensors. Trends Anal. Chem. 2013, 51, 79–86. [Google Scholar] [CrossRef]
- Kamel, A.H.; Amr, A.E.; Al-Omar, M.A.; Almehizia, A.A. Solid-State Membrane Sensors Based on Man-Tailored Biomimetic Receptors for Selective Recognition of Isoproturon and Diuron Herbicides. Membranes 2020, 10, 279. [Google Scholar] [CrossRef] [PubMed]
- Abd-Rabboh, H.S.M.; Kamel, A.H. Novel potentiometric screen-printed carbon electrodes for bisphenol S detection in commercial plastic samples. Anal. Sci. 2020, 36, 1359–1364. [Google Scholar] [CrossRef] [PubMed]
- Abd-Rabboh, H.S.M.; Amr, A.E.; Almehizia, A.A.; Kamel, A.H. Paper-Based Potentiometric Device for Rapid and Selective Determination of Salicylhydroxamate as a Urinary Struvite Stone Inhibitor. ACS Omega 2021, 6, 27755–27762. [Google Scholar] [CrossRef]
- Ye, L.; Haupt, K. Molecularly imprinted polymers as antibody and receptor mimics for assays, sensors and drug discovery. Anal. Bioanal. Chem. 2004, 378, 1887–1897. [Google Scholar] [CrossRef]
- Özcan, N.; Karaman, C.; Atar, N.; Karaman, O.; Yola, M.L. A Novel Molecularly Imprinting Biosensor Including Graphene Quantum Dots/Multi-Walled Carbon Nanotubes Composite for Interleukin-6 Detection and Electrochemical Biosensor Validation. ECS J. Solid State Sci. Technol. 2020, 9, 121010. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Yola, M.L.; Atar, N.; Orooji, Y.; Karimi, F.; Kumar, P.S.; Rouhi, J.; Baghayeri, M. A novel detection method for organophosphorus insecticide fenamiphos: Molecularly imprinted electrochemical sensor based on core-shell Co3O4@MOF-74 nanocomposite. J. Colloid Interface Sci. 2021, 592, 174–185. [Google Scholar] [CrossRef]
- Karaman, C.; Karaman, O.; Atar, N.; Yola, M.L. A molecularly imprinted electrochemical biosensor based on hierarchical Ti2Nb10O29 (TNO) for glucose detection. Microchim. Acta 2022, 189, 24–34. [Google Scholar] [CrossRef]
- Özcan, A.; Atar, N.; Yola, M.L. Enhanced surface plasmon resonance (SPR) signals based on immobilization of core-shell nanoparticles incorporated boron nitride nanosheets: Development of molecularly imprinted SPR nanosensor for anticancer drug, etoposide. Biosens. Bioelectron. 2019, 130, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Yola, M.L.; Atar, N. Development of molecular imprinted sensor including graphitic carbon nitride/N-doped carbon dots composite for novel recognition of epinephrine. Compos. Part B Eng. 2019, 175, 107113. [Google Scholar] [CrossRef]
- Liang, R.N.; Song, D.A.; Zhang, R.M.; Qin, W. Potentiometric sensing of neutral species based on a uniform-sized molecularly imprinted polymer as a receptor. Angew. Chem. Int. Ed. 2010, 49, 2556–2559. [Google Scholar] [CrossRef] [PubMed]
- Meesters, R.; Voswinkel, S. Bioanalytical method development and validation: From the USFDA 2001 to the USFDA 2018 Guidance for Industry. J. Appl. Bioanal. 2018, 4, 67–73. [Google Scholar] [CrossRef]
- Bakker, E. Determination of Improved Selectivity Coefficients of Polymer Membrane Ion-Selective Electrodes by Conditioning with a Discriminated Ion. J. Electrochem. Soc. 1996, 143, L83–L85. [Google Scholar] [CrossRef]
- Bobacka, J. Potential stability of all-solid-state ion-selective electrodes using conducting polymers as ion-to-electron transducers. Anal. Chem. 1999, 71, 4932–4937. [Google Scholar] [CrossRef]
- USP-NF Standard; USP30-NF25. US Pharmacopoeial Convention, Inc.: Rockville, MD, USA, 2007.



| Parameters | MIP/o,NPOE | MIP/DOP | MIP/DBS | NIP/o,NPOE |
|---|---|---|---|---|
| Slope (mv/decade) | 51.2 ± 0.9 | 43.6 ± 0.5 | 45.4 ± 1.3 | 28.1 ± 0.9 |
| Detection limit, (M) | 3.0 × 10−6 | 4.0 × 10−6 | 4.5 × 10−6 | 1.7 × 10−5 |
| Correlation coefficient (r2) | 0.997 | 0.999 | 0.999 | 0.997 |
| Linear range, (mol/L) | 4.5 × 10−6 to 1.0 × 10−3 | 7.7 × 10−6 to 1.0 × 10−3 | 8.0 × 10−6 to 1.0 × 10−3 | 4.5 × 10−5–1.0 × 10−3 |
| Response time, (s) | <5 | <5 | <5 | <5 |
| pH range | 4.3–8.5 | 4.3–8.5 | 4.3–8.5 | 4.3–8.5 |
| Precision, (%) | 1.7 | 1.6 | 0.6 | 1.2 |
| Accuracy, (%) | 99.0 | 99.9 | 99.4 | 99.2 |
| Standard deviation, (mv) | ±2.4 | ±2.9 | ±1.2 | ±2.6 |
| Interfering Ion | log K pot caffeine, J ±SD * |
|---|---|
| K+ | −4.9± 0.1 |
| Na+ | −5.8 ± 0.4 |
| Mg2+ | −5.3 ± 0.3 |
| Ca2+ | −5.2 ± 0.1 |
| Arginine | −5.1 ± 0.2 |
| Alanine | −4.3 ± 0.3 |
| Glycine | −4.6 ± 0.4 |
| Nicotine | −3.3 ± 0.2 |
| Glucose | −5.4 ± 0.1 |
| Lactose | −5.2 ± 0.4 |
| Aspirin | −3.3 ± 0.2 |
| Codeine | −3.1 ± 0.7 |
| Paracetamol | −3.4 ± 0.5 |
| Creatinine | −3.7 ± 0.1 |
| Camylofine | −2.9 ± 0.3 |
| Ephedrine | −2.4 ± 0.1 |
| Chlorpheniramine | −3.05 ± 0.3 |
| Pharmaceutical Product and Source | Nominal Content Taken, mg/Tablet | Found, mg/Tablet a | b F-Test | b t-Student Test | |||
|---|---|---|---|---|---|---|---|
| Proposed Method | Recovery% ±SD | Reference Method [67] | Recovery% ±SD | ||||
| Pirafene caffeine, Memphis Co., Egypt | 20 | 21.3 ± 0.6 | 106.5 ± 0.4 | 20.1 ± 0.4 | 100.5 ± 0.5 | 2.2 | 1.6 |
| Allergex caffeine, Eipico Co., Egypt | 50 | 48.9 ± 0.9 | 97.8 ± 0.6 | 49.5 ± 0.5 | 99.0 ± 0.7 | 9.3 | 0.3 |
| Alertin, Pharco Co., Egypt | 20 | 19.5 ± 2.2 | 97.5 ± 1.5 | 19.8 ± 0.2 | 99.0 ± 0.9 | 2.6 | 3.6 |
| Vegaskine d, Alexandria Co., Egypt | 25 | 23.8 ± 1.3 | 95.2 ± 1.1 | 25.2 ± 0.7 | 100.8 ± 0.2 | 6.7 | 2.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd-Rabboh, H.S.M.; E. Amr, A.E.-G.; Almehizia, A.A.; Naglah, A.M.; H. Kamel, A. New Potentiometric Screen-Printed Platforms Modified with Reduced Graphene Oxide and Based on Man-Made Imprinted Receptors for Caffeine Assessment. Polymers 2022, 14, 1942. https://doi.org/10.3390/polym14101942
Abd-Rabboh HSM, E. Amr AE-G, Almehizia AA, Naglah AM, H. Kamel A. New Potentiometric Screen-Printed Platforms Modified with Reduced Graphene Oxide and Based on Man-Made Imprinted Receptors for Caffeine Assessment. Polymers. 2022; 14(10):1942. https://doi.org/10.3390/polym14101942
Chicago/Turabian StyleAbd-Rabboh, Hisham S. M., Abdel El-Galil E. Amr, Abdulrahman A. Almehizia, Ahmed M. Naglah, and Ayman H. Kamel. 2022. "New Potentiometric Screen-Printed Platforms Modified with Reduced Graphene Oxide and Based on Man-Made Imprinted Receptors for Caffeine Assessment" Polymers 14, no. 10: 1942. https://doi.org/10.3390/polym14101942
APA StyleAbd-Rabboh, H. S. M., E. Amr, A. E.-G., Almehizia, A. A., Naglah, A. M., & H. Kamel, A. (2022). New Potentiometric Screen-Printed Platforms Modified with Reduced Graphene Oxide and Based on Man-Made Imprinted Receptors for Caffeine Assessment. Polymers, 14(10), 1942. https://doi.org/10.3390/polym14101942








