Effect of the Composition of Copolymers Based on Glycidyl Methacrylate and Fluoroalkyl Methacrylates on the Free Energy and Lyophilic Properties of the Modified Surface
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Random GMA and FMA Copolymers and Modification of Materials
2.3. Attachment of Synthesized Copolymers to the Glass Surface
2.4. Attachment of Synthesized Copolymers to the Textured Aluminum Surface
2.5. Methods
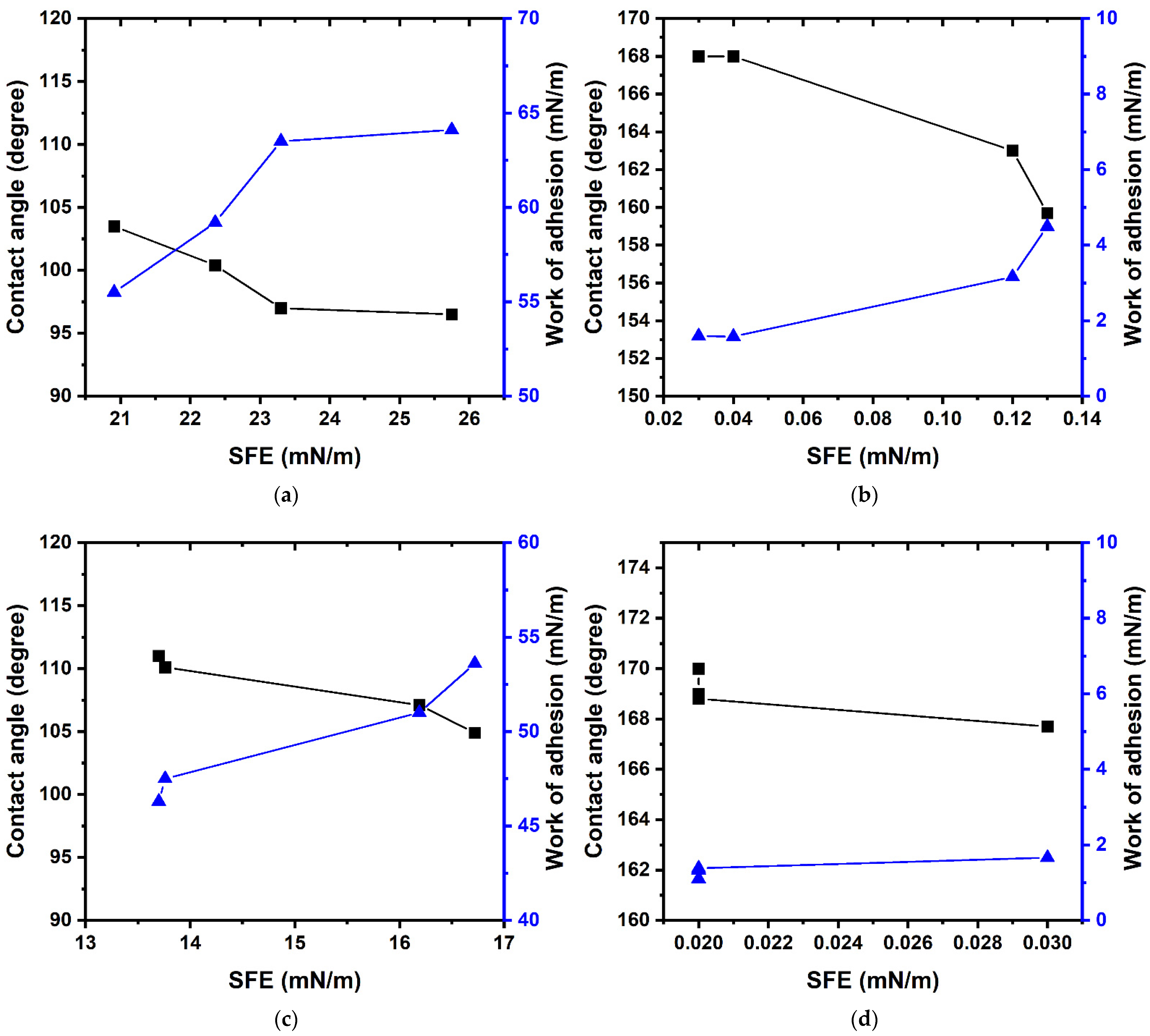
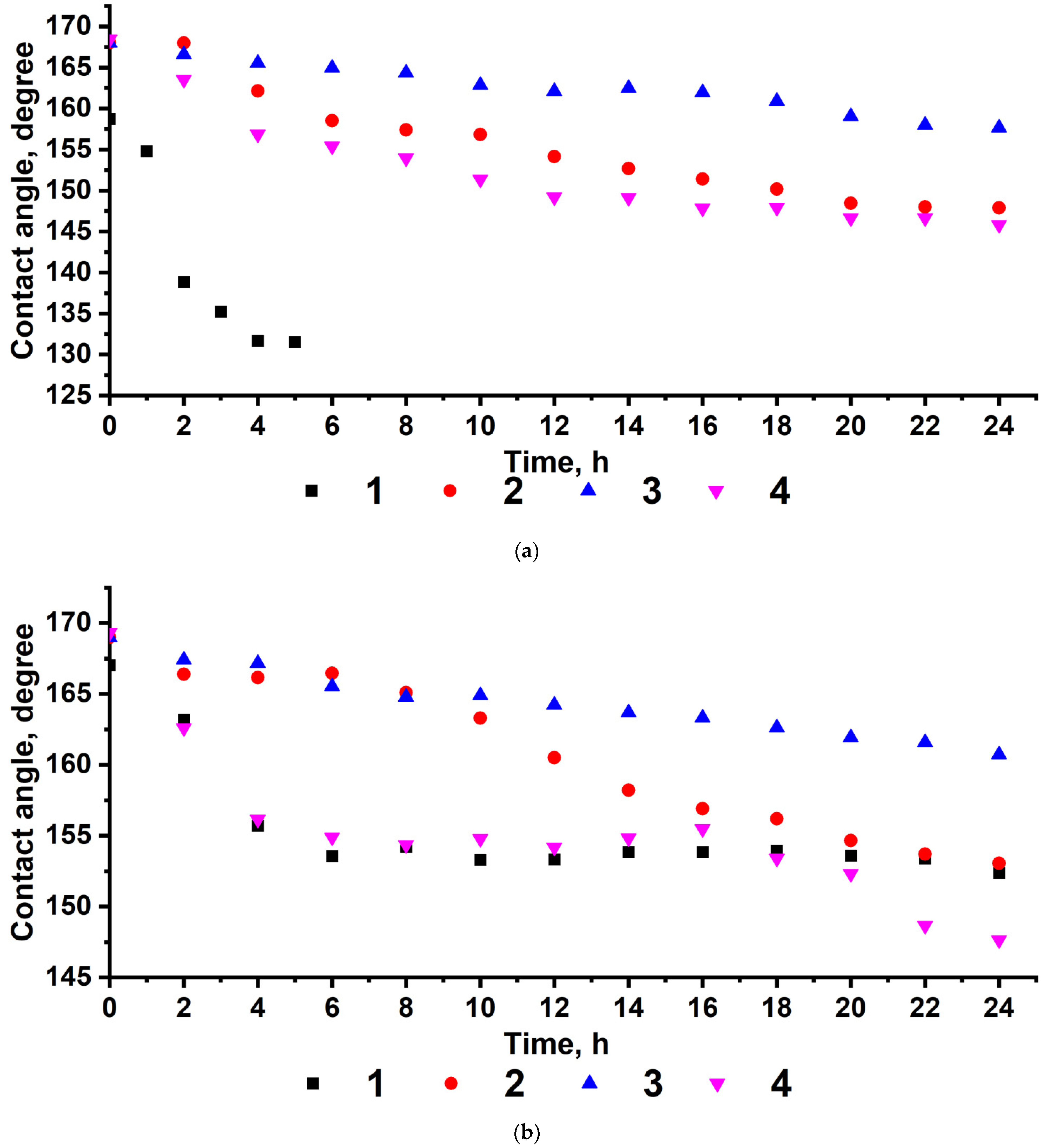
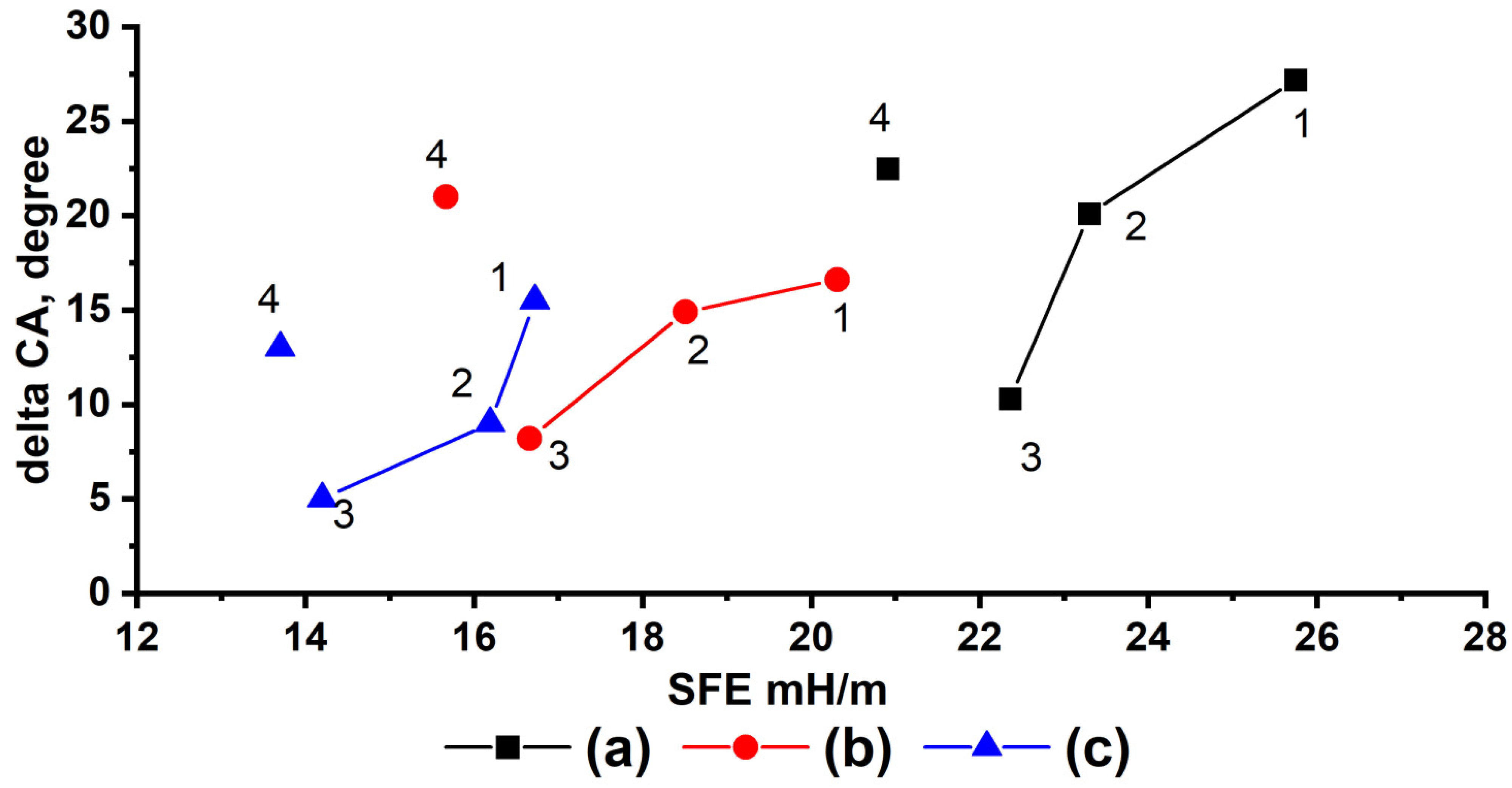
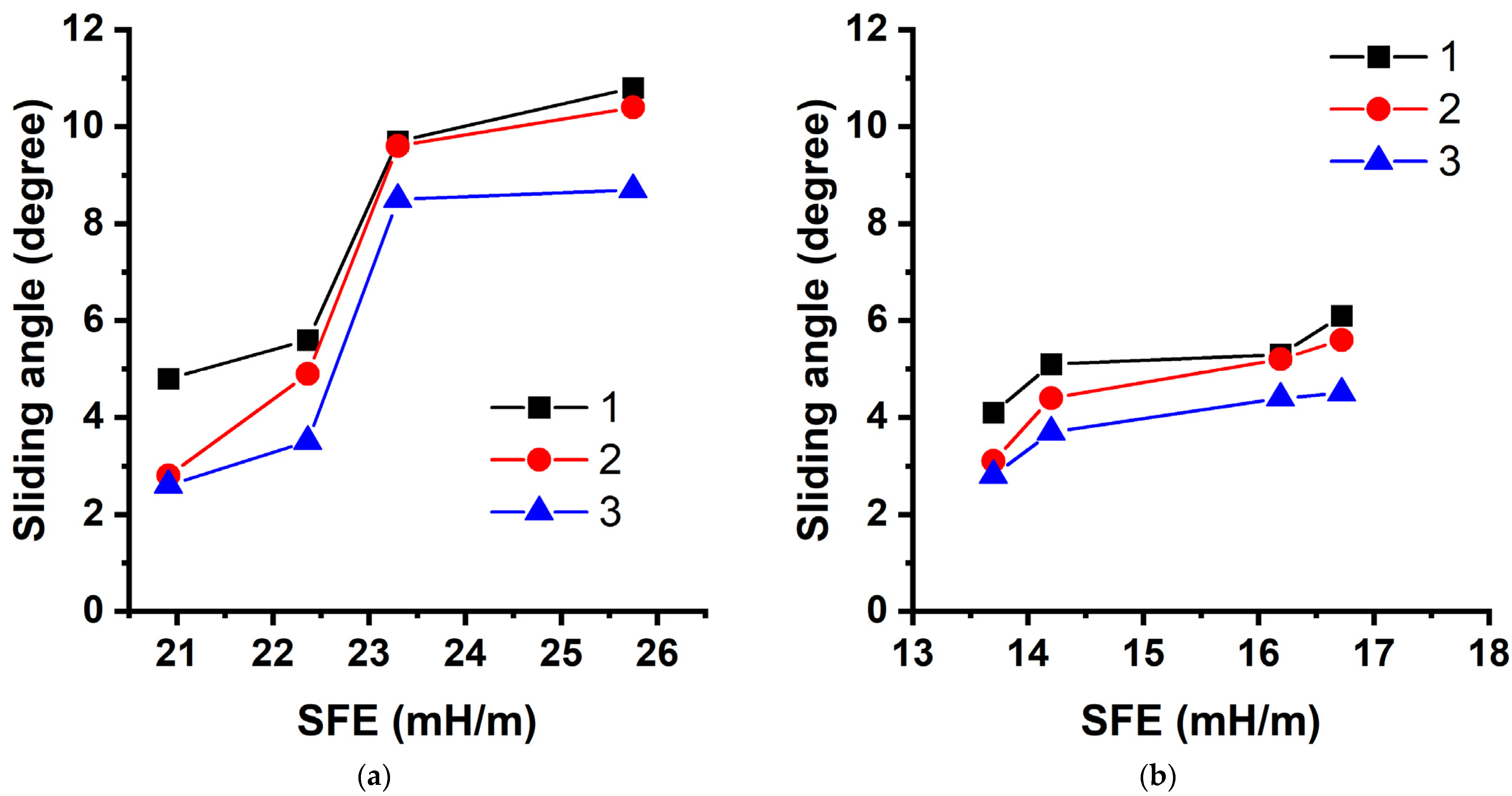
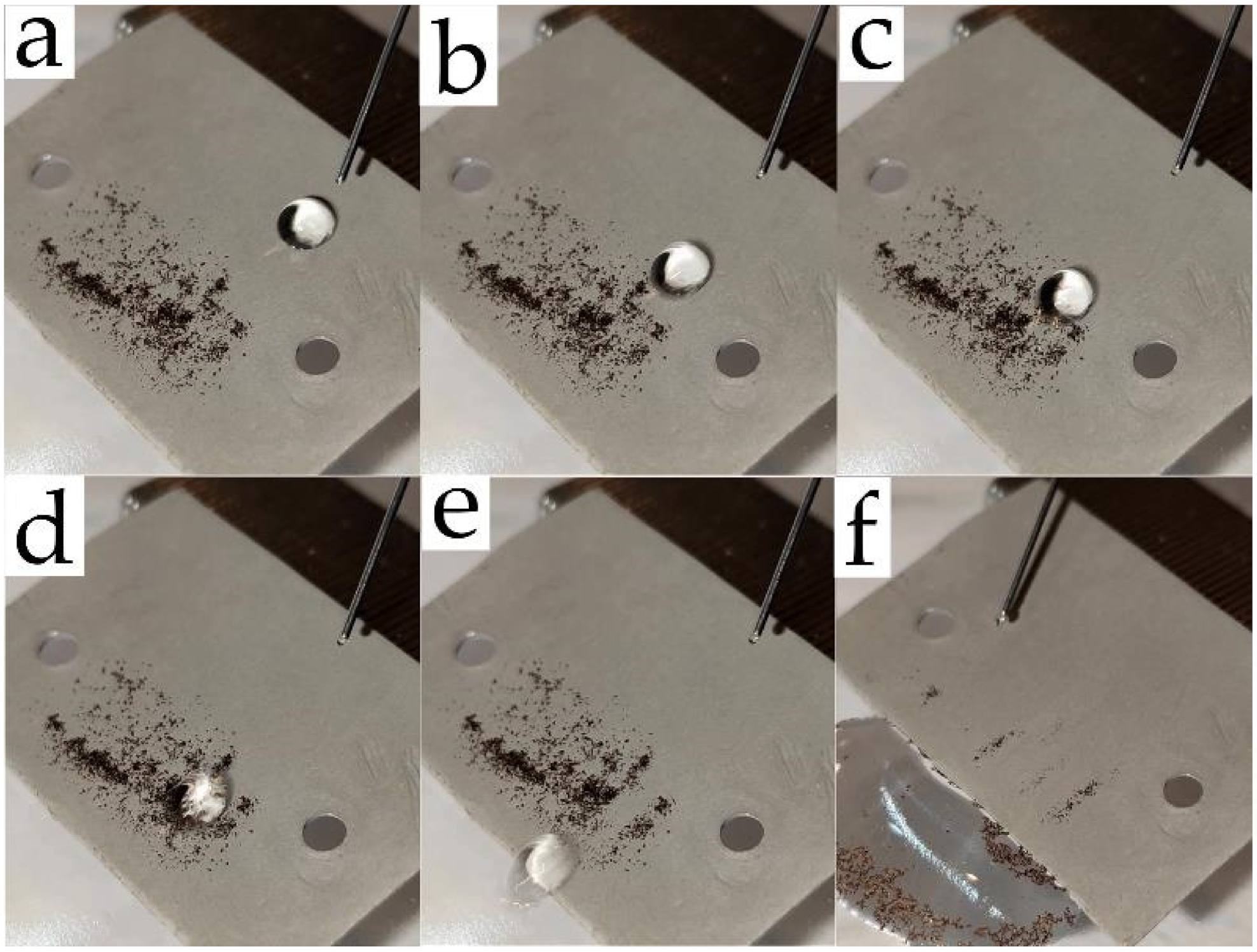
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abdelaziz, E.A.; Saidur, R.; Mekhilef, S. A Review on Energy Saving Strategies in Industrial Sector. Renew. Sustain. Energy Rev. 2011, 15, 150–168. [Google Scholar] [CrossRef]
- Swain, N.; Mishra, S. A Review on the Recovery and Separation of Rare Earths and Transition Metals from Secondary Resources. J. Clean. Prod. 2019, 220, 884–898. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; Martínez-Huitle, C.A. The Use of Renewable Energies Driving Electrochemical Technologies for Environmental Applications. Curr. Opin. Electrochem. 2020, 22, 211–220. [Google Scholar] [CrossRef]
- Erbil, H.Y. Practical Applications of Superhydrophobic Materials and Coatings: Problems and Perspectives. Langmuir 2020, 36, 2493–2509. [Google Scholar] [CrossRef]
- Boinovich, L.B.; Emelyanenko, A.M. Hydrophobic Materials and Coatings: Principles of Design, Properties and Applications. Russ. Chem. Rev. 2008, 77, 583–600. [Google Scholar] [CrossRef]
- Lafuma, A.; Quéré, D. Superhydrophobic States. Nat. Mater. 2003, 2, 457–460. [Google Scholar] [CrossRef]
- Yilbas, B.S.; Al-Sharafi, A.; Ali, H.; Al-Aqeeli, N. Dynamics of a Water Droplet on a Hydrophobic Inclined Surface: Influence of Droplet Size and Surface Inclination Angle on Droplet Rolling. RSC Adv. 2017, 7, 48806–48818. [Google Scholar] [CrossRef] [Green Version]
- Varshney, P.; Mohapatra, S.S.; Kumar, A. Fabrication of Mechanically Stable Superhydrophobic Aluminium Surface with Excellent Self-Cleaning and Anti-Fogging Properties. Biomimetics 2017, 2, 2. [Google Scholar] [CrossRef] [Green Version]
- Zheng, S.; Li, C.; Fu, Q.; Hu, W.; Xiang, T.; Wang, Q.; Du, M.; Liu, X.; Chen, Z. Development of Stable Superhydrophobic Coatings on Aluminum Surface for Corrosion-Resistant, Self-Cleaning, and Anti-Icing Applications. Mater. Des. 2016, 93, 261–270. [Google Scholar] [CrossRef]
- Zheng, S.; Li, C.; Zhang, Y.; Xiang, T.; Cao, Y.; Li, Q.; Chen, Z. A General Strategy towards Superhydrophobic Self-Cleaning and Anti-Corrosion Metallic Surfaces: An Example with Aluminum Alloy. Coatings 2021, 11, 788. [Google Scholar] [CrossRef]
- Young, T., III. An Essay on the Cohesion of Fluids. Philos. Trans. R. Soc. Lond. 1805, 95, 65–87. [Google Scholar] [CrossRef]
- Wenzel, R.N. Surface Roughness and Contact Angle. J. Phys. Colloid Chem. 1949, 53, 1466–1467. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Large Contact Angles of Plant and Animal Surfaces. Nature 1945, 155, 21–22. [Google Scholar] [CrossRef]
- Kwok, D.Y.; Neumann, A.W. Contact Angle Measurement and Contact Angle Interpretation. Adv. Colloid Interface Sci. 1999, 81, 167–249. [Google Scholar] [CrossRef]
- Kung, C.H.; Sow, P.K.; Zahiri, B.; Mérida, W. Assessment and Interpretation of Surface Wettability Based on Sessile Droplet Contact Angle Measurement: Challenges and Opportunities. Adv. Mater. Interfaces 2019, 6, 1900839. [Google Scholar] [CrossRef]
- Jeevahan, J.; Chandrasekaran, M.; Britto Joseph, G.; Durairaj, R.B.; Mageshwaran, G. Superhydrophobic Surfaces: A Review on Fundamentals, Applications, and Challenges. J. Coat. Technol. Res. 2018, 15, 231–250. [Google Scholar] [CrossRef]
- Marmur, A. Soft Contact: Measurement and Interpretation of Contact Angles. Soft Matter 2006, 2, 12–17. [Google Scholar] [CrossRef]
- Marmur, A. Equilibrium and Spreading of Liquids on Solid Surfaces. Adv. Colloid Interface Sci. 1983, 19, 75–102. [Google Scholar] [CrossRef]
- Rilda, Y.; Meranti, A.; Citra, Y.; Refinel, R.; Eka Putri, Y.; Agustien, A.; Pardi, H. Self-Cleaning and Superhidrofilic Surface Cottonby Nanocomposite TiO2-SiO2-Chitosan. Mater. Res. Innov. 2021, 25, 348–353. [Google Scholar] [CrossRef]
- Vazirinasab, E.; Jafari, R.; Momen, G. Application of Superhydrophobic Coatings as a Corrosion Barrier: A Review. Surf. Coatings Technol. 2018, 341, 40–56. [Google Scholar] [CrossRef]
- Nosonovsky, M.; Rohatgi, P.K. Biomimetics in Materials Science: Self-Healing, Self-Lubricating, and Self-Cleaning Materials; Springer Science & Business Media: New York, NY, USA, 2012; Volume 152, pp. 1–409. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.B.; Park, S.; Lim, H. Effects of Morphology Parameters on Anti-Icing Performance in Superhydrophobic Surfaces. Appl. Surf. Sci. 2018, 435, 585–591. [Google Scholar] [CrossRef]
- Choi, S.-J.; Huh, S.-Y. Direct Structuring of a Biomimetic Anti-Reflective, Self-Cleaning Surface for Light Harvesting in Organic Solar Cells. Macromol. Rapid Commun. 2010, 31, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Michielsen, S. Lotus Effect: Superhydrophobicity. J. Text. Inst. 2006, 97, 455–462. [Google Scholar] [CrossRef]
- Sethi, S.K.; Manik, G. Recent Progress in Super Hydrophobic/Hydrophilic Self-Cleaning Surfaces for Various Industrial Applications: A Review. Polym. Plast. Technol. Eng. 2018, 57, 1932–1952. [Google Scholar] [CrossRef]
- Sethi, S.K.; Shankar, U.; Manik, G. Fabrication and Characterization of Non-Fluoro Based Transparent Easy-Clean Coating Formulations Optimized from Molecular Dynamics Simulation. Prog. Org. Coat. 2019, 136, 105306. [Google Scholar] [CrossRef]
- Drelich, J.W. Contact Angles: From Past Mistakes to New Developments through Liquid-Solid Adhesion Measurements. Adv. Colloid Interface Sci. 2019, 267, 1–14. [Google Scholar] [CrossRef]
- Liu, M.; Wang, S.; Jiang, L. Nature-Inspired Superwettability Systems. Nat. Rev. Mater. 2017, 2, 17036. [Google Scholar] [CrossRef]
- Iqbal, M.; Dinh, D.K.; Abbas, Q.; Imran, M.; Sattar, H.; Ul Ahmad, A. Controlled Surface Wettability by Plasma Polymer Surface Modification. Surfaces 2019, 2, 349–371. [Google Scholar] [CrossRef] [Green Version]
- Kota, A.K.; Kwon, G.; Tuteja, A. The Design and Applications of Superomniphobic Surfaces. NPG Asia Mater. 2014, 6, e109–e116. [Google Scholar] [CrossRef] [Green Version]
- Hare, E.F.; Shafrin, E.G.; Zisman, W.A. Properties of Films of Adsorbed Fluorinated Acids. J. Phys. Chem. 1954, 58, 236–239. [Google Scholar] [CrossRef]
- Caliskan, T.D.; Luzinov, I. Effect of Number of -CF3 Groups in Tails of Polyester on Surface Wettability of Coatings: Synthesis and Characterization of PFPE Based Polyesters with Three -CF3 Groups in Tails. J. Polym. Res. 2020, 27, 128. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the Surface Free Energy of Polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Lee, S.; Park, J.S.; Lee, T.R. The Wettability of Fluoropolymer Surfaces: Influence of Surface Dipoles. Langmuir 2008, 24, 4817–4826. [Google Scholar] [CrossRef] [PubMed]
- Chan, R.K. Surface Tension of Fluoropolymers. J. Colloid Interface Sci. 1970, 32, 499–504. [Google Scholar] [CrossRef]
- Tuteja, A.; Choi, W.; Ma, M.; Mabry, J.M.; Mazzella, S.A.; Rutledge, G.C.; McKinley, G.H.; Cohen, R.E. Designing Superoleophobic Surfaces. Science 2007, 318, 1618–1622. [Google Scholar] [CrossRef] [Green Version]
- Mabry, J.M.; Vij, A.; Iacono, S.T.; Viers, B.D. Fluorinated Polyhedral Oligomeric Silsesquioxanes (F-POSS). Angew. Chemie Int. Ed. 2008, 47, 4137–4140. [Google Scholar] [CrossRef]
- Li, Y.; Li, B.; Zhao, X.; Tian, N.; Zhang, J. Totally Waterborne, Nonfluorinated, Mechanically Robust, and Self-Healing Superhydrophobic Coatings for Actual Anti-Icing. ACS Appl. Mater. Interfaces 2018, 10, 39391–39399. [Google Scholar] [CrossRef]
- Su, F.; Yao, K. Facile Fabrication of Superhydrophobic Surface with Excellent Mechanical Abrasion and Corrosion Resistance on Copper Substrate by a Novel Method. ACS Appl. Mater. Interfaces 2014, 6, 8762–8770. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, H.; Chen, S.; Wang, D. Ag Nanoparticle-Loaded Hierarchical Superamphiphobic Surface on an Al Substrate with Enhanced Anticorrosion and Antibacterial Properties. J. Phys. Chem. C 2015, 119, 25449–25456. [Google Scholar] [CrossRef]
- Bai, N.; Li, Q.; Dong, H.; Tan, C.; Cai, P.; Xu, L. A Versatile Approach for Preparing Self-Recovering Superhydrophobic Coatings. Chem. Eng. J. 2016, 293, 75–81. [Google Scholar] [CrossRef]
- Ma, M.; Hill, R.M. Superhydrophobic Surfaces. Curr. Opin. Colloid Interface Sci. 2006, 11, 193–202. [Google Scholar] [CrossRef]
- Kousaalya, A.B.; Garg, N.; Kumar, R. Silica-Based Superhydrophobic Coating by a Single-Step Process. Surf. Innov. 2013, 1, 173–180. [Google Scholar] [CrossRef] [Green Version]
- Brostow, W.; Cassidy, P.E.; Macossay, J.; Pietkiewicz, D.; Venumbaka, S. Connection of Surface Tension with Multiple Tribological Properties in Epoxy + Fluoropolymer Systems. Polym. Int. 2003, 52, 1498–1505. [Google Scholar] [CrossRef]
- Kota, A.K.; Tuteja, A. Superoleophobic Surfaces. ACS Symp. Ser. 2012, 1106, 171–185. [Google Scholar] [CrossRef]
- Goharshenas Moghadam, S.; Parsimehr, H.; Ehsani, A. Multifunctional Superhydrophobic Surfaces. Adv. Colloid Interface Sci. 2021, 290, 102397. [Google Scholar] [CrossRef]
- Bryuzgin, E.V.; Klimov, V.V.; Repin, S.A.; Navrotskiy, A.V.; Novakov, I.A. Aluminum Surface Modification with Fluoroalkyl Methacrylate-Based Copolymers to Attain Superhydrophobic Properties. Appl. Surf. Sci. 2017, 419, 454–459. [Google Scholar] [CrossRef]
- Bryuzgin, E.; Klimov, V.; Le, M.D.; Navrotskiy, A.; Novakov, I. The Superhydrophobic State Stability of Coatings Based on Copolymers of Glycidyl Methacrylate and Alkyl Methacrylates on Cotton Fabric Surface. Fibers Polym. 2020, 21, 1032–1038. [Google Scholar] [CrossRef]
- Klimov, V.V.; Bryuzgin, E.V.; Navrotskiy, A.V.; Novakov, I.A. Superhydrophobic Behavior of Coatings Based on Fluoroalkyl Methacrylate Copolymers on a Textured Aluminum Surface. Surf. Interfaces 2021, 25, 101255. [Google Scholar] [CrossRef]
- Bryuzgin, E.V.; Klimov, V.V.; Zaytsev, S.D.; Nikolitchev, D.E.; Navrotskiy, A.V.; Novakov, I.A. Synthesis of Grafted Functional Polymer Coatings on the Aluminum Surface by the Methods of Controlled Radical Polymerization. Russ. Chem. Bull. 2014, 63, 1610–1614. [Google Scholar] [CrossRef]
- Barshilia, H.C.; Mohan, D.K.; Selvakumar, N.; Rajam, K.S. Effect of Substrate Roughness on the Apparent Surface Free Energy of Sputter Deposited Superhydrophobic Polytetrafluoroethylene Thin Films. Appl. Phys. Lett. 2009, 95, 033116. [Google Scholar] [CrossRef]
- Stahl, T.; Mattern, D.; Brunn, H. Toxicology of Perfluorinated Compounds. Environ. Sci. Eur. 2011, 23, 38. [Google Scholar] [CrossRef] [Green Version]
- Lindstrom, A.B.; Strynar, M.J.; Libelo, E.L. Polyfluorinated Compounds: Past, Present, and Future. Environ. Sci. Technol. 2011, 45, 7954–7961. [Google Scholar] [CrossRef] [PubMed]
- Zdyrko, B.; Swaminatha Iyer, K.; Luzinov, I. Macromolecular Anchoring Layers for Polymer Grafting: Comparative Study. Polymer 2006, 47, 272–279. [Google Scholar] [CrossRef]
- Köthe, M.; Müller, M.; Simon, F.; Komber, H.; Jacobasch, H.J.; Adler, H.J. Examination of Poly(Butadiene Epoxide)-Coatings on Inorganic Surfaces. Colloids Surf. A Physicochem. Eng. Asp. 1999, 154, 75–85. [Google Scholar] [CrossRef]
- Avossa, J.; Bifulco, A.; Amendola, E.; Gesuele, F.; Oscurato, S.L.; Gizaw, Y.; Mensitieri, G.; Branda, F. Forming Nanostructured Surfaces through Janus Colloidal Silica Particles with Nanowrinkles: A New Strategy to Superhydrophobicity. Appl. Surf. Sci. 2019, 465, 73–81. [Google Scholar] [CrossRef]
- Ellison, A.H.; Zisman, W.A. Wettability of Halogenated Organic Solid Surfaces. J. Phys. Chem. 1954, 58, 260–265. [Google Scholar] [CrossRef]
- Piscitelli, F.; Chiariello, A.; Dabkowski, D.; Corraro, G.; Marra, F.; Di Palma, L. Superhydrophobic Coatings as Anti-Icing Systems for Small Aircraft. Aerospace 2020, 7, 2. [Google Scholar] [CrossRef] [Green Version]
- De Santis, S.; Rossi, E.; Sebastiani, M.; Sennato, S.; Bemporad, E.; Orsini, M. A Nanoindentation Approach for Time-Dependent Evaluation of Surface Free Energy in Micro- and Nano-Structured Titanium. Materials 2021, 15, 287. [Google Scholar] [CrossRef]
- Marmur, A. The Lotus Effect: Superhydrophobicity and Metastability. Langmuir 2004, 20, 3517–3519. [Google Scholar] [CrossRef]
- Boinovich, L.; Emelyanenko, A. A Wetting Experiment as a Tool to Study the Physicochemical Processes Accompanying the Contact of Hydrophobic and Superhydrophobic Materials with Aqueous Media. Adv. Colloid Interface Sci. 2012, 179–182, 133–141. [Google Scholar] [CrossRef]
- Bharathidasan, T.; Kumar, S.V.; Bobji, M.S.; Chakradhar, R.P.S.; Basu, B.J. Effect of Wettability and Surface Roughness on Ice-Adhesion Strength of Hydrophilic, Hydrophobic and Superhydrophobic Surfaces. Appl. Surf. Sci. 2014, 314, 241–250. [Google Scholar] [CrossRef]
- Miwa, M.; Nakajima, A.; Fujishima, A.; Hashimoto, K.; Watanabe, T. Effects of the Surface Roughness on Sliding Angles of Water Droplets on Superhydrophobic Surfaces. Langmuir 2000, 16, 5754–5760. [Google Scholar] [CrossRef]
- Geyer, F.; D’Acunzi, M.; Sharifi-Aghili, A.; Saal, A.; Gao, N.; Kaltbeitzel, A.; Sloot, T.-F.; Berger, R.; Butt, H.-J.; Vollmer, D. When and How Self-Cleaning of Superhydrophobic Surfaces Works. Sci. Adv. 2020, 6, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smyth, K.M.; Paxson, A.T.; Kwon, H.-M.; Varanasi, K.K. Visualization of Contact Line Motion on Hydrophobic Textures. Surf. Innov. 2013, 1, 84–91. [Google Scholar] [CrossRef]




| Copolymer | Molar Content [FMA], % | Elemental Content, % | ||
|---|---|---|---|---|
| Theoretical | Experimental | C, % | H, % | |
| Poly-(TEMA-co-GMA) | 33.3 | 29.0 | 53.80 | 6.55 |
| 50.0 | 44.4 | 51.19 | 5.75 | |
| 66.7 | 62.1 | 48.35 | 4.50 | |
| Poly-(HFMA-co-GMA) | 33.3 | 29.0 | 50.50 | 6.08 |
| 50.0 | 41.7 | 47.96 | 4.76 | |
| 66.7 | 61.7 | 43.80 | 3.78 | |
| Poly-(HIMA-co-GMA) | 33.3 | 29.8 | 49.50 | 6.13 |
| 50.0 | 42.7 | 46.09 | 4.69 | |
| 66.7 | 64.9 | 41.37 | 3.08 | |
| Poly-(HBMA-co-GMA) | 33.3 | 29.3 | 48.75 | 4.89 |
| 50.0 | 46.7 | 44.56 | 3.94 | |
| 66.7 | 65.8 | 40.82 | 3.22 | |
| Modifier | Molar Content [FMA], % | Contact Angle of Wetting, ° | SE, mN/m | D, mN/m | p, mN/m | RQ * | ||
|---|---|---|---|---|---|---|---|---|
| Water | n-Decane | Diiodomethane | ||||||
| Poly-GMA | 0 | 72.2 ± 1.1 | 14.0 ± 0.9 | 32.7 ± 2.1 | 40.24 ± 0.84 | 29.49 ± 0.38 | 10.75 ± 0.47 | 0.95 |
| Poly-(TEMA-co-GMA) | 29.0 | 96.5 ± 1.0 | 28.6 ± 1.0 | 61.0 ± 0.4 | 25.75 ± 0.41 | 23.38 ± 0.15 | 2.36 ± 0.25 | 0.98 |
| 44.4 | 97.0 ± 1.5 | 31.7 ± 0.5 | 70.4 ± 0.3 | 23.30 ± 0.43 | 20.55 ± 0.13 | 2.75 ± 0.38 | 0.99 | |
| 62.1 | 100.4 ± 1.4 | 32.6 ± 1.0 | 71.1 ± 0.6 | 22.36 ± 0.45 | 20.47 ± 0.18 | 1.89 ± 0.27 | 0.99 | |
| Poly-TEMA | 100 | 103.5 ± 1.4 | 35.5 ± 0.4 | 74.1 ± 0.4 | 20.91 ± 0.33 | 19.53 ± 0.14 | 1.38 ± 0.22 | 0.99 |
| Poly-(HFMA-co-GMA) | 29.0 | 92.0 ± 1.0 | 13.0 ± 0.3 | 57.3 ± 1.1 | 28.59 ± 0.42 | 25.33 ± 0.26 | 3.25 ± 0.30 | 0.99 |
| 41.7 | 98.1 ± 1.0 | 30.3 ± 0.4 | 71.4 ± 0.6 | 22.97 ± 0.32 | 20.52 ± 016 | 2.46 ± 0.19 | 0.99 | |
| 61.7 | 101.9 ± 0.7 | 39.8 ± 0.7 | 81.8 ± 0.7 | 19.08 ± 0.35 | 16.91 ± 0.14 | 2.17 ± 0.16 | 0.96 | |
| Poly-HFMA | 100 | 104.2 ± 0.4 | 44.2 ± 0.7 | 84.5 ± 1.0 | 17.65 ± 0.52 | 15.79 ± 0.41 | 1.86 ± 0.11 | 0.94 |
| Poly-(HIMA-co-GMA) | 29.8 | 99.5 ± 1.3 | 39.3 ± 2.5 | 78.6 ± 2.3 | 20.31 ± 0.68 | 17.65 ± 0.84 | 2.66 ± 0.14 | 0.99 |
| 42.7 | 103.0 ± 1.0 | 42.3 ± 0.6 | 82.4 ± 1.2 | 18.51 ± 0.49 | 16.49 ± 0.40 | 2.02 ± 0.17 | 0.96 | |
| 64.9 | 109.9 ± 1.0 | 44.1 ± 0.7 | 86.1 ± 1.8 | 16.66 ± 0.69 | 15.88 ± 0.64 | 0.79 ± 0.10 | 0.92 | |
| Poly-HIMA | 100 | 110.3 ± 1.6 | 50.5 ± 0.5 | 86.5 ± 1.7 | 15.67 ± 0.44 | 14.77 ± 0.40 | 0.90 ± 0.27 | 0.97 |
| Poly-(HBMA-co-GMA) | 29.3 | 104.9 ± 0.6 | 49.0 ± 1.4 | 86.0 ± 0.8 | 16.72 ± 0.36 | 14.83 ± 0.38 | 1.90 ± 0.40 | 0.95 |
| 46.7 | 107.1 ± 0.5 | 50.2 ± 0.5 | 86.1 ± 0.4 | 16.19 ± 0.32 | 14.73 ± 0.23 | 1.46 ± 0.10 | 0.96 | |
| 65.8 | 110.1 ± 0.8 | 55.2 ± 0.6 | 93.7 ± 1.2 | 13.76 ± 0.42 | 12.46 ± 0.35 | 1.29 ± 0.09 | 0.90 | |
| Poly-HBMA | 100 | 111.0 ± 0.6 | 57.0 ± 0.6 | 91.6 ± 0.3 | 13.70 ± 0.32 | 12.74 ± 0.13 | 0.96 ± 0.20 | 0.91 |
| Modifier | Molar Content [FMA], % | Concentration, At.% | |||
|---|---|---|---|---|---|
| Al | O | C | F | ||
| Initial Al | --- | 91.9 | 3.1 | 4.9 | --- |
| Textured Al | --- | 92.4 | 7.1 | --- | |
| Poly-GMA | 0 | 17.3 | 50.1 | 32.5 | --- |
| Poly-(TEMA-co-GMA) | 29.0 | 20.5 | 54.1 | 22.4 | 2.9 |
| 44.4 | 26.6 | 52.4 | 18.1 | 3 | |
| 62.1 | 23.6 | 51.7 | 20.6 | 4.2 | |
| Poly-TEMA | 100 | 22.6 | 50.9 | 20.4 | 6.1 |
| Poly-(HFMA-co-GMA) | 29.0 | 21.3 | 60.5 | 13.7 | 4.5 |
| 41.7 | 24 | 51.3 | 19.8 | 4.9 | |
| 61.7 | 19.4 | 49.3 | 24.8 | 6.6 | |
| Poly-HFMA | 100 | 21.9 | 62.4 | 8.4 | 7.3 |
| Poly-(HIMA-co-GMA) | 29.8 | 23.1 | 52.4 | 21.1 | 3.4 |
| 42.7 | 23.7 | 52.8 | 19 | 4.5 | |
| 64.9 | 22.6 | 50.5 | 21.4 | 5.5 | |
| Poly-HIMA | 100 | 24.2 | 50 | 18.2 | 7.6 |
| Poly-(GMA-co-HBMA) | 29.3 | 27.5 | 48.9 | 19.9 | 3.6 |
| 46.7 | 23.4 | 53.2 | 18 | 5.3 | |
| 65.8 | 19.7 | 48.4 | 25 | 6.8 | |
| Poly-HBMA | 100 | 19.6 | 51.4 | 19.9 | 9.1 |
| Modifier | Molar Content [FMA], % | Contact Angle of Wetting, ° | SE, mN/m | D, mN/m | p, mN/m | |
|---|---|---|---|---|---|---|
| Water | Diiodomethane | |||||
| Poly-GMA | 0 | 144.2 ± 2 | 53.5 ± 2 | 45.89 | 38.44 | 7.45 |
| Poly-(TEMA-co-GMA) | 29.0 | 159.7 ± 3 | 154.6 ± 2 | 0.13 | 0.11 | 0.02 |
| 44.4 | 163.0 ± 2 | 154.6 ± 2 | 0.12 | 0.12 | 0.00 | |
| 62.1 | 168.0 ± 3 | 160.7 ± 3 | 0.04 | 0.04 | 0.00 | |
| Poly-TEMA | 100 | 168.0 ± 3 | 161.9 ± 2 | 0.03 | 0.03 | 0.00 |
| Poly-(HFMA-co-GMA) | 29.0 | 163.8 ± 2 | 160.7 ± 3 | 0.05 | 0.04 | 0.01 |
| 41.7 | 165.5 ± 3 | 161.6 ± 3 | 0.03 | 0.03 | 0.00 | |
| 61.7 | 166.1 ± 3 | 163.5 ± 3 | 0.02 | 0.02 | 0.00 | |
| Poly-HFMA | 100 | 168.7 ± 2 | 163.6 ± 3 | 0.02 | 0.02 | 0.00 |
| Poly-(HIMA-co-GMA) | 29.8 | 166.3 ± 2 | 161.1 ± 3 | 0.04 | 0.04 | 0.00 |
| 42.7 | 167.0 ± 3 | 162.3 ± 3 | 0.03 | 0.03 | 0.00 | |
| 64.9 | 169.0 ± 3 | 163.4 ± 3 | 0.02 | 0.02 | 0.00 | |
| Poly-HIMA | 100 | 169.2 ± 2 | 163.6 ± 3 | 0.02 | 0.02 | 0.00 |
| Poly-(GMA-co-HBMA) | 29.3 | 167.7 ± 3 | 162.1 ± 3 | 0.03 | 0.03 | 0.00 |
| 46.7 | 168.8 ± 3 | 163.1 ± 3 | 0.02 | 0.02 | 0.00 | |
| 65.8 | 169.0 ± 3 | 163.8 ± 3 | 0.02 | 0.02 | 0.00 | |
| Poly-HBMA | 100 | 170.0 ± 2 | 163.8 ± 2 | 0.02 | 0.02 | 0.00 |
| Modifier | Molar Content [FMA], % | Roll-off Angle as a Function of the Rate of Inclination of the Plane to the Horizon (°/s), ° | ||
|---|---|---|---|---|
| 0.37°/s | 0.61°/s | 1.1°/s | ||
| Poly-(GMA-co-TEMA) | 29.0 | 10.8 ± 3.5 | 10.4 ± 3.2 | 8.7 ± 2.0 |
| 44.4 | 9.7 ± 1.1 | 9.6 ± 2.3 | 8.9 ± 1.0 | |
| 62.1 | 5.6 ± 1.8 | 4.9 ± 2.2 | 3.5 ± 1.4 | |
| Poly-TEMA | 100 | 4.8 ± 1.6 | 2.8 ± 1.6 | 2.6 ± 1.2 |
| Poly-(GMA-co-HFMA) | 29.0 | 15.7 ± 1.1 | 9.7 ± 2.8 | 8.6 ± 2.4 |
| 41.7 | 7.8 ± 3.3 | 6.6 ± 2.6 | 5.7 ± 1.1 | |
| 61.7 | 5.4 ± 1.6 | 5.3 ± 1.6 | 4.8 ± 1.0 | |
| Poly-HFMA | 100 | 4.7 ± 1.2 | 4.0 ± 2.0 | 3.5 ± 2.0 |
| Poly-(GMA-co-HIMA) | 29.8 | 8.8 ± 2.5 | 6.4 ± 2.5 | 5.8 ± 2.3 |
| 42.7 | 7.8 ± 2.2 | 5.8 ± 2.0 | 5.0 ± 1.8 | |
| 64.9 | 5.8 ± 2.1 | 4.2 ± 2.0 | 3.4 ± 1.8 | |
| Poly-HIMA | 100 | 3.7 ± 1.2 | 2.9 ± 1.5 | 2.3 ± 1.4 |
| Poly-(GMA-co-HBMA) | 29.3 | 6.1 ± 1.7 | 5.6 ± 2.5 | 4.5 ± 2.0 |
| 46.7 | 5.3 ± 1.3 | 5.2 ± 0.6 | 4.5 ± 1.7 | |
| 65.8 | 5.4 ± 1.1 | 4.4 ± 1.6 | 3.7 ± 1.7 | |
| Poly-HBMA | 100 | 4.1 ± 1.1 | 3.1 ± 1.3 | 2.8 ± 1.0 |
| Modifier | Percentage of Spontaneous rolling off of Water Droplets When They Are Dropped Onto a Plane with a Given Angle of Inclination to the Horizon, % | ||
|---|---|---|---|
| 3° | 5° | 7° | |
| Poly-(GMA-co-TEMA) | 83% | 93% | 100% |
| Poly-(GMA-co-HFMA) | 80% | 91% | 96% |
| Poly-(GMA-co-HIMA) | 80% | 93% | 95% |
| Poly-(GMA-co-HBMA) | 80% | 93% | 98% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klimov, V.V.; Kolyaganova, O.V.; Bryuzgin, E.V.; Navrotsky, A.V.; Novakov, I.A. Effect of the Composition of Copolymers Based on Glycidyl Methacrylate and Fluoroalkyl Methacrylates on the Free Energy and Lyophilic Properties of the Modified Surface. Polymers 2022, 14, 1960. https://doi.org/10.3390/polym14101960
Klimov VV, Kolyaganova OV, Bryuzgin EV, Navrotsky AV, Novakov IA. Effect of the Composition of Copolymers Based on Glycidyl Methacrylate and Fluoroalkyl Methacrylates on the Free Energy and Lyophilic Properties of the Modified Surface. Polymers. 2022; 14(10):1960. https://doi.org/10.3390/polym14101960
Chicago/Turabian StyleKlimov, Viktor V., Olga V. Kolyaganova, Evgeny V. Bryuzgin, Alexander V. Navrotsky, and Ivan A. Novakov. 2022. "Effect of the Composition of Copolymers Based on Glycidyl Methacrylate and Fluoroalkyl Methacrylates on the Free Energy and Lyophilic Properties of the Modified Surface" Polymers 14, no. 10: 1960. https://doi.org/10.3390/polym14101960
APA StyleKlimov, V. V., Kolyaganova, O. V., Bryuzgin, E. V., Navrotsky, A. V., & Novakov, I. A. (2022). Effect of the Composition of Copolymers Based on Glycidyl Methacrylate and Fluoroalkyl Methacrylates on the Free Energy and Lyophilic Properties of the Modified Surface. Polymers, 14(10), 1960. https://doi.org/10.3390/polym14101960






